Abstract
Urolithiasis is a multifactorial disease, the onset and severity of which is influenced by both genetic and environmental factors. This study represents an investigation of the role of vitamin D receptor (VDR) gene polymorphisms (ApaI, BsmI, and TaqI) and combined genotypes in urolithiasis in a Turkish population. We studied 110 patients with urinary stones and 150 control subjects. The polymorphic regions were amplified using polymerase chain reaction, followed by digestion with restriction enzymes BsmI, ApaI, and TaqI, and analyzed electrophoretically. Genotype and allele frequencies were calculated, and the association with urolithiasis, family history, and recurrence of stone was investigated. Our data provide no evidence for an association between urolithiasis and VDR ApaI, BsmI, and TaqI genotypes. We also analyzed the effects of VDR ApaI, BsmI, and TaqI genotypes in combination; the “GTT” VDR haplotype, constructed from three adjacent restriction fragment length polymorphisms was overrepresented among the urolithiasis patients. However, no significant differences between heterozygous carriers (OR 1.302; 95% CI 0.527–3.215) and homozygous carriers (OR 3.39; 95% CI 0.719–15.985) were observed in our study population. A significant association was found only between the ApaI polymorphism and family history (P=0.017; χ 2=5.657). Our data indicate that the VDR ApaI, BsmI, and TaqI polymorphisms do not confer a significant risk for urolithiasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin D plays an important role in skeletal muscle metabolism, including calcium absorption and bone loss, and has also been shown to play an important role in other metabolic pathways, including those involved in immune response and cancer [1].
Vitamin D receptor (VDR) gene (OMIM 601769) can have profound effects on mineral metabolism and bone mineral density [2–4]. The 3′ untranslated (UTR) region of the VDR gene includes a cluster of linked polymorphisms: BsmI, ApaI, and TaqI sites [4–6]. TaqI, BsmI, and ApaI polymorphisms of the VDR gene do not cause any amino acid change in the protein [7].
The effect of vitamin D on target cells is mediated by the interaction between its active metabolite, calcitriol, and its cellular receptor, VDR [8]. In view of their potential influence on the hormonal signal, VDR gene polymorphisms have been recently studied in disorders of calcium metabolism [7, 9, 10] and in urinary calcium stone disease [11, 12]. There are few studies on the correlation of VDR polymorphism with urolithiasis [13–15]. Because the genetic effect may be different in different populations, the aim of this study was to investigate the relationship between urolithiasis, and the BsmI, ApaI, and TaqI polymorphisms of the VDR gene in a Turkish population. Thus, we first analyzed the relationship between urolithiasis and BsmI, ApaI, and TaqI polymorphisms, and then studied the combined influence of polymorphisms in the VDR gene.
Patients and methods
The study was designed as a case–control study. The control subjects and patients attended the Urology Clinic between February 2003 and September 2005. The study group consisted of 110 unrelated Turkish patients (67 men and 43 women; mean age 49.22±1.33, range 17–71) with urolithiasis, who had radioopaque stones on abdominal X-rays, and 150 healthy unrelated volunteers as the control group (73 men and 77 women; mean age 48.15±1.62, range 30–68), who reside in the same geographic area as the patients and had no history of familial stone. All subjects were of Turkish origin from the Black Sea Coastal Region. The exclusion criteria for patients and controls were the presence of chronic urinary tract infection, renal failure, other metabolic diseases, chronic diarrhea or gout, primary and secondary hyperparathyroidism, cancer, and osteoporosis. A family history of urolithiasis was sought from each patient. Family history was considered positive if any of siblings, parents, grandparents, or parental siblings had a history of renal-stone disease. Forty-six patients (46%) reported a family history of stones. Fifty-seven patients (52%) had recurrent stones. Written and oral informed consent was obtained from all subjects in accordance with the Helsinki Declaration 1975 (revised 2000).
Genomic DNA was isolated from the blood samples by a “salting out” method [16].
Three polymorphisms, dbSNP BsmI (rs1544410), ApaI (rs11168271), and TaqI (rs731236) in VDR were identified from the National Center for Biotechnology Information LocusLink database (www.ncbi.nih.gov/LocusLink). The genotypes for three restriction fragment length polymorphisms of the VDR were determined by polymerase chain reaction (PCR; Techne Gradient, Cambridge, UK) and enzymatic digestion of the products with BsmI, ApaI, and TaqI restriction enzymes.
The primer sequences (Iontec, Bursa, Turkey) were: intron 8, BsmI polymorphic site 5′-CAA CCA AGA CTA CAA GTA CCG CGT CAT GA-3′ forward and 5′-AAC CAG CGG GAA GAG GTC AAG G G-3′ reverse; intron 8 and exon 9, ApaI and TaqI polymorphic sites: 5′-CAG AGC ATG GAC AGG GAG CAA-3′ forward, 5′-CAC TTC GAG CAC AAG GGG CGT TAG C-3′ reverse.
An 825-bp fragment encompassing the BsmI polymorphic site was amplified. PCR reaction was performed in 25 μl; 1× PCR buffer containing 20 pmol of each primer, 2.5 mM MgCl2, 200 mM of each dNTP (MBI, Fermentas, Lithuania), 50 ng DNA, and 1.25 U Taq polymerase (MBI). Following initial denaturation at 94°C for 5 min, amplification was performed by 35 cycles of denaturation at 94°C for 30 s, annealing at 57°C for 30 s, and extension at 72°C for 30 s. Final extension was allowed to proceed at 72°C for 5 min [7]. Eight microliters of the PCR products were digested with 10 U of BsmI (MBI) at 37°C.
Amplification of the 490-bp fragment encompassing ApaI and TaqI polymorphic sites was performed in 25 μl; 1× PCR buffer containing 20 pmol of each primer, 2.5 mM MgCl2, 200 mM of each dNTP, 50 ng DNA, and 1.25 U Taq polymerase. Following initial denaturation at 94°C for 5 min, amplification was performed by 35 cycles of denaturation at 94°C for 30 s, annealing at 64°C for 30 s, and extension at 72°C for 30 s. The reaction was terminated by extension at 72°C for 5 min. Eight microliters of the PCR products were digested with 10 U of ApaI (MBI) at 22°C and 10 U of TaqI (MBI) at 65°C [17].
All of the digested products were resolved on 2% agarose gel and analyzed in a video gel documentation system (Vilbert Lourmat, Cedex, France) after staining with ethidium bromide.
We then carried out haplotype analysis as the SNPs are frequently inherited together. ApaI, BsmI, and TaqI SNPs were assessed in relation to each other by a direct molecular haplotyping PCR procedure [18, 19]. For the comparison of carriage rate of VDR genotypes, we made reference, homozygote, and heterozygote groups for VDR alleles. Among the triple combinations, genotypes AAGAGG (0.77%), GGGTTT (0.77%), GGGTTC (0.77%), AAGGTC (1.9%), and GATTTT (0.77%) accounted each for less than 2% of the study population. Thus, we excluded the 11 patients containing one of these genotypes from this haplotype analysis. Because there was an overrepresentation of family history in urolithiasis patients with the “GTT” (formerly baT) haplotype, we grouped patients according to the carrier status for this VDR haplotype as homozygous carriers (GGTTTT) and heterozygous carriers (including GAGTTC and GAGGTC genotypes) of the risk haplotype and compared with patients not carrying the haplotype (including GGGTTT, AAGGCC, and GGGGTT genotypes).
The statistical analysis was performed using a commercially available software program (SPSS 12.0, SPSS Inc., Chicago, IL, USA). Associations between the disease and genotypes were assessed by calculating odds ratios (OR) and 95% confidence intervals (CI). VDR genotype distribution in urolithiasis was compared with that in controls using the χ 2 test (two-sided) according to Hardy–Weinberg equilibrium. The expected values were compared with those observed in the controls and cases by the χ 2 test contingency tables. The likelihood ratio test statistic was used to test for genotype distribution in urolithiasis patients with and without family histories. ORs and 95% CIs were calculated by multivariate logistic regression analysis to estimate the relative risk of family history by genotype. A probability of less than 0.05 was required for statistical significance.
Results
Genotype and allele frequencies for ApaI, BsmI, and TaqI
According to the single nucleotide polymorphisms (SNP) database VDR BB, Bb, and bb genotypes are referred to as AA, AG, and GG; VDR AA, Aa, and aa genotypes are referred to as GG, GT, and TT, and VDR TT, Tt, and tt genotypes are referred to as TT, TC, and CC, respectively.
BsmI, ApaI, and TaqI polymorphisms of VDR were investigated by PCR/RFLP. After digestion with BsmI, the three possible genotypes were defined by the three distinct banding patterns: AA (825 bp), GA (175, 650, and 825 bp), and GG (175 and 650 bp) (Fig. 1).
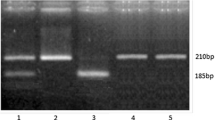
The primer pair used to analyze ApaI and TaqI polymorphisms produces 490-bp fragment, which was cut into 210 bp and 280-bp fragments with ApaI digestion or into 200 bp and 290-bp fragments by TaqI digestion. After digestion with ApaI, the three possible genotypes were defined by the three distinct banding patterns: GG (490 bp), GT (490, 280, and 210 bp), and TT (200 and 290 bp). After digestion with TaqI, the three possible genotypes were defined by the three distinct banding patterns: TT (490 bp), TC (490, 290, and 200 bp), and CC (200 and 290 bp) (Figs. 2, 3).
The genotype frequencies and distribution of alleles are given in Table 1. The genotype frequencies of the three VDR polymorphisms in the control group were in Hardy–Weinberg equilibrium. Allele effects were calculated using AA (formerly BB), GG (formerly AA), and TT as reference groups. No significant differences were observed between genotype frequencies of the controls and the patients for ApaI (P=0.742; χ 2=0.595), BsmI (P=0.297; χ 2 =2.429), and TaqI (P=0.248; χ 22 =1.34) polymorphisms of VDR gene.
We also investigated the associations between VDR genotypes and family histories and the recurrence of stones. There was no association between the BsmI (P=0.247; χ 2=2.794) and TaqI (P=0.425; χ 2=0.570) genotypes and family histories for urolithiasis, although there was a higher prevalence of the TT (formerly aa) genotype amongst patients reporting positive family history (GG vs TT; OR 5.57; 95% CI 1.104–31.51, P=0.017; χ 2=5.657). No significant differences were observed between recurrence of stone and BsmI (P=0.307; χ 2 =4.810), ApaI (P=0.817; χ 2=1.553), and TaqI (P=0.665; χ 2 =2.385) genotypes of VDR gene.
Haplotype and combined genotype frequencies
As the three SNPs that we studied showed strong linkage disequilibrium (LD) [7], we also investigated the effects of combined genotypes on urolithiasis. The distribution of combined genotype frequencies showed statistically non-significant differences (P=0.463) between urolithiasis and control groups (Table 2). Although the patients homozygous for the risk haplotype had risk for urolithiasis that was close to statistical significance (OR 3.39; 95% CI 0.719–15.989) compared with the risk of the patients not carrying the haplotype, heterozygous carriers had a risk of 1.302 (0.502–3.215) (Table 3). There was no association between the haplotype and family history (P=0.725, χ 2=0.725) and disease recurrence (P=8.288, χ 2=0.874).
Discussion
Urolithiasis is a complex disease involving multifactorial causes. Different genes may influence different aspects of the disease pathology. The VDR plays a role in regulating calcium homeostasis by affecting bone resorption and increasing calcium absorption. The mechanism of stone formation via the VDR is not resolved yet. Allelic differences in the 3′ UTR region may alter the regulation of messenger RNA stability and/or translation and thus affect vitamin D activity, hence predisposing to stone disease [14]. Polymorphic variations in VDR gene are associated with stone disease in some but not all studies [13–15, 20–22]. Besides the ApaI, BsmI, and TaqI polymorphisms, other allelic variation in the VDR gene, such as FokI may [22–24] or may not [21, 25] also be associated with calcium oxalate stone disease. Relan et al. [25] stated that there might be an association between F allele and higher calcium excretion in nephrolithiatic subjects. Mossetti et al. [26] reported a genetic association between BsmI and TaqI polymorphisms and idiopathic hypocitraturia in calcium-oxalate recurrent stone formers. These findings may reflect genetic association between urolithiaisis and VDR polymorphisms.
In the present study, we investigated the association of ApaI, BsmI, and TaqI polymorphisms of the VDR gene in urolithiasis patients and controls in a Turkish population. In our study, we did not observe a significant difference between the frequencies of ApaI, BsmI, and TaqI alleles in the patient and control groups. The findings of this study indicate that the VDR gene ApaI alleles were not associated with urolithiasis (GG vs GT+TT; OR 1.13; 95% CI 0.66–1.95; P=0.62; χ 2=0.2). Although a report supports an association between the ApaI polymorphism and calcium nephrolithiasis in another Turkish population [15], our results are more in line with a report suggesting the lack of association in a Japanese population [14]. On the other hand, we observed a significant positive association of the TT genotype for ApaI amongst patients reporting positive family history (P=0.017). This data is consistent with the findings of Özkaya et al. [15].
The frequencies of the TaqI genotypes were not significantly different in patient and control groups (TT vs TC+CC; OR 1.35; 95% CI 0.78–2.33; P=0.248; χ 2=1.34). Two studies [14, 15], reporting no difference in the distribution of TaqI genotypes between the study and control groups, indicated that TaqI genotypes were associated with more aggressive stone disease [14], family history, and recurrence of the disease [15].
We also found no statistically significant difference in the allelic and genotypic frequencies of BsmI in the patient and control groups (AA vs GA+GG; OR 0.92; 95% CI 0.42–2.02; P=0.819; χ 2=0.05). Several studies have also not found any significant association between BsmI VDR polymorphism and stone formation [15, 25, 27]. Gennari et al. reported that intestinal calcium absorption was significantly lower in the AA genotype than in the GG genotype in the presence of similar levels of vitamin D [28]. Ruggiero et al. reported that the GG genotype shows a higher calcium excretion than the AA genotype [29]. It is possible that the opposing effects of AA and GG genotypes on calcium metabolism may cancel out each other in heterozygous individuals.
Studies on the VDR gene polymorphisms associated with osteoporosis, osteomalacia, breast cancer, and hypercalcemia have been reported in a Turkish population [30–33]. Kaya et al. investigated the association between VDR gene polymorphism and psoriasis in a Turkish population [34]. The genotypes and their frequencies in our control group for ApaI, BsmI, and TaqI were similar to the frequencies in the control group of the above-mentioned study, confirming the accuracy of our results (G: 69%, A: 41%, and T: 67%) [34]. The frequencies of BsmI, ApaI, and TaqI alleles may vary among different populations. We found the frequencies of G, A, and T alleles at 63, 38, and 65%, respectively. The frequencies of G and A alleles in the present study were in between the frequencies of Caucasian (44% for G allele, 74% for A allele) and Asian (42% for A allele, 7% for G allele) populations. The T allele of the TaqI polymorphism has a higher frequency compared to those of Caucasians and Asians (43 and 8%, respectively) [35].
The conflicting results may stem from the complexity of urolithiasis etiology, genetic heterogeneity of disease, ethnicity, differences in population characteristics, including interaction with environmental factors, selection of control group, sample size, and gene–gene and gene–environment interactions. The VDR polymorphisms we have investigated are probably non-functional and are unlikely to be directly involved with stone disease. However, they could be in LD with one or more functional polymorphisms yet to be identified elsewhere in the VDR gene.
Strong LD at 3′ end of the gene has been observed for the BsmI, ApaI, and TaqI. LD measures a co-occurrence of alleles of adjacent polymorphisms with each other [36]. This means that one polymorphism can predict the other adjacent “linked” one or display statistical dependence because very little recombination has occurred between them. In this study, the distribution of combined genotype frequencies showed statistically non-significant differences (P=0.463) between the urolithiasis and control groups (Table 2). Although heterozygous carriers had a risk of 1.302 (0.502–3.215) as compared with the frequency of patients with reference genotype, the frequency of homozygous [GGTTTT (bbaaTT)] patients was close to the statistical significance level for urolithiasis (OR 3.39; 95% CI 0.719–15.989), compared with patients not carrying the haplotype.
Susceptible genes for stone disease are being sought by serial association studies that screen DNA polymorphisms in genes including the VDR gene, p21 gene, and calcitonin receptor gene [19, 37, 38]. There is evidence that multiple genes contribute to disease susceptibility, each of which may confer a small increase in risk [39]. Further understanding of the biological mechanism underlying these genetic differences and their relationship with environmental factors will help define the role of the VDR gene and urolithiasis.
In conclusion, we found no specific causal role of the ApaI, BsmI, and TaqI VDR gene polymorphism in urolithiasis.
References
Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, Dominguez CE, Jurutka PW (1998) The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res 13:325
Lin NU, Malloy PJ, Sakati N, al-Ashwal A, Feldman D (1996) A novel mutation in the deoxyribonucleic acid-binding domain of the vitamin D receptor causes hereditary 1,25-dihydroxyvitamin D-resistant rickets. J Clin Endocrinol Metab 81:2564
Malloy PJ, Eccleshall TR, Gross C, Van Maldergem L, Bouillon R, Feldman D (1997) Hereditary vitamin D resistant rickets caused by a novel mutation in the vitamin D receptor that results in decreased affinity for hormone and cellular hyporesponsiveness. J Clin Invest 99:297
Taylor JA, Hirvonen A, Watson M, Pittman G, Mohler JL, Bell DA (1996) Association of prostate cancer with vitamin D receptor gene polymorphism. Cancer Res 56(18):4108
Morrison NA, Yeoman R, Kelly PJ, Eisman JA (1992) Contribution of trans-acting factor alleles to normal physiological variability: vitamin D receptor polymorphisms and circulating osteocalcin. Proc Natl Acad Sci USA 89:6665
Feldman D (1997) Androgen and vitamin D receptor gene polymorphisms: the long and short of prostate cancer risk. J Natl Cancer Inst 89:109
Morrison NA, Qi JC, Tokita A, Kelly PJ, Crofts L, Nguyen TV, Sambrook PN, Eisman JA (1994) Prediction of bone density from vitamin D receptor alleles. Nature 367:284
Jones G, Strugnell SA, DeLuca H (1998) Current understanding of the molecular actions of vitamin D. Physiol Rev 78:1193
Dawson-Hughes B, Harris SS, Finneran S (1995) Calcium absorption on high and low calcium intakes in relation to vitamin D receptor genotype. J Clin Endocrinol Metab 80:3657
Sainz J, Van Tornout JM, Loro ML, Sayre Jroe TF, Gilsanz V (1997) Vitamin D-receptor gene polymorphisms and bone density in prepubertal American girls of Mexican descent. N Engl J Med 337:77
Scott P, Ouimet D, Valiquette L, Guay G, Proulx Y, Trouve ML, Gagnon B, Bonnardeaux A (1999) Suggestive evidence for a susceptibility gene near the vitamin D receptor locus in idiopathic calcium stone formation. J Am Soc Nephrol 10:1007
Zerwekh JE, Hughes MR, Reed BJ, Breslau NA, Helleer HJ, Lemke M, Nasonkin I, Pak CY (1995) Evidence for normal vitamin D receptor messenger ribonucleic acid and genotype in absorptive hypercalciuria. J Clin Endocrinol Metab 80:2960
Jackman SV, Kibel AS, Ovuworie CA, Moore RG, Kavosussi LR, Jarrett TW (1999) Familial calcium stone disease: TaqI polymorphism and the vitamin D receptor. J Endourol 13(4):313
Nishijima S, Sugaya K, Naito A, Morozumi M, Hatano T, Ogawa Y (2002) Association of vitamin D receptor gene polymorphism with urolithiasis. J Urol 167:2188
Özkaya O, Söylemezoğlu O, Mısırlıoğlu M, Gönen S, Buyan N, Hasanoğlu E (2003) Polymorphisms in the vitamin D receptor gene and risk of calcium nephrolithiasis in children. Eur Urol 44:150
Miller SA, Dykes DD (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16:1215
Habuchi T, Suzuki T, Sasaki R, Wang L, Sato K, Satoh S, Akao T, Tsuchiya N, Shimoda N, Wada Y, Koizumi A, Chihara J, Ogawa O, Kato T (2000) Association of vitamin D receptor gene polymorphism with prostate cancer and benign prostatic hyperplasia in a Japanese population. Cancer Res 60(2):305
Uitterlinden AG, Pols HA, Burger H, Huang Q, Van Daele PL, Van Duijn CM, Hofman A, Birkenhager JC, Van Leeuwen JP (1996) A large-scale population-based study of the association of vitamin D receptor gene polymorphisms with bone mineral density. J Bone Miner Res 11(9):1241
Uitterlinden AG, Weel AE, Burger H, Fang Y, van Duijn CM, Hofman A, van Leeuwen JP, Pols HA (2001) Interaction between the vitamin D receptor gene and collagen type Ialpha1 gene in susceptibility for fracture. J Bone Miner Res 16(2):379
Heilberg IP, Teixeira SH, Martini LA, Boim MA (2002) Vitamin D receptor gene polymorphism and bone mineral density in hypercalciuric calcium-stone-forming patients. Nephron 90(1):51
Vezzoli G, Soldati L, Proverbio MC, Adamo D, Rubinacci A, Bianchi G, Mora S (2002) Polymorphism of vitamin D receptor gene start codon in patients with calcium kidney stones. J Nephrol 15(2):158
Chen WC, Chen HY, Lu HF, Hsu CD, Tsai FJ (2001) Association of the vitamin D receptor gene start codon Fok I polymorphism with calcium oxalate stone disease. BJU Int 87(3):168
Chen WC, Chen HY, Lu HF, Hsu CD, Tsai FJ (2001) Association of vitamin D receptor gene start codon FokI polymorphism with calcium oxalate stone disease. Br J Urol 87:168
Bid HK, Kumar A, Kapoor R, Mittal RD (2005) Association of vitamin D receptor-gene (FokI) polymorphism with calcium oxalate nephrolithiasis. J Endourol 19(1):111
Relan V, Khullar M, Singh SK, Sharma SK (2004) Association of vitamin D receptor genotypes with calcium excretion in nephrolithiatic subjects in northern India. Urol Res 32(3):236
Mossetti G, Vuotto P, Rendina D, Numis FG, Viceconti R, Giordano F, Cioffi M, Scopacasa F, Nunziata V (2003) Association between vitamin D receptor gene polymorphisms and tubular citrate handling in calcium nephrolithiasis. J Intern Med 253(2):194
Chan WC, Chan HY, Hsu CD, Wu JY, Tsai FJ (2001) No association of vitamin D receptor gene BsmI polymorphisms with calcium oxalate stone formation. Mol Urol 5(1):7
Gennari L, Becherini L, Masi L, Gonnelli S, Cepollaro C, Martini S, Mansani R, Brandi ML (1997) Vitamin D receptor genotypes and intestinal calcium absorption in postmenopausal women. Calcif Tissue Int 61(6):460
Ruggiero M, Pacini S, Amato M, Aterini S, Chiarugi V (1999) Association between vitamin D receptor gene polymorphism and nephrolithiasis. Miner Electrolyte Metab 25(3):185
Duman BS, Tanakol R, Erensoy N, Ozturk M, Yilmazer S (2004) Vitamin D receptor alleles, bone mineral density and turnover in postmenopausal osteoporotic and healthy women. Med Princ Pract 13(5):260
Kahraman H, Duman BS, Alagol F, Tanakol R, Yilmazer S (2004) Lack of association between vitamin D receptor gene polymorphism (BsmI) and osteomalacia. J Bone Miner Metab 22(1):39
Akcay A, Ozdemir FN, Sezer S, Micozkadioglu H, Arat Z, Atac FB, Verdi H, Sahin F, Haberal M (2005) Association of vitamin D receptor gene polymorphisms with hypercalcemia in peritoneal dialysis patients. Perit Dial Int 3:S52
Buyru N, Tezol A, Yosunkaya-Fenerci E, Dalay N (2003) Vitamin D receptor gene polymorphisms in breast cancer. Exp Mol Med 35(6):550
Kaya TI, Erdal ME, Tursen U, Camdeviren H, Gunduz O, Soylemez F, Ikizoglu G (2002) Association between vitamin D receptor gene polymorphism and psoriasis among the Turkish population. Arch Dermatol Res 294(6):286
Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP (2004) Genetics and biology of vitamin D receptor polymorphisms. Gene 338(2):143
Wall JD, Pritchard JK (2003) Assessing the performance of the haplotype block model of linkage disequilibrium. Am J Hum Genet 73(3):502
Chen WC, Wu HC, Lu HF, Chen HY, Tsai FJ (2001) Arginine form of p21 gene codon 31 is less prominent in patients with calcium oxalate stone. Urol Res 29:94
Chen WC, Wu HC, Lin WC, Wu MC, Hsu CD, Tsai FJ (2001) The association of androgen receptor gene and estrogen receptor gene polymorphisms with urolithiasis in males. Br J Urol 88:432
Mathew C (2001) Post genomic technologies: hunting the genes for common disorders. BMJ 322:1031
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gunes, S., Bilen, C.Y., Kara, N. et al. Vitamin D receptor gene polymorphisms in patients with urolithiasis. Urol Res 34, 47–52 (2006). https://doi.org/10.1007/s00240-005-0033-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-005-0033-1