Abstract
A comparison is made among all the models proposed to explain the origin of the tRNA molecule. The conclusion reached is that, for the model predicting that the tRNA molecule originated after the assembly of two hairpin-like structures, molecular fossils have been found in the half-genes of the tRNAs of Nanoarchaeum equitans. These might be the witnesses of the transition stage predicted by the model through which the evolution of the tRNA molecule passed, thus providing considerable corroboration for this model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Corroboration of the Theories in the Framework of Evolutionary Biology and Molecular Fossils
The corroboration/falsification of theories in the framework of evolutionary biology can present enormous difficulties because many of these theories make predictions that are difficult to support, as they refer to events from the distant past and hence are extremely difficult to analyze. For instance, in the framework of the early evolution of life, the events proposed to explain the origin of the genetic code may be difficult or impossible to reconstruct because they refer to a time in the very distant past. Nevertheless, the theory of the coevolution of the genetic code (Wong 1975), which considers the genetic code as an imprint of the biosynthetic relationships between amino acids, is able to make a prediction on the possible existence of biosynthetic pathways linking up the amino acids and taking place on tRNA molecules. The mechanism underlying this theory is based on the existence, at the time when the genetic code organization was defined, of biosynthetic pathways linking up amino acids and taking place on tRNAs like molecules, which might have enabled the exchange of codons between the first amino acids to occupy the genetic code early on and those which evolved from them at a later biosynthetic date. Therefore, the main prediction of this theory is that these biosynthetic pathways taking place on tRNAs might still be identifiable in living organisms. This prediction finds extraordinary confirmation in the existence of biosynthetic pathways between amino acids on tRNAs in actual organisms (Yuan et al. 2008). Evolutionary analyses have shown that these pathways are very probably molecular fossils of the mechanism suggested by the coevolution theory (Di Giulio 2002). It is clear that, if these pathways were molecular fossils, then the coevolution theory would be considerably corroborated, as these molecular fossils might have been charged with the evolutionary history of organisms and, therefore, might effectively constitute the most important evidence attributable to this theory. In other words, and more generally, if molecular fossils are identified as such in a rigorous evolutionary analysis, they can provide considerable corroboration for a theory, as they would be the ‘eye witnesses’ of the mechanism on which that theory is based. Moreover, it is obvious how the concept of molecular fossils contains the evolutionary history, for instance, of that particular mechanism that makes the molecular fossil a unique and extraordinarily rich piece of evidence able to support theories.
I therefore think that more attention needs to be paid to molecular fossils in the framework of the early evolution of life and, more generally, in evolutionary biology, because the findings might significantly corroborate the theories and models, as seems to be the case for a model of the origin of the tRNA molecule.
Models and Suggestions for the Origin of the tRNA Molecule
The Precursor of Hopfield: The Hairpin
Hopfield (1978) was the first to suggest that a hairpin structure of RNA might have been the precursor of the tRNA molecule, although Woese (1969) suggests that a primitive and smaller subunit of tRNA might have played a major role in its origin. In order to bring the anticodon into close contact with the amino acid, Hopfield (1978) performed the simplest refolding pattern of the tRNA structure (Fig. 1). In this way he identified the hairpin (Fig. 1) as the most likely structure for the precursor of the tRNA molecule. The hairpin was assumed as a precursor in many models of tRNA origin, with or without the anticodon placed on its 5′ end (Fig. 1).
The hairpin suggested by Hopfield (1978)
The Eigen and Winkler-Oswatitsch Model
In one of the earliest analyses of tRNA sequences, Eigen and Winkler-Oswatitsch (1981) reconstructed the sequences of ancestral tRNA. In particular, they suggested that a hairpin structure with the anticodon on the 5′ end might have been the precursor of the tRNA molecule (Fig. 2a). Moreover, in reconstructing the master sequence of ancestral tRNA, they suggested that the 3′ half of the ancestral tRNA molecule was paired with the 5′ half, as shown in Fig. 2b, and that the subsequent evolution of this structure, as organized in Fig. 2c, might have eventually given rise to the tRNA molecule. In other words, Eigen and Winkler-Oswatitsch (1981) proposed that the 3′ half of primordial tRNA acted as a template, creating the 5′ half, and subsequent evolution generated the entire tRNA molecule. This mechanism for the origin of tRNA formally involves an indirect duplication that is substantially different, for instance, from the direct one introduced by Di Giulio (1992). Furthermore, the hairpin precursor in Fig. 2a is ‘identical’ to the one suggested by Hopfield (1978). Note that although the hairpin was suggested as precursor in this model (Fig. 2a), it does not actually seem to intervene in the model of Eigen and Winkler-Oswatitsch (1981).
The Eigen and Winkler-Oswatitsch (1981) model. See text and their Fig. 6 for further details and information
Bloch, McArthur, and Mirrop’s Model of the Replication of a Small Hairpin
Bloch et al. (1985) hypothesized successive duplications of a small hairpin structure as a model of tRNA origin (Fig. 3). This model generated a cruciform structure after three cycles of hairpin replication (Fig. 3). The final cloverleaf structure is generated through an unusual form of direct duplication, as the two halves of the final molecule are not perfectly equal (imperfect direct duplication) (Fig. 3) (Di Giulio 1992).
Formation of a hairpin and its three replication cycles that eventually produce the typical cloverleaf structure, as originally suggested by Bloch et al. (1985). See their Fig. 3 for further information
The Model of Moller and Janssen
In a statistical analysis of sequences of tRNA, Moller and Janssen (1990, 1992) provided evidence of the presence of codons in positions 3, 4, and 5 of the acceptor stem of tRNAs specific for certain amino acids. Furthermore, as illustrated in Fig. 4, the anticodon located in the acceptor stem opposite positions 3, 4, and 5 of the codon can be transferred into the anticodon loop by means of duplication, cleavage, and ligation (Fig. 4). In actual fact, those authors (Moller and Janssen 1990, 1992) do not seem to have realized that the long hairpin structure deriving from the union of the two molecules (Fig. 4, right) can be arranged so as to assume the secondary cloverleaf structure typical of the tRNA molecule. This is because it derives, unlike that of Eigen and Winkler-Oswatitsch (1981), from a hairpin structure whose duplication will clearly form a double hairpin that is potentially capable of assuming not only the figure shown in Fig 4. (right) but also the secondary cloverleaf structure.
The hairpin structure of Moller and Janssen (1990, 1992) having the codon 5′GUC3′ in its stem (anticodon 5′GAC3′). Through a duplication, a cleavage, and a ligation, the model would predict the transfer of the anticodon from the stem to the anticodon loop (right). See Fig. 2 of Moller and Jansssen (1992) for further information
The model of Di Giulio (1992) has been enhanced by the suggestion of Moller and Janssen (1990, 1992) that the anticodon located in the stem of a hairpin structure might have been transferred into the anticodon loop (Di Giulio 1994, 1995, 2004).
The Assembly of Two Hairpin Structures Might Have Originated the tRNA Molecule
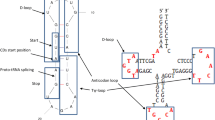
Di Giulio (1992) suggested that the direct duplication of an RNA hairpin might have created the conditions for the origin of the tRNA molecule (Fig. 5). In particular, if two hairpins are joined to form the structure shown in Fig. 5, then this dimer—intended as covalently closed between only two ends (Di Giulio 1992)—might have created the complete tRNA molecule through successive evolutions (Fig. 5) (Di Giulio 1992, 1995, 1999, 2004). Subsequently, Dick and Schamel (1995) practically suggested the same model.
The Suggestion of Maizels and Weiner
Maizels and Weiner (1994) suggested an idea, which became extremely popular, that the ‘top half’ of the tRNA molecule appeared at an evolutionarily earlier date and is therefore more ancient than the ‘bottom half’ (Fig. 6). Maizels and Weiner (1993, 1994) do not discuss how this could have happened in a real model but they are the first to acknowledge that the three-dimensional structure of tRNA, which is formed of two hairpins (Fig. 6), might contain traces of its origin (Di Giulio 1995).
The secondary structure (left) and the tertiary structure (right) of tRNA. The figure shows the main suggestions proposed by Maizels and Weiner (1994)
An ‘identical’ suggestion was later made by Schimmel and Ribas de Pouplana (1995), who reinforced the idea that the structure of tRNAs can be seen as being formed of two domains with separate functions (Fig. 7).
The suggestion of Schimmel and Ribas de Pouplana (1995). See text and their Fig. 1 for further information
The Suggestion of Rodin, Rodin, and Ohno
Rodin et al. (1996) also maintain that the hairpin might have been the precursor of the tRNA molecule. However, their suggestion regards, above all, the presence of codon-anticodon pairs in the acceptor stem of tRNAs and the hypothesis of a concerted origin of tRNAs with complementary anticodons, which might have been codified on complementary strands (Rodin et al. 1993, 1996). Therefore, the suggestion of Rodin et al. (1996) is compatible with that of Moller and Janssen (1992), albeit different in the details; together they strengthen the hypothesis of the presence of an ancient code in the acceptor stem of tRNAs (de Duve 1988; Schimmel et al. 1993; Rodin and Rodin 2006).
Tanaka and Kikuchi’s Suggestion of the Secondary Double Hairpin Structure
On the basis of analyses of tRNAs, Tanaka and Kikuchi (2001) suggested that a double hairpin, as shown in Fig. 8, might have been the intermediate stage preceding the cloverleaf structure of current tRNAs. In actual fact, this extremely useful suggestion simply gives a secondary structure to the intermediate in Fig. 5 suggested by Di Giulio (1992), as the double hairpin is also a characteristic of the latter model (Fig. 5) (see legend to Fig. 1 of Di Giulio 1992).
Schematic representation of the secondary structure of the double hairpin of the tRNA molecule, as suggested by Tanaka and Kikuchi (2001). See their Fig. 3 for further information
A Ring as the Ancestor of the tRNA Molecule
On the basis of their reconstruction of ancestral sequences, Demongeot and Moreira (2007) suggested that a ring might have been the precursor of the tRNA molecule (Fig. 9). In other words, they suggest that regions of an ancestral loop might have been the precursors of some regions of the tRNA molecule, as shown in Fig. 9.
The evolutionary relationship between the regions of an ancestral loop and the homologous regions of a tRNA, as suggested by Demongeot and Moreira (2007). See their Fig. 2 for further information
The Model of the Origin of tRNA Based on the Progressive Increase in the Number of Stem-Loop Structures in This Molecule
Sun and Caetano-Anolles (2008) suggested a model for the origin of tRNA that considers the cloverleaf structure of tRNA to have originated through the gradual addition of structural components to the growing molecule (Fig. 10). This took place either by insertion of single or multiple nucleotides or by partial or total duplication (Sun and Caetano-Anolles 2008). The molecule originated from a hairpin of RNA containing a single stem-loop structure, homologous to the acceptor arm of today’s tRNAs (Fig. 10) and consistent with what has been suggested by numerous authors. However, unlike other authors and as shown in Fig. 10, it is the gradual addition through several stages of stem-loop structures to this initial hairpin that might have eventually originated tRNAs, and not, for instance, a single duplication event that completed the tRNA molecule.
The Strengths of the Models of tRNA Origin
The strengths of the models of tRNA origin are, in my view, as follows.
-
(i)
Hopfield’s RNA hairpin (1978) seems to possess two characteristics that make it the most likely candidate, as a precursor, for the origin of the tRNA molecule. First, RNA hairpins must have been the structures that formed most easily during the very earliest polymerizations of RNA because, once the chain of RNA has reached a certain length, it can start to copy itself, thus producing an RNA hairpin (Orgel 1968). Therefore, the hairpin structures must have been very common in a certain stage of protocellular life. This might have favored, very early on, the use of these structures in some protocellular activities. However, the main reason for considering the hairpin the most likely precursor of tRNA origin is that the latter’s three-dimensional structure is practically formed of two hairpins (Figs. 6 and 7), which might recall the stages of the molecule’s origin (Maizels and Weiner 1993, 1994; Di Giulio 1994, 1995).
-
(ii)
The presence of anticodons in the stem of the RNA hairpin precursor is a feature of several models (Hopfield 1978; Eigen and Winkler-Oswatitsch 1981; Moller and Janssen 1990, 1992; Di Giulio 1994, 1995; Rodin et al. 1993, 1996). However, only the models predicting the presence of the anticodon near the 3′ end of the hairpin (Moller and Janssen 1990, 1992; Di Giulio 1994, 1995; Rodin et al. 1993, 1996) seem to be correct because, from this position, the anticodon might have been transferred into the anticodon loop, thus creating it (Moller and Janssen 1990, 1992 [Fig. 4]; Di Giulio 1994, 1995 [Fig. 5]), by means of a covalent dimerisation of the RNA hairpin precursor (Di Giulio 1992), for instance. Therefore the hairpin precursor most probably had the anticodon near its 3′ end, which seems compatible with the presence of an ancient code in this position, which might have predated the genetic code (de Duve 1988; Schimmel et al. 1993; Rodin and Rodin 2006).
It is noteworthy that hairpin dimerisation can transfer the anticodon from the stem of a hairpin to the anticodon loop of a potential tRNA (Fig. 5). This property seems to strongly corroborate the latter model because it would link the stages of the tRNA molecule origin (Fig. 5) to particular aspects regarding specific regions of the evolving tRNA, i.e., to the origin of the anticodon loop.
-
(iii)
The model of Di Giulio (1992, 1995, 1999, 2004) (Fig. 5) seems to encapsulate many of the suggestions made for tRNA molecule origin and, therefore, seems to be a very promising model. Indeed, this model (Fig. 5) (a) has the hairpin as its precursor, (b) can take into account the transfer of the anticodon from the stem to the anticodon loop (Figs. 4 and 5), and (c) explains why the three-dimensional structure of tRNA is formed of two hairpins (Figs. 6 and 7) which might have derived through assembly of two hairpin structures during tRNA molecule evolution (Di Giulio 1992, 1994, 1995, 2004). This interpretation is also highly compatible and consistent with the position of introns in the genes of tRNAs (see below) (Di Giulio 1992, 1995, 1999, 2006a, 2008a). Finally, (d) the intermediate of this model (Fig. 5, center) has a secondary structure which is the one suggested by Tanaka and Kikuchi (2001). Therefore, this model (Fig. 5) enjoys many properties that allow it to be given a more than positive assessment.
The Models Compared: A Synthesis
Of the models proposed to explain the origin of the tRNA molecule, that of Demongeot and Moreira (2007) seems to be unique in that this molecule might come from a ring of RNA that created different and noncontiguous parts of the tRNA molecule (Fig. 9). Nevertheless, the intervention of a circular RNA in the maturation of the permuted genes of tRNAs (Soma et al. 2007; Di Giulio 2008a) provides some support to this model. In the same way, the presence of multiple introns in the genes of tRNAs (Randau and Soll 2008) might perhaps provide some corroboration for this model if the origin of these genes was read in the light of the exon theory of genes (Darnell 1978; Gilbert 1978; Doolittle 1978; Di Giulio 1999). However, these arguments seem to me to be extremely weak, because there is no clear relationship among permuted genes, intron positions, and this model but, above all, because the relationship between this model’s RNA ring and the circular RNA of permuted genes is neither clear nor immediately obvious.
A truly fascinating model is that of Bloch et al. (1985), which manages to create the tRNA molecule through three rounds of replication of a small hairpin structure (Fig. 3). However, the trouble with this model is that it cannot provide observations in its favor, given that the sequence data can also be interpreted in favor of other models (Nazarea et al. 1985; Di Giulio 1992, 1995; Widmann et al. 2005). Therefore, albeit fascinating, it is difficult to falsify. However, the observation that the timing of the appearance of the complete tRNA molecule might be much more recent than was at first believed (Di Giulio 1999, 2006a, 2008a, b) would seem to falsify the model of Bloch et al. (1985), as it seems to refer to a much more ancient time for the appearance of this molecule, i.e., to the RNA world.
The Eigen and Winkler-Oswatitsch (1981) model is based on the transformation of a large hairpin (Fig. 2b) into the secondary structure of tRNA (Fig. 2c) by means of puntiform substitutions and therefore seems to be much less parsimonious than, for instance, the model of Di Giulio (1992), which produces the potential secondary structure of tRNA by simple dimerization of the same or a similar hairpin structure. Furthermore, the Eigen and Winkler-Oswatitsch model also seems to refer to a time for the appearance of the tRNA molecule that is attributable to the RNA world, and it is therefore incompatible with the observations indicating that the complete tRNA molecule originated more recently in evolution (Di Giulio 1999, 2006a, 2008a, b).
Similar to the Eigen and Winkler-Oswatitisch model, but certainly more complete than this, is the model of Moller and Janssen (1990, 1992) (Fig. 4). These two models are based on an indirect duplication that is also substantially different from that of the model of Di Giulio (1992). However, the model of Moller and Janssen (1992), unlike that of Eigen and Winkler-Oswatitsch (1981), can produce a potential secondary structure of tRNA (albeit not represented in Fig. 4, right), although it is different from that of Di Giulio (1992) in that, in the latter, the loop regions are not complementary but homologous, while those in the model of Moller and Janssen (1992) are complementary. Analysis of the tRNA sequences has indicated that the loop regions of tRNA are homologous and not complementary (Nazarea et al. 1985; Di Giulio 1992, 1995; Widmann et al. 2005). The model of Moller and Janssen (1990, 1992) also seems to refer to the RNA world for the appearance of the tRNA molecule, which is incompatible with what seems to be its much more recent origin (Di Giulio 1999, 2006a, 2008a, b).
In the model of Sun and Caetano-Anolles (2008), it is not clearly specified how the gradual addition of other stem-loop structures are added to the initial hairpin (Fig. 10). That is, it is unclear how, for instance, the different stem-loop structures were added (Fig. 10) and why the different classes of ancestral tRNA were not produced, as the model seems to predict, given the ease with which the model predicts that stem-loop structures should have been added to the initial hairpin (Fig. 10). Furthermore, the model of Sun and Caetano-Annolles (2008) is less parsimonious than other models because it might involve both the insertion of single or multiple nucleotides and the partial or total duplication for the origin of tRNA. While other models (Moller and Janssen 1990, 1992; Di Giulio 1992) predict the potential formation of the typical cloverleaf structure of tRNA in a more parsimonious number of events; in particular, for the model of Di Giulio (1992) the intermediate stage of the double hairpin (Tanaka and Kikuchi 2001) (Fig. 8) might have favored the transition from the initial hairpin to the final cloverleaf structure of tRNA (Tanaka and Kikuchi 2001; Di Giulio 2004), thus preventing the violation of the principle of evolutionary continuity.
All the considerations and observations provided so far in favor or against the various models are, in my opinion, of minor importance with respect to the existence of molecular fossils which are witnesses of the transition stages through which the tRNA molecule passed and thus strongly corroborate one of these models.
The model of Di Giulio (1992, 1994, 1995, 1999) predicts, in accordance with the exon theory of genes (Darnell 1978; Gilbert 1978; Doolittle 1978; Di Giulio 1992, 1999), that if the introns were important in assembling the earliest genes, then they should have made the point of suture between the two hairpin structures called to form the complete tRNA molecule. Therefore, this model predicts that the introns are located in the anticodon loop because in this position they could have joined up the two hairpin structures (Fig. 5). The most highly conserved intron of tRNA is located in the anticodon loop (Fig. 5) (Di Giulio 1992, 1995, 1999, 2006a, b).
This point of view makes another prediction: that there existed half-genes of tRNA codifying only half of the tRNA molecule, i.e., codifying for the RNA hairpins which were later joined to form the entire tRNA molecule (Di Giulio 1992, 1995, 1999, 2006a, b). This prediction finds an extraordinary confirmation in the existence in Nanoarchaeum equitans of minigenes of tRNAs codifying only for the 5′ and 3′ halves of tRNAs (Randau et al. 2005). These half-genes have the main characteristics possessed by the hairpin of this model (Di Giulio 2006a). Therefore, there might have existed minigenes separately codifying for the 5′ and 3′ halves of the tRNA molecule, given that in N. equitans these genes still exist today. Evolutionary analyses have shown that these genes for the 5′ and 3′ halves of tRNAs are the missing links, i.e., the transition stages through which evolution of the tRNA molecule passed (Di Giulio 2006a, b, 2008b). Therefore, the main prediction of the model of Di Giulio of the existence of genes codifying only for the 5′ and 3′ halves of tRNAs is confirmed, and this represents a very high level of corroboration for this model (see the first section). Moreover, the permuted genes of tRNA of Cynidioschyzon merolae have strengthened this conclusion (Di Giulio 2008a). Finally, it is fascinating that the study of half-genes of the tRNAs of N. equitans and of the permuted genes of C. merolae has opened the possibility of identification of the evolutionary timing of the appearance of this molecule (Di Giulio 2006a, b, 2008a, b; Fujishima et al. 2008).
References
Bloch DP, McArthur B, Mirrop S (1985) tRNA-rRNA sequence omologies: evidence from an ancient modular format shared by tRNAs and rRNAs. BioSystems 17:209–225
Darnell JE Jr (1978) Implications of RNA-RNA splicing in evolution of eukaryotic cells. Science 202:1250–1260
de Duve C (1988) The second genetic code. Nature 33:117–118
Demongeot J, Moreira A (2007) A possible circular RNA at the origin of life. J Theor Biol 249:314–324
Dick TP, Schamel WA (1995) Molecular evolution of transfer RNA from two precursor hairpins: implications for the origin of protein synthesis. J Mol Evol 41:1–9
Di Giulio M (1992) On the origin of the transfer RNA molecule. J Theor Biol 159:199–214
Di Giulio M (1994) On the origin of proteins synthesis: a speculative model based on hairpin RNA structures. J Theor Biol 171:303–308
Di Giulio M (1995) Was it an ancient gene codifyng for a hairpin RNA that, by means of direct duplication, gave rise to the primitive tRNA molecule? J Theor Biol 177:95–101
Di Giulio M (1999) The non-monophyletic origin of tRNA molecule. J Theor Biol 197:403–414
Di Giulio M (2002) Genetic code origin: Are the pathways of the type Glu-tRNAGln->Gln-tRNAGln molecular fossils or not? J Mol Evol 55:616–622
Di Giulio M (2004) The origin of the tRNA molecule: implications for the origin of protein synthesis. J Theor Biol 226:89–93
Di Giulio M (2006a) The non-monophyletic origin of the tRNA molecule and the origin of genes only after the evolutionary stage of the Last Universal Common Ancestor (LUCA). J Theor Biol 240:343–352
Di Giulio M (2006b) Nanoarchaeum equitans is a living fossil. J Theor Biol 242:257–260
Di Giulio M (2008a) Permuted tRNA genes of Cyanidioschyzon merolae, the origin of the tRNA molecule and the root of the Eukarya domain. J Theor Biol 253:587–592
Di Giulio M (2008b) Split genes, ancestral genes. In: Tze-Fei Wong J, Lazcano A (eds) Prebiotic evolution and astrobiology, chap 13. Landes Bioscience, Austin, TX
Doolittle WF (1978) Genes in pieces: were they ever together? Nature 272:581–582
Eigen M, Winkler-Oswatitsch R (1981) Transfer-RNA, an early gene? Naturwissenschaften 68:282–292
Fujishima K, Sugahara J, Tomita M, Kanai A (2008) Sequence evidence in the archaeal genomes that tRNAs emerged through the combination of ancestral genes as 5′ and 3′ tRNA halves. PLoS ONE 3(2):e1622
Gilbert W (1978) Why genes in pieces? Nature 271:501
Hopfield JJ (1978) Origin of the genetic code: a testable hypothesis based on tRNA structure, sequence and kinetic proofreading. Proc Natl Acad Sci USA 75:4334–4338
Maizels N, Weiner AM (1993) The genomic tag hypothesis: modern viruses as molecular fossils of ancient strategies for genomic replication. In: Gesteland RF, Atkins JF (eds) The RNA world. Cold Spring Harbor Laboratory Press, Plainview, NY, pp 577–602
Maizels N, Weiner AM (1994) Phylogeny from function: evidence from the molecular fossil record that tRNA originated in replication, not translation. Proc Natl Acad Sci USA 91:6729–6734
Moller W, Janssen GMC (1990) Transfer RNAs for primordial amino acids contains remnants of a primitive code at position 3 to 5. Biochimie 72:361–368
Moller W, Janssen GMC (1992) Statistical evidence for remnants of primordial code in the acceptor stem of prokaryotic transfer RNA. J Mol Evol 34:471–477
Nazarea AD, Bloch DP, Semrau AC (1985) Detection of a fundamental modular format common to transfer and ribosomal RNAs. Proc Natl Acad Sci USA 82:5337–5341
Orgel LE (1968) Evolution of the genetic apparatus. J Mol Biol 38:381–383
Randau L, Munch R, Hohn M, Jahn D, Soll D (2005) Nanoarchaeum equitans creates functional tRNAs from separate genes for their 5′- and 3′-halves. Nature 433:537–541
Randau L, Soll D (2008) Transfer RNA genes in pieces. EMBO Rep 9:623–628
Rodin S, Rodin A (2006) Origin of the genetic code: first aminoacyl-tRNA synthetases could replace isofunctional ribozymes when only the second base of codons was established. DNA Cell Biol 25:365–375
Rodin S, Ohno S, Rodin A (1993) Transfer RNA with complementary anticodon: could they reflect early evolution of discrimative genetic code adaptors? Proc Natl Acad Sci USA 90:4723–4727
Rodin S, Rodin A, Ohno S (1996) The presence of codon-anticodon pairs in the acceptor stem of tRNAs. Proc Natl Acad Sci USA 93:4537–4542
Schimmel P, Giege R, Morras D, Yokoyama S (1993) An operational RNA code for amino acids and possible relationship to genetic code. Proc Natl Acad Sci USA 90:8763–8768
Schimmel P, Ribas de Pouplana L (1995) Transfer RNA: from minihelix to genetic code. Cell 81:983–986
Soma A, Onodera A, Sugahara J, Kanai A, Yachie N, Tomita M, Kawamura F, Sekine Y (2007) Permuted tRNA genes expressed via a circular RNA intermediate in Cyanidioschyzon merolae. Science 318:450–453
Sun FJ, Caetano-Anolles G (2008) The origin and evolution of tRNA inferred from phylogenetic analysis of structure. J Mol Evol 66:21–35
Tanaka T, Kikuchi Y (2001) Origin of cloverleaf of transfer RNA—the double-hairpin model: implication for the role of tRNA intron and the long extra loop. Viva Origino 29:134–142
Widmann J, Di Giulio M, Yarus M, Knight R (2005) tRNA creation by hairpin duplication. J Mol Evol 61:524–530
Woese CR (1969) The biological significance of the genetic code. Prog Mol Subcell Biol 1:5–46
Wong JT (1975) A co-evolution theory of the genetic code. Proc Natl Acad Sci USA 72:1909–1912
Yuan J, Shepperd K, Soll D (2008) Amoni acid modifications on tRNA. Acta Biochim Biophys Sin 40:539–553
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Di Giulio, M. A Comparison Among the Models Proposed to Explain the Origin of the tRNA Molecule: A Synthesis. J Mol Evol 69, 1–9 (2009). https://doi.org/10.1007/s00239-009-9248-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00239-009-9248-z