Abstract
Purpose
Degenerative cervical myelopathy (DCM) is a common cause of spinal cord dysfunction. In this study, we explored the potential of magnetization transfer ratio (MTR) for evaluating the structural integrity of spinal cord tracts in patients with clinically significant DCM.
Methods
Fifty-three patients with DCM and 41 patients with cervical radiculopathy were evaluated using high-resolution cervical spinal cord magnetic resonance imaging (MRI), which included the magnetization transfer technique. MRI data were analyzed with the Spinal Cord Toolbox (v5.5); MTR values in each spinal tract were calculated and compared between groups after correction for patient age and sex. Correlations between MTR values and patients’ clinical disability rate were also evaluated.
Results
A statistically significant reduction in the average MTR of the spinal cord white matter, as well as the MTR of the ventral columns and lateral funiculi, was revealed in the DCM group (adjusted p < 0.01 for all comparisons). Furthermore, reductions in MTR values in the fasciculus cuneatus, spinocerebellar, rubrospinal, and reticulospinal tracts were found in patients with DCM (adjusted p < 0.01 for all comparisons). Positive correlations between the JOA score and the MTR within the ventral columns of the spinal cord (R = 0.38, adjusted p < 0.05) and the ventral spinocerebellar tract (R = 0.41, adjusted p < 0.05) were revealed.
Conclusion
The findings of our study indicate that demyelination in patients with DCM primarily affects the spinal tracts of the extrapyramidal system, and the extent of these changes is related to the severity of the condition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Degenerative cervical myelopathy (DCM) is the predominant cause of spinal cord dysfunction in developed countries, and its prevalence is increasing due to the increase in life expectancy [1]. As the cervical spine canal undergoes degenerative changes, it becomes narrower and compresses the spinal cord, resulting in gradual damage [2]. Symptoms of DCM can include gait impairment, lack of coordination, dysesthesia, and bladder dysfunction. If left untreated, this can lead to severe spinal cord injury and tetraparesis [3]. The sole efficient treatment for patients with DCM is surgical decompression. Nevertheless, despite successful surgery, some patients may not experience any improvement in their clinical condition or may even experience a decline [4].
Cervical spine magnetic resonance imaging (MRI) is a standard and very useful diagnostic tool for patients with DCM [5]. The use of sophisticated neuroimaging techniques, such as diffusion tensor imaging (DTI), the magnetization transfer ratio (MTR), the myelin water fraction (MWF), and MR spectroscopy (MRS), can yield quantitative information on the microstructural changes that occur in the spinal cord and can offer us greater insight into the pathophysiology of this disease [6]. A number of studies have focused on utilizing these techniques in individuals with DCM to evaluate the severity of spinal cord injury and predict the outcome of surgical interventions [2, 6,7,8,9,10]. However, the ability to monitor DCM development and predict the potential for recovery with existing neuroimaging methods is still limited.
Neuroimaging research on spinal cord structural reorganization in patients with DCM has focused primarily on the application of techniques such as cervical cord morphometry (according to T2-WI) and DTI [5, 6]. Thus, the evaluation of white matter tracts demyelination with myelin-sensitive techniques, such as MTR, appears to be understudied in this group of patients. However, myelin damage due to chronic spinal cord compression and ischemia plays an important role in the pathobiology of DCM and the development of neurological disability in patients with this condition [11, 12]. It is also interesting what tracts of the spinal cord are the most prone to degenerative demyelination and how this is associated with clinical symptoms.
To date, only a few papers have been published dedicated to examining changes in myelin integrity within the white matter tracts of the spinal cord in patients with DCM compared to patients without spinal cord compression. Some of them showed great potential for the MTR technique [11, 12] under these conditions but were carried out with small sample sizes and did not selectively evaluate each tract. Therefore, our objective was to assess the presence of microstructural (demyelinating) changes within different spinal tracts in patients with DCM using the MTR technique. We hypothesized that patients with DCM would show a reduction in MTR in the white matter regions of the cervical spinal cord examined and that some spinal cord tracts would be more vulnerable to this damage than others. Furthermore, we assumed that the changes in MTR values would correlate with disease severity.
Methods
Patients
The participants in this study were patients diagnosed with DCM according to the diagnostic criteria [13] and patients diagnosed with cervical radiculopathy (without clinical or neuroimaging myelopathy signs) who were surgically treated at our hospital from January 2022 to September 2023. Fifty-three patients with DCM (30 males and 23 females) and 41 patients with cervical radiculopathy due to disk extrusion (22 males and 19 females) participated in the study. All patients underwent high-resolution cervical spine and brain MRI before surgery. Neurological assessment was conducted for all patients before spine surgery; disease severity was evaluated with the JOA scale [14] for DCM patients. Detailed information on the patients is provided in Table 1. Each patient signed a written informed consent form before participating in the study. The study was carried out according to the Declaration of Helsinki and was approved by the local Ethics Committee (protocol no. 7 dated 05–25-2021).
MRI data acquisition
MR imaging data were acquired using a 3 T system (Ingenia, Philips Healthcare, The Netherlands) equipped with a 16-channel receiver head and neck coil. The MRI protocol was performed according to an early published guide [15] and included high-resolution T2-WI, T2 GRE, MT, and DTI. The magnetization transfer technique (in the axial plane) had the following parameters: TR—57 ms, TE—2 ms, FOV—220*220 mm, matrix—256*256, MT on and off, number of slices—22, and slice thickness—5 mm.
MRI data processing
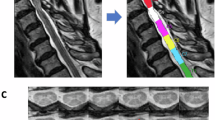
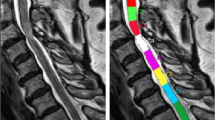
Spinal cord MRI postprocessing was performed using the Spinal Cord Toolbox (https://spinalcordtoolbox.com/index.html) after converting the DICOM files to the NIfTI format. Initially, spinal cord segmentation based on high-resolution T2-weighted imaging (T2-WI) was performed with manual vertebral labeling, followed by registration on the PAM50 template [16]. After that, multimodal registration and spinal cord gray matter segmentation were performed using the T2 GRE sequence. Finally, magnetization transfer image processing included the calculation of magnetization transfer ratio (MTR) maps, coregistration with T2-WI and T2 GRE, followed by atlas-based segmentation of the spinal cord in the axial plane on 36 different ROIs [17]. The average values throughout the 2nd to 5th vertebra levels were extracted for the following analysis; additionally, the average values between the right and left sides were used. In all the patients, the vertebrae labeling, multimodal registration, and segmentation results were visually verified by a neuroradiologist. Examples of data processing in the Spinal Cord Toolbox are shown in Fig. 1 (vertebrae labeling and gray matter segmentation) and Fig. 2 (atlas-based MTR maps segmentation).
Schematic representation of the magnetization transfer MRI postprocessing algorithm with the Spinal Cord Toolbox: a, b original sagittal axial magnetization transfer images; c calculated magnetization transfer ratio map; d registration on a high-resolution T2w template; e the results of white matter delineation; f the results of atlas-based spinal tract segmentation
Statistical analysis
Statistical analysis was performed using R software (www.r-project.org). Our data were normally distributed (Shapiro‒Wilk test). Covariance analyses (ANCOVAs) were performed to assess the differences in MTR values and included group (DCM, radiculopathy) as the between-subject factor, ROI average signal intensity as the dependent variable, and patient age and sex as covariates. Correlation analyses (Pearson’s correlation test) were performed among different ROI MTR data, disease severity (according to the JOA scale), and disease duration. A p value less than 0.05 was regarded as statistically significant (after FDR correction).
Results
Clinical and demographic data
The clinical and demographic information of the participants enrolled is presented in Table 1. Fifty-three patients with DCM (30 males and 23 females, 39–82 years of age, average 56.4 years) and 41 patients with cervical radiculopathy due to disk extrusion (22 males and 19 females, 30–72 years of age, average 48.3 years) ultimately participated in the study. The DCM and radiculopathy groups did not differ in sex (p > 0.1) but differed in age (p < 0.05); however, this difference was corrected during the following analysis by including age as a covariate in the ANCOVA model. None of the patients in the radiculopathy group had spinal cord compression or signal changes according to MRI data or clinical signs of myelopathy. In the DCM group, 23 patients had compression of the upper part of the cervical cord (at the level of the C3–C4 vertebrae), and 30 patients had compression of the lower part of the cervical cord (at the level of the C5–C7 vertebrae). None of the patients had previously undergone surgical treatment for DCM.
Cervical spinal cord MTR values in patients with DCM and radiculopathy
Statistically significant reductions in the average MTR values of the spinal cord white matter, as well as the MTR values of the ventral columns and lateral funiculi, were revealed in the DCM group compared to the radiculopathy group (p < 0.01 in all cases, FDR corrected; Fig. 3). Furthermore, reductions in MTR values in the fasciculus cuneatus, ventral spinocerebellar, rubrospinal, and reticulospinal tracts were found in patients with DCM (p < 0.01, p < 0.001, p < 0.01, and p < 0.01, respectively; FDR corrected; Fig. 4). Furthermore, a trend toward a decrease in MTR values within the ventral corticospinal tract was found (p = 0.077, FDR corrected, not shown). All the results are shown in Supplementary File 1.
MTR values in different spinal tracts of patients with DCM and cervical radiculopathy (results of ANCOVA): A ventral spinocerebellar tract (adjusted p < 0.001); B rubrospinal tract (adjusted p < 0.01); C ventral reticulospinal tract (adjusted p < 0.001); D lateral reticulospinal tract (adjusted p < 0.01)
Correlations between MTR and JOA score
Positive correlations between the JOA score and the MTR values within the ventral columns of the spinal cord (R = 0.38, adjusted p < 0.05; Fig. 5B) and between the JOA score and the ventral spinocerebellar tract (R = 0.41, adjusted p < 0.05; Fig. 5C) were revealed, as was a trend toward a positive association between the JOA score and the average white matter MTR (R = 0.36; adjusted p = 0.06; Fig. 5A). All the results are shown in Supplementary File 1.
Relationships (Pearson’s correlation) between spinal cord MTR values and JOA scores: A correlation between the JOA score and white matter spinal cord MTR values; B between the JOA score and ventral columns of the spinal cord MTR; C correlation between the JOA score and MTR values within the ventral spinocerebellar tract
Correlations between MTR and disease duration
No statistically significant correlations were revealed between the MTR values within any of the analyzed ROIs and disease duration (p > 0.1, not shown). All the results are shown in Supplementary File 1.
Discussion
The search for valuable neuroimaging markers to track disease progression and assess the severity of spinal cord damage in patients with DCM is an important part of the research in this field. The main objective of the current study was to compare the MTR values in different spinal tracts between patients with DCM and those without DCM. In line with our hypothesis, a statistically significant decrease in MTR in different cervical spinal cord regions was found in patients with DCM compared to patients with radiculopathy. Furthermore, these changes were correlated with clinical symptom severity (measured as the JOA score).
Our results generally confirm the existing data, showing that decreased MTR in various regions of the spinal cord could serve as a useful biomarker reflecting disease-associated myelin damage in the spinal cord. Chronic spinal cord compression is believed to lead to demyelination and axonal destruction [18]. The magnetization transfer technique has been shown to be useful in monitoring the progression of symptoms in patients with DCM as a part of multiparametric MRI [19]. Cloney et al. (2018) revealed a decrease in MTR values within the anterior (but not lateral or posterior) spinal cord region in patients with DCM, which was associated with hyperreflexia [12]. Additionally, changes in the MTR were correlated with clinical disability scale scores (such as the neck disability index, pain interference scale, and modified JOA) according to another study [11]. Furthermore, an association between a decrease in MTR and a decrease in DTI parameters within the fasciculus cuneatus and the lateral corticospinal tract has also been demonstrated [10]. However, it should be noted that all of these studies were carried out with very small sample sizes (the number of patients in the DCM group varied from 7 to 26).
To our knowledge, this study is the first attempt to evaluate differences in the vulnerability of the spinal tracts to myelin damage in patients with DCM in a relatively large sample. The most prominent decreases in MTR were observed in the ventral columns and lateral funiculi of the cervical spinal cord. The pathobiology of the disease may account for this observation, as mechanical cord compression typically affects the anterior part of the vertebral canal, leading to chronic ischemia in the circulation of the anterior spinal artery [12]. Specifically, structural impairments were more prominent within regions such as the spinocerebellar, rubrospinal, ventral, and lateral reticulospinal tracts. All of these tracts belong to the circulation of the anterior spinal artery [20]. In addition, we found that MTR values tended to decrease within the ventral corticospinal tract, which is also part of the ventral columns. There were no differences in MTR within the gray matter of the spinal cord between the groups, confirming that this region is less vulnerable to degenerative demyelination than white matter tracts are.
Thus, chronic microstructural impairment of white matter at the level of anterior spinal artery circulation can result in loss of myelin integrity (reflected in a decrease in MTR values) due to both direct compression damage and the combination of chronic ischemia and Wallerian degeneration mechanisms [18]. The primary involvement of the components of the extrapyramidal system (specifically the reticulospinal, rubrospinal, and spinocerebellar tracts) in the myelin damage process revealed in our study generally explains the clinical picture of DCM disease [21]. One of the earliest signs of DCM is impaired sensory perception, as well as difficulties with locomotion and gait, which can lead to clumsiness [21]. The reticulospinal tract is known to be responsible for regulating the proximal and axial muscles, primarily in gross movements such as posture, reaching, and locomotion [22]. The rubrospinal system is essential for motor control and contributes to the modulation of muscle tone, motor reactions, motor skill acquisition, and sensory perception [23]. Additionally, the ventral spinocerebellar tract contributes to driving the generation and maintenance of locomotor behavior [24]. However, it should be noted that all the aforementioned white matter tracts are very thin, which is why they are difficult to delineate with even 3 T MRI systems [17]; thus, further studies with higher field MRI usage may be very helpful from this perspective.
In our study, correlations between the severity of clinical symptoms and the values of MTR within the ventral columns (and specifically the ventral spinocerebellar tract) were also revealed. These data generally confirm previously published results [10,11,12]. Therefore, progression of the disease may be indicated by degenerative demyelination in the ventral regions of the cervical spinal cord. However, the duration of the disease was not associated with the decrease in MTR in any region of the spinal cord. We assumed that the variability in the clinical course and history of DCM, which is common in DCM patients (1), along with the subjective nature of this metric, could provide an explanation.
Together, the results of our study provide additional evidence supporting the occurrence of structural reorganization of the cervical cord white matter in patients with DCM that appears secondary to chronic compression of the cervical cord. Furthermore, we revealed that this reorganization is associated with disease severity and therefore could reflect the progression of DCM. Comparisons of MTR with other quantitative metrics (such as SCA, DTI, and T2 GRE measurements) are beyond the scope of the present study but may constitute a subject of further research.
Another potential avenue for further exploration in this field involves leveraging convolutional neural networks (CNN) and other contemporary artificial intelligence techniques for analyzing spinal cord images in patients with DCM. In recent years, there has been a growing interest in utilizing this approach in radiology [25], as it holds promise for facilitating early disease detection and prognosis assessment. While numerous studies have employed CNN-based image analysis for spinal pathologies, research specific to DCM remains limited [26]. A key challenge lies in the requirement for a substantial volume of high-quality cervical spine MRI data to effectively deploy these algorithms, necessitating prospective acquisition with standardized protocols. Additionally, investigating the diagnostic and prognostic capabilities of various radiomics metrics in patients with DCM could offer valuable insights [27].
This study has several limitations. First, due to the moderate sample size (n = 53 in the DCM group), we were only able to draw preliminary conclusions. Second, our study lacked a healthy control group, which limits our comparisons to only patients with DCM and cervical radiculopathy. Furthermore, there were no follow-up data in our study. Further studies with larger sample sizes, detailed follow-up evaluations, and the application of other quantitative neuroimaging techniques are needed to validate our findings and evaluate their utility in patient care.
Conclusion
We revealed a significant reduction in MTR values within the spinal white matter tracts in patients with DCM compared to patients with radiculopathy, suggesting that chronic spinal cord injury results in myelin damage, which specifically affects the spinal tracts of the extrapyramidal system primarily in the regions of the ventral and lateral cervical cord. Furthermore, these changes were correlated with disease severity. These findings argue that the spinal cord MTR technique may serve as an indicator of the ongoing progression of DCM.
Data availability
The raw data were generated at the Federal Neurosurgical Center Novosibirsk. Derived data supporting the findings of this study are available from the corresponding author upon request.
References
Yurac R, Matamala JM, Zamorano JJ, Harrop JS, Davies BM, Nouri A et al (2022) Degenerative cervical myelopathy. Rev Med Chil 150(3):339–52. https://pubmed.ncbi.nlm.nih.gov/36156719/
Nouri A, Tessitore E, Molliqaj G, Meling T, Schaller K, Nakashima H et al (2022) Degenerative cervical myelopathy: development and natural history [AO Spine RECODE-DCM Research Priority Number 2]. Global Spine J 12(1 Suppl):39S
Fehlings MG, Tetreault LA, Wilson JR, Skelly AC (2013) Cervical spondylotic myelopathy: current state of the art and future directions. Spine 38(22 Suppl 1):S1. https://pubmed.ncbi.nlm.nih.gov/23962994/
Fehlings MG, Smith JS, Kopjar B, Arnold PM, Yoon ST, Vaccaro AR et al (2012) Perioperative and delayed complications associated with the surgical treatment of cervical spondylotic myelopathy based on 302 patients from the AOSpine North America Cervical Spondylotic Myelopathy Study: presented at the 2011 Spine Section Meeting. J Neurosurg Spine. 16(5):425–32. https://thejns.org/spine/view/journals/j-neurosurg-spine/16/5/article-p425.xml
Nouri A, Martin AR, Mikulis D, Fehlings MG (2016) Magnetic resonance imaging assessment of degenerative cervical myelopathy: a review of structural changes and measurement techniques. Neurosurg Focus 40(6):1
Sasiadek MJ, Szewczyk P, Bladowska J (2012) Application of diffusion tensor imaging (DTI) in pathological changes of the spinal cord. Med Sci Monit 18(6):73
Liu H, MacMillian EL, Jutzeler CR, Ljungberg E, MacKay AL, Kolind SH et al (2017) Assessing structure and function of myelin in cervical spondylotic myelopathy: evidence of demyelination. Neurology 89(6):602
Martin AR, De Leener B, Cohen-Adad J, Cadotte DW, Kalsi-Ryan S, Lange SF et al (2017) A novel MRI biomarker of spinal cord white matter injury: T2*-weighted white matter to gray matter signal intensity ratio. AJNR Am J Neuroradiol 38(6):1266–73. https://pubmed.ncbi.nlm.nih.gov/28428212/
He B, Sheldrick K, Das A, Diwan A (2022) Clinical and research MRI techniques for assessing spinal cord integrity in degenerative cervical myelopathy—a scoping review. Biomedicines 10(10):2621
Yang HE, Kim WT, Kim DH, Kim SW, Yoo WK, Yang HE et al (2022) Utility of diffusion and magnetization transfer MRI in cervical spondylotic myelopathy: a pilot study. Diagnostics 12(9):2090
Paliwal M, Weber KA, Hopkins BS, Cantrell DR, Hoggarth MA, Elliott JM et al (2020) Magnetization transfer ratio and morphometrics of the spinal cord associates with surgical recovery in patients with degenerative cervical myelopathy. World Neurosurg 144:e939
Cloney MB, Smith ZA, Weber KA, Parrish TB (2018) Quantitative magnetization transfer MRI measurements of the anterior spinal cord region are associated with clinical outcomes in cervical spondylotic myelopathy. Spine 43(10):675
Hilton B, Gardner EL, Jiang Z, Tetreault L, Wilson JRF, Zipser CM et al (2022) Establishing diagnostic criteria for degenerative cervical myelopathy [AO Spine RECODE-DCM Research Priority Number 3]. Global Spine J 12(1 Suppl):55S
Kato S, Oshima Y, Matsubayashi Y, Taniguchi Y, Tanaka S, Takeshita K (2019) Minimum clinically important difference and patient acceptable symptom state of Japanese Orthopaedic Association Score in degenerative cervical myelopathy patients. Spine 44(10):691–7. https://pubmed.ncbi.nlm.nih.gov/30395093/
Cohen-Adad J, Alonso-Ortiz E, Abramovic M, Arneitz C, Atcheson N, Barlow L et al (2021) Open-access quantitative MRI data of the spinal cord and reproducibility across participants, sites and manufacturers. Sci Data 8(1):1–17. https://www.nature.com/articles/s41597-021-00941-8
De Leener B, Fonov VS, Collins DL, Callot V, Stikov N, Cohen-Adad J (2018) PAM50: unbiased multimodal template of the brainstem and spinal cord aligned with the ICBM152 space. Neuroimage 165:170–9. https://pubmed.ncbi.nlm.nih.gov/29061527/
Lévy S, Benhamou M, Naaman C, Rainville P, Callot V, Cohen-Adad J (2015) White matter atlas of the human spinal cord with estimation of partial volume effect. Neuroimage 119:262–71. https://pubmed.ncbi.nlm.nih.gov/26099457/
Akter F, Yu X, Qin X, Yao S, Nikrouz P, Syed YA et al (2020) The pathophysiology of degenerative cervical myelopathy and the physiology of recovery following decompression. Front Neurosci 14:138. https://www.frontiersin.org
Martin AR, De Leener B, Cohen-Adad J, Kalsi-Ryan S, Cadotte DW, Wilson JR et al (2018) Monitoring for myelopathic progression with multiparametric quantitative MRI. PLoS One 13(4):e0195733
Hardy TA (2021) Spinal cord anatomy and localization. CONTINUUM Lifelong Learning in Neurology 27(1):12–29
Milligan J, Ryan K, Fehlings M, Bauman C (2019) Degenerative cervical myelopathy: diagnosis and management in primary care. Can Fam Physician 65(9):619
Baker SN (2011) The primate reticulospinal tract, hand function and functional recovery. J Physiol 589(23):5603–5612
Olivares-Moreno R, Rodriguez-Moreno P, Lopez-Virgen V, Macías M, Altamira-Camacho M, Rojas-Piloni G (2021) Corticospinal vs rubrospinal revisited: an evolutionary perspective for sensorimotor integration. Front Neurosci 15
Chalif JI, de Martínez-Silva M (2022) Control of mammalian locomotion by ventral spinocerebellar tract neurons. Cell 185(2):328-344.e26
Yamashita R, Nishio M, Do RKG, Togashi K (2018) Convolutional neural networks: an overview and application in radiology. Insights Imaging 9(4):611–629
Baur D, Kroboth K, Heyde CE, Voelker A (2022) Convolutional neural networks in spinal magnetic resonance imaging: a systematic review. World Neurosurg 1(166):60–70
Zhang MZ, Ou-Yang HQ, Jiang L, Wang CJ, Liu JF, Jin D et al (2021) Optimal machine learning methods for radiomic prediction models: clinical application for preoperative T2*-weighted images of cervical spondylotic myelopathy. JOR Spine 4(4)
Acknowledgements
The authors thank Aria Nouri (Switzerland) for inspiration for this study and for providing valuable advice.
Funding
No funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The data were acquired and published in accordance with the principles outlined in the Declaration of Helsinki. The study was approved by the local Ethics Committee of the Federal Center for Neurosurgery, Novosibirsk, Russia (protocol no. 7 dated 05–25-2021).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Filimonova, E., Abdaev, M., Vasilenko, I. et al. Evaluation of the structural integrity of different spinal cord tracts with magnetization transfer ratio in degenerative cervical myelopathy. Neuroradiology 66, 839–846 (2024). https://doi.org/10.1007/s00234-024-03327-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-024-03327-w