Abstract
The objectives of this study are to assess the efficacy and safety of retrograde ureteroscopic holmium laser lithotripsy for intrarenal calculi greater than 2 cm in diameter. A total of 24 patients with a stone burden >2 cm were treated with retrograde ureteroscopic laser lithotripsy. Primary study endpoints were number of treatments until the patient was stone free and perioperative complications with a follow-up of at least 3 months after intervention. In 24 patients (11 women and 13 men, 20–78 years of age), a total of 40 intrarenal calculi were treated with retrograde endoscopic procedures. At the time of the initial procedure, calculi had an average total linear diameter of 29.75 ± 1.57 mm and an average stone volume of 739.52 ± 82.12 mm3. The mean number of procedures per patient was 1.7 ± 0.8 (range 1–3 procedures). The overall stone-free rate was 92%. After 1, 2 and 3 procedures 54, 79 and 92% of patients were stone free, respectively. There were no major complications. Minor postoperative complications included pyelonephritis in three cases (7.5%), of whom all responded immediately to parenteral antibiotics. In one patient the development of steinstrasse in the distal ureter required ureteroscopic fragment disruption and basketing. Ureteroscopy with holmium laser lithotripsy represents an efficient treatment option and allows the treatment of large intrarenal calculi of all compositions and throughout the whole collecting system even for patients with a stone burden of more than 2 cm size.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Percutaneous nephrolithotomy (PCNL) represents the gold standard for the management of large renal calculi due to its stone-free rate of more than 90% independent of stone size, location or composition [1–5]. The morbidity associated with PCNL is acceptable for the majority of patients [5–7]. However, the low but significant rate of major complications include acute renal loss, chronic renal failure and prolonged urine leakage [7].
However, for a certain group of patients with large renal stones, PCNL is not considered to be the ideal treatment [8, 9]. The indication for a different surgical approach (e.g., retrograde ureteroscopic treatment) of large upper urinary tract calculi includes morbid obesity (i.e., body mass index > 30), bleeding diathesis, severe cardiopulomonary disease, certain anatomic factors such as severe kyphoscoliosis and renal ektopia and the inability to tolerate the potential morbidities and/or complications of PCNL, as well as failed prior PCNL [8–10].
A new generation of flexible ureteroscopes with reduced distal tip and midshaft size are now widely available. Compared to their predecessors access to the complete upper urinary tract in up to 94% of cases is facilitated [11]. Due to these improvements the therapeutic role of ureteroscopic treatment is may be extended.
Ureterorenoscopy in combination with Ho:YAG laser lithotripsy has become a widely recognized technique for the treatment of all types of ureteral and intrarenal calculi, including the treatment of complex calculi, previously preserved for primary percutaneous approaches [10, 11].
Endoscopic ureteroscopy in combination with Ho: YAG laser lithotripsy as a viable treatment has found its way into recent literature even for large renal calculi with a diameter of more than 2 cm. In these small series stone-free rates comparable to those of PCNL with less complications have been reported [8, 9, 12–14].
We evaluated the efficacy of ureteroscopic Ho:YAG laser lithotripsy in a single-center experience with regard to stone-free rate and morbidity for intrarenal stone burden >2 cm.
Materials and methods
We retrospectively analyzed our database of patients treated between December 2004 and June 2009 at our institution for urinary calculi. During this period 1,279 ureteroscopic procedures were performed and a total of 24 patients (11 women and 13 men) presenting with an upper urinary tact calculus burden with a total linear calculus diameter on standard imaging or CT scans of >2 were identified and included in the study.
All patients were either poor candidates for standard percutaneous approach and were therefore referred to our institution, or had an unsatisfactory experience from previous PCNL or ESWL treatments and preferred the ureteroscopic approach (Table 1).
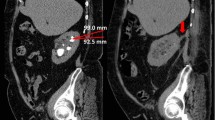
Stone size was obtained from patient chart and radiographic film review. Total linear calculus diameter was determined by measuring the largest linear dimension (transverse or cranial caudal section). In patients presenting with multiple calculi in one collecting system, sums of linear measurements of individual calculi were totaled.
Postoperative evaluation included plain abdominal films taken immediately after treatment before leaving the operation theater. In the postoperative follow-up patients received either abdominal ultrasonography, plain films or CT scans depending on the doctor’s choice and availability. In these imaging modalities any component of obstruction, hydronephrosis or residual stone burden was assessed. Control evaluation 3 months after intervention included clinical assessment and either renal ultrasonography plain films or CT scan at our institution.
The outcome was assessed with regard to stone-free and complication rates. Immediate success was based on X-ray imaging at the end of the ureteroscopic treatment. Stone free was defined as pulverization of all stones to fine dust and the absence of any stone fragments larger than 2 mm, considered to be too small to extract and to pass spontaneously [9].
Patients requiring complementary procedures such as follow-up ureteroscopy or ESWL were not considered to be successful.
Patients who were classified to be stone free were followed as described by the primary care physician or attending urologist.
Operation setup and technique
Patients were placed under general anesthesia in the dorsal or low lithotomy position and prepared and draped in a sterile fashion. The bladder was entered with a 22 Fr. cystoscope, allowing overall cystoscopy and visualization of the ureteral orifice. Under fluoroscopic guidance, the ureteral orifice was cannulated with a 0.038-in. hydrophilic guide wire that was passed into the renal pelvis. 7.5 Fr. flexible ureteroscopes implying a two-way active tip deflection mechanism and maintaining a 3.6 Fr. working channel were used to access and endoscopically inspect the amount of stone burden and the location(s) in the kidney. Larger, mobile fragments were directed into an easily accessible calyx, mostly in the upper pole or central region, to fragment to small debris or even pulverize to dust.
The flexible ureteroscope was positioned in the upper urinary tract via a 14 Fr. ureteral access sheath (Cook Medical Bloomington, Illinois, USA) that was placed to the level of the proximal ureter/the ureteropelvic junction in a monorail fashion over the guide wire. Ureteral access sheaths were used routinely to allow frequent passage of the ureteroscope to the collecting system, to enable optimal visualization, to maintain low intrarenal pressure as well as to facilitate calculi fragments extraction.
Because of the primary stone location or after calculus direction from lower or mid-calyxes to better accessible positions of the collecting system (e.g., renal pelvis or upper calyx), in selected cases, the flexible ureteroscopes were changed intraoperatively to semirigid ureteroscopes. The larger working and irrigation channels of the latter facilitate higher irrigation flow, allowing better visibility and the option to use a 365 μm fiber to gain faster fragmentation compared to the flexible ureteroscope in combination with the 220 μm fiber. Semirigid ureteroscopy was conducted with fiber optic ureteroscopes (diameter from 7–9.5 Fr.) using 16 Fr. transurethral bladder drainages to prevent bladder distension and facilitate continuous irrigation in all patients. Sterile saline irrigant was applied through the endoscope-working channel without the use of a syringe system.
We defined a timeframe for ureteroscopic laser lithotripsy that should not exceed 3.0 h; the reasons were limited endoscopic visibility, hindering efficient therapy after debulking large amounts of stone burden and prevent the patient to develop a compartment syndrome due to prolonged lithotomy position. Operative time was defined as the time span from cystoscope placement until the removal of all equipment.
The decision to place a stent postoperatively was based on the following criteria: prolonged procedures (more than 60 min), large amount of debris or evident of ureteral trauma or edema.
Laser lithotripsy
A high power Ho:YAG laser system (Auriga XL, Starmedtec, Starnberg, Germany) was used in all cases. The 220 μm fiber was used with the flexible ureteroscopes in order to allow access to the entire collecting system including the lower pole calices without compromising the maximal endoscope tip deflection to a greater amount. Laser fibers with 365 μm in diameter were used with the semirigid ureteroscopes.
Basketing of fragments was considered in cases with residual fragments more than 2 mm after laser fragmentation and to achieve samples for stone composition analysis. If visibility became a limiting factor which made further access to the fragments or remaining stone burden for defined laser lithotripsy impossible, a staged therapy was mandatory.
Statistical analysis
All data were expressed as mean ± standard error of mean (SEM) calculated using standard statistical methods.
Results
In this study 24 patients (11 male and 13 female) were included and treated by retrograde ureteroscopy in 40 procedures. Patient’s age ranged from 20 to 78 years (mean 55.8 years). Eleven of the 24 patients (45.8%) had prior treatments: eight had multiple ESWL treatments (range 2–8), two patients had previous percutaneous attempts and one patient had had an unsuccessful URS. 58.3% of the patients presented stone burden in the renal pelvis, 29.2% of the patients in the upper pole and central region and 12.5% in the lower pole. At the time of the initial procedure calculi presented with a total linear diameter of 29.75 ± 1.57 mm and an estimated stone volume of 739.52 ± 82.12 mm2. Nine patients presented with multiple, 15 with solitary stones.
Patient demographics and pretreatment stone characteristics are listed in Table 2.
Mean operative time was 114.1 min (range 50–215 min) for a total of 40 treatments. Complete fragmentation with a single procedure was achieved in 13 patients (54%), 6 patients required two treatments (25%) and 5 required three treatments (21%) for stone clearance. The mean number of laser procedures was 1.7 ± 0.8 (range 1–3 procedures).
The primary intervention was performed in 12 patients (50%) using a flexible ureteroscope, in 8 patients (33%) using a combination of flexible and semirigid ureteroscopes and in 4 patients (17%) using semirigid ureteroscope exclusively. All sequence interventions were conducted with flexible ureterocopes to gain access to the complete collecting system. In 37 of 40 (92.5%) procedures a ureteral stent was placed at the end of the procedure to ensure drainage thus preventing transient obstruction.
During the displacement of the ureteral stent 7 (29.2%) patients were evaluated after the final procedure by an attending control ureteroscopic treatment to visually inspect the collecting system for residual fragments.
The overall stone-free rate was 92%. After 1, 2, 3 procedures 54, 79 and 92% of the patients were stone free, respectively. Two patients (8%) were treated with a total of 3 ESWL sessions after the final laser treatment course.
The most common urinary stone composition was calcium oxalate with the monohydrate variety (COM) being the major type in 13 patients (54.16%). Stones analyzed of four patients (16.66%) had a significant amount (greater than 50%) of uric acid, one had pure uric acid and seven patients had compositions of calcium phosphate (16%) with apatite being the major type.
The holmium laser was capable of fragmenting all stone compositions to acceptable amounts of debris. There were no major intraoperative complications noted in this series. In one patient breakage of the Ho:YAG fiber occurred intraoperatively, yet complete extraction was still possible. Minor postoperative complications included pyelonephritis in three cases (7.5%), of whom all responded immediately to parenteral antibiotics. In one patient the development of steinstrasse in the distal ureter required ureteroscopic fragment disruption and basketing.
Discussion
The current treatment options for upper urinary tract calculi include ESWL, retrograde ureteroscopic therapy and the percutaneous antegrade approach.
The American Urological Association guidelines on staghorn calculi recommend PCNL as first line treatment for intrarenal calculi of more than 2 cm and ESWL for stones smaller than 2 cm [3]. With emerging new generation flexible ureteroscopes with greater angles of maximum active tip deflection and improved durability, the complete collecting system was made accessible for ureteroscopic laser lithotripsy. In combination with Ho:YAG laser and its precise and powerful thermal decomposition mechanism, even larger stones can be treated ureteroscopically [9, 10, 14–16].
Besides the improved accessibility of the collection system Ho:YAG laser lithotripsy has another main advantage: the generated stone debris are finer and therefore more likely to pass spontaneously than that of any other lithotripsy device [14, 17]. In addition, soft tissue injury and bleeding are less likely due to the low penetration depth (0.4 mm) of the Ho:YAG laser.
Therefore, the combination of the retrograde ureteroscopic approach and the holmium laser as the lithotrite of choice appears to be an adequate tool to disintegrate urinary calculi larger than 2 cm. On the downside, generating small fragments or fine debris is a time-consuming procedure resulting in long operative time making staged therapy especially for large stone burdens necessary in some cases.
Ho:YAG laser lithotripsy has become the procedure of choice for patients who are poor candidates (e.g., bleeding diathesis, severe cardiopulomonary disease, certain anatomic factors and body mass index > 30), for standard percutaneous therapy. In these selected patients, ureteroscopy combined with Ho:YAG laser lithotripsy may allow a decrease in morbidity and hospital stay with a stone-free rate reaching that of the PCNL approach.
Our own data support the notion that the stone-free rate following ureteroscopic treatment of calculi larger than 2 cm is comparable to that of PCNL [8, 9, 12–14, 18]. We showed a stone-free rate after 1, 2, 3 procedures to be 54, 79 and 92%. This is in line with previous reports showing a stone-free rate of 60–77% after the first stage and of 86.6–91% after second stage [9, 12, 13]. Table 3 overviews the published data of other series [9].
In our series the mean operative time was 114.1 min (range 50–215). We attribute this time span to our effort to completely “melt down” the calculi with the use of the holmium laser and the effort to try to basket as much significant stone debris as possible in order not to leave ‘insignificant’ fragments because they are likely to progress to larger stone burdens and therefore requiring retreatment. The time-consuming combination of flexible and semirigid ureteroscopy in 33% of the patients in this series also contributes to prolonged operative time.
Comparing our results to previous findings, a mean operative time of 30–240 min had been reported [8, 12, 14].
A disadvantage of ureteroscopy to PCNL is that multiple procedures may be required to clear large stone burdens and to extract the fragments as opposed to PCNL which can offer a 95% stone-free rate after the first treatment [12].
In our series patients underwent an average of 1.7 procedures to become stone free, which seems to be reasonable in light of other studies (1.3–2.3 procedures).
The disadvantage of PCNL despite its high success rate is a higher and more severe complication rate compared to ureteroscopy. In the literature the total complication rate of PCNL is up to 83% [7, 19, 20].
Most of these complications are minor complications such as insignificant bleeding or fever, hemorrhage resulting in blood transfusion, renal artery embolization or the need for an emergent nephrectomy, but still remain a major concern [5]. The frequency of major complications is as high as 0.9–4.7% for septicemia and 0.6–1.4% for renal haemorrhage [7]. Major complications resulting from access procedures include pleural injury in 2.3–3.1% and colonic injury in 0.2–08% [7]. The reported blood transfusion rates in earlier reports varied from 14 to 24% [21–23]. In more recent series the overall transfusion rate is denoted to be as low as 0.8%. Postulated factors to explain this fact are the increased use of balloon dilatation compared to Amplatz dilatators in conjunction with the use of multiple correctly placed tracts, reducing excessive torque to the kidney [5].
Major complications secondary to ureteroscopy are less common and ureteroscopy has proved to be safe in patients where shock wave lithotripsy (SWL) is likely to fail or in whom PCNL may be contraindicated (e.g., pregnancy, obese patients, patients with coagulopathy or scoliosis or other body deformities) [5, 10, 24]. Due to the decrease of ureteroscope size, significant complications like ureteral avulsion and stricture formation are extremely rare and noted as low as 1.5% [25].
Grasso et al. stratified complications in their series as intraoperative, postoperative (minor versus major) or long term. In their series of 53 patients, they noted no intraoperative and no long-term complications [9]. So did Ricchiuti et al. [8] in their series of 23 patients.
Breda et al. and El-Anany et al. did not experience major intraoperative or postoperative complications in their series of 15 and 30 patients. Breda described three minor complications, including one case of fever and pain and two cases of haematuria postoperatively of which neither required transfusion [12].
In our series of 24 patients neither experienced major intraoperative nor postoperative complications. One patient (4.1%) who presented with flank pain and steinstrasse was successfully treated with one final ureteroscopy.
The routine practice of a staged ureteroscopic treatment for large renal calculi burden has some drawbacks. The procedure can be time and material consuming and requires intense patient compliance.
In a recent survey comparing PCNL and flexible ureteroscopy, Hyams et al. stated that a second stage URS or ESWL for residual fragments after a primary ureteroscopic approach improves the stone-free rate, but “it is not clear whether the trade-off in the morbidity of another procedure as well as the additional costs counterbalance the risks of observations of small fragments, particularly in a compliant population” [26].
The precise size threshold for safe residual fragments is open to debate. Some data suggest that observation of residual fragments in some cases maybe safe. On the other hand, the risk of symptomatic episodes, and therefore the need for further interventions, increases [26–29].
In order to achieve stone-free status post surgery, we performed subsequent basketing of residual fragments, which were regarded to be too big for spontaneous passage in 70% of all cases. This additional procedure after primary laser lithotripsy contributed to the prolonged operation time and the need for staged therapy in some cases.
When treating large upper urinary tract calculi, the prevention of inadvertent laser firing, patience and clear visibility are essential. Decreased visibility during fragmentation leads to a prolonged operative time and increases the potential risk of injuring the surrounding tissue or the flexible ureteroscope, making a staged fashion ultimately necessary in several cases.
Therefore, the foremost dilemma associated with this method seems to be whether the risks of multiple ureteroscopic approaches and administration of general anesthesia outweigh the benefits of avoiding the associated morbidity of PCNL [8].
Our study does have certain limitations including the retrospective data collection in a small series of patients from a single institution and the lack of a randomized trail. Another limitation of this study is the relative short follow-up period. Therefore larger series with longer follow-up are necessary to confirm the long-term value of this procedure.
Conclusion
The combination of flexible ureteroscopes and the holmium laser using advanced endourological equipment allows the treatment of large intrarenal calculi of all compositions throughout the whole collecting system. Based on medical comorbidities, anatomical factors and the patient’s preference, all treatment options (PCNL, URS and shockwave lithotripsy) should be discussed prior to definitive treatment decision in complex urinary calculi.
References
Albala DM, Assimos DG, Clayman RV et al (2001) Lower pole I: a prospective randomized trial of extracorporeal shock wave lithotripsy and percutaneous nephrostolithotomy for lower pole nephrolithiasis—initial results. J Urol 166:2072–2080
Desai M, Jain P, Ganpule A, et al (2009) Developments in technique and technology: the effect on the results of percutaneous nephrolithotomy for staghorn calculi. BJU Int
Preminger GM, Assimos DG, Lingeman JE et al (2005) Chapter 1: AUA guideline on management of staghorn calculi: diagnosis and treatment recommendations. J Urol 173:1991–2000
Shah H, Khandkar A, Sodha H et al (2009) Tubeless percutaneous nephrolithotomy: 3 years of experience with 454 patients. BJU Int 104:840–846
Soucy F, Ko R, Duvdevani M, et al (2009) Percutaneous nephrolithotomy for staghorn calculi: a single center’s experience over 15 years. J Endourol
Hafron J, Fogarty JD, Boczko J, Hoenig DM (2005) Combined ureterorenoscopy and shockwave lithotripsy for large renal stone burden: an alternative to percutaneous nephrolithotomy? J Endourol 19:464–468
Michel MS, Trojan L, Rassweiler JJ (2007) Complications in percutaneous nephrolithotomy. Eur Urol 51:899–906 discussion 06
Ricchiuti DJ, Smaldone MC, Jacobs BL et al (2007) Staged retrograde endoscopic lithotripsy as alternative to PCNL in select patients with large renal calculi. J Endourol 21:1421–1424
Grasso M, Conlin M, Bagley D (1998) Retrograde ureteropyeloscopic treatment of 2 cm or greater upper urinary tract and minor staghorn calculi. J Urol 160:346–351
Busby JE, Low RK (2004) Ureteroscopic treatment of renal calculi. Urol Clin North Am 31:89–98
Grasso M, Ficazzola M (1999) Retrograde ureteropyeloscopy for lower pole caliceal calculi. J Urol 162:1904–1908
Breda A, Ogunyemi O, Leppert JT, Lam JS, Schulam PG (2008) Flexible ureteroscopy and laser lithotripsy for single intrarenal stones 2 cm or greater—is this the new frontier? J Urol 179:981–984
El-Anany FG, Hammouda HM, Maghraby HA, Elakkad MA (2001) Retrograde ureteropyeloscopic holmium laser lithotripsy for large renal calculi. BJU Int 88:850–853
Mariani AJ (2007) Combined electrohydraulic and holmium:YAG laser ureteroscopic nephrolithotripsy of large (greater than 4 cm) renal calculi. J Urol 177:168–173, discussion 73
Johnson GB, Portela D, Grasso M (2006) Advanced ureteroscopy: wireless and sheathless. J Endourol 20:552–555
Vassar GJ, Chan KF, Teichman JM et al (1999) Holmium: YAG lithotripsy: photothermal mechanism. J Endourol 13:181–190
Teichman JM, Vassar GJ, Bishoff JT, Bellman GC (1998) Holmium:YAG lithotripsy yields smaller fragments than lithoclast, pulsed dye laser or electrohydraulic lithotripsy. J Urol 159:17–23
Schuster TG, Hollenbeck BK, Faerber GJ, Wolf JS Jr (2002) Ureteroscopic treatment of lower pole calculi: comparison of lithotripsy in situ and after displacement. J Urol 168:43–45
Kim SC, Kuo RL, Lingeman JE (2003) Percutaneous nephrolithotomy: an update. Curr Opin Urol 13:235–241
Segura JW, Patterson DE, LeRoy AJ et al (1985) Percutaneous removal of kidney stones: review of 1,000 cases. J Urol 134:1077–1081
Stoller ML, Wolf JS Jr, St Lezin MA (1994) Estimated blood loss and transfusion rates associated with percutaneous nephrolithotomy. J Urol 152:1977–1981
Lang EK (1987) Percutaneous nephrostolithotomy and lithotripsy: a multi-institutional survey of complications. Radiology 162:25–30
Clayman RV, Surya V, Hunter D et al (1984) Renal vascular complications associated with the percutaneous removal of renal calculi. J Urol 132:228–230
Miller NL, Lingeman JE (2007) Management of kidney stones. BMJ 334:468–472
Harmon WJ, Sershon PD, Blute ML, Patterson DE, Segura JW (1997) Ureteroscopy: current practice and long-term complications. J Urol 157:28–32
Hyams ES, Shah O (2009) Percutaneous nephrostolithotomy versus flexible ureteroscopy/holmium laser lithotripsy: cost and outcome analysis. J Urol 182:1012–1017
Tan YH, Wong M (2005) How significant are clinically insignificant residual fragments following lithotripsy? Curr Opin Urol 15:127–131
Streem SB, Yost A, Mascha E (1996) Clinical implications of clinically insignificant store fragments after extracorporeal shock wave lithotripsy. J Urol 155:1186–1190
Raman JD, Bagrodia A, Gupta A et al (2009) Natural history of residual fragments following percutaneous nephrostolithotomy. J Urol 181:1163–1168
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bader, M.J., Gratzke, C., Walther, S. et al. Efficacy of retrograde ureteropyeloscopic holmium laser lithotripsy for intrarenal calculi >2 cm. Urol Res 38, 397–402 (2010). https://doi.org/10.1007/s00240-010-0258-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-010-0258-5