Abstract
Study design
Prospective case-control study.
Objectives
We investigated the use of the magnetization transfer saturation (MTsat) technique to assess the structural integrity of the spinal cord tracts in individuals with clinically significant degenerative cervical myelopathy (DCM) and associated disability.
Setting
Novosibirsk Neurosurgery Centre, Russia.
Methods
A total of 53 individuals diagnosed with DCM and 41 patients with cervical radiculopathy underwent high-resolution MRI of the cervical spinal cord via the magnetization transfer technique. The MRI data were processed using the Spinal Cord Toolbox (v5.5), with MTsat values determined for each spinal tract and compared between the two groups. Furthermore, associations between MTsat values and the clinical disability rates of patients were investigated.
Results
A significant decrease in the MTsat of the ventral spinocerebellar tract was observed in the DCM group compared to the control group (adjusted p < 0.001). There was a trend towards lower MTsat values in the rubrospinal tract in the DCM group (adjusted p = 0.08). Additionally, a decrease in MTsat values in the lateral funiculi of the spinal cord was found in patients with DCM (adjusted p < 0.01). Furthermore, a trend toward a positive correlation was observed between the JOA score and the MTsat values within the ventral spinocerebellar tract (R = 0.33, adjusted p = 0.051).
Conclusions
The findings of our study indicate that demyelination in patients with DCM affects mainly the ventral spinocerebellar and rubrospinal tracts, and the extent of changes in the ventral spinocerebellar tract is related to the severity of the condition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Degenerative cervical myelopathy (DCM) is the leading cause of spinal cord dysfunction in developed countries, and its incidence is increasing due to increased life expectancy [1]. As degenerative changes occur in the cervical spine canal, the canal narrows and puts pressure on the spinal cord, causing progressive damage [2]. Symptoms of DCM include gait impairment, coordination problems, abnormal sensations, and bladder dysfunction. Without surgical decompression, this condition can progress to severe spinal cord injury and tetraparesis [3]. However, despite successful surgery, some patients may not experience any improvement in their clinical status or may even deteriorate [4].
Cervical spine magnetic resonance imaging (MRI) is a commonly used and valuable diagnostic tool for patients with DCM [5]. In addition to conventional sequences, numerous sophisticated neuroimaging methods, such as diffusion tensor imaging (DTI), magnetization transfer (MT), myelin water fraction (MWF), and magnetic resonance spectroscopy (MRS), offer in-depth quantitative information on microstructural alterations in the spinal cord, providing an enhanced understanding of the disease pathophysiology [6, 7]. Several studies have explored the application of these techniques in patients with DCM to assess spinal cord injury severity and predict the outcome of surgical interventions [2, 6, 8,9,10,11]. However, unfortunately, the ability to monitor DCM development and predict the potential for recovery is still limited, even with advanced neuroimaging techniques.
Neuroimaging research on spinal cord structural reorganization in patients with DCM has focused mainly on methods such as cervical cord morphometry based on T2-weighted imaging and DTI [5, 6]. For example, Vallonton et al. (2021) demonstrated the presence of microstructural changes in the grey and white matter of the cervical spinal cord, as indicated by the DTI data, in patients with DCM. Intriguingly, their study also revealed similar changes in white matter at the lumbar cord level [12]. Another DTI-based study demonstrated similarity in the pattern of spinal cord atrophy between patients with spinal cord traumatic injury and DCM [13]. Nevertheless, studies examining spinal cord white matter using myelin-sensitive techniques in this population are lacking. However, myelin damage resulting from chronic spinal cord compression and ischemia is a significant factor in the pathophysiology of DCM and the progression of neurological impairment in affected individuals [14, 15]. Understanding which spinal cord tracts are most vulnerable to degenerative demyelination and how this correlates with clinical symptoms is also an intriguing area for further exploration.
There are several studies dedicated to the use of myelin-specific neuroimaging modalities in patients with traumatic cervical cord injury and have demonstrated promising results [16, 17]. In addition, some previous reports have shown the utility of MT imaging in patients with DCM [15, 18]. Our previous results demonstrated impaired myelin integrity in various spinal cord tracts in patients with DCM, as revealed by the magnetization transfer ratio (MTR) technique, and its association with disease severity [19]. Specifically, extrapyramidal tracts such as the spinocerebellar, rubrospinal, and reticulospinal systems exhibited the most severe decreases in MTR. However, it is important to acknowledge that each myelin-sensitive technique has limitations, with MTR being particularly reliant on both cross-relaxation and longitudinal relaxation in tissues, potentially introducing bias [20, 21]. Thus, it will be interesting to confirm the MTR-based data with other quantitative myelin-sensitive MRI techniques. One such method involves assessing the magnetization transfer saturation (MTsat) metric, which is believed to be independent of T1 and B1 effects [21].
To date, the role of myelin damage within the different white matter tracts of the spinal cord in patients with DCM appears to be underresearched. Most existing papers have focused on the MTR technique and highlighted its significant potential [14, 15, 19]. Nevertheless, only a limited number of studies have investigated the possibilities offered by other myelin-sensitive techniques [22, 23]. Therefore, our objective was to assess the demyelinating changes within different spinal tracts in patients with DCM using the MTsat technique. We hypothesized that patients with DCM would show a reduction in MTsat in the white matter regions of the cervical spinal cord examined and that some spinal cord tracts would be more vulnerable to this damage than others. Furthermore, we assumed that the changes in MTsat values would correlate with disease severity.
Methods
Patients
In this study, the participants included patients who were diagnosed with DCM according to the diagnostic criteria [24], as well as patients who were diagnosed with cervical radiculopathy (without evident signs of myelopathy in clinical or neuroimaging evaluations) and who underwent surgical treatment at our hospital between January 2022 and September 2023. A total of 53 DCM patients (30 men and 23 women) and 41 patients with cervical radiculopathy caused by disk extrusion (22 men and 19 women) were enroled in the present study. Before surgery, all participants underwent high-resolution cervical spine and brain MRI. Neurological assessment was conducted for all patients before spine surgery; disease severity was evaluated with the Japanese orthopaedic association (JOA) scale [25] for DCM patients. Detailed information on the patients is provided in Table 1. Each patient signed a written informed consent form before participating in the study. The study was carried out according to the Declaration of Helsinki and was approved by the local Ethics Committee (protocol no. 7 dated 05-25-2021).
MRI data acquisition
MR imaging data were acquired using a 3 T system (Ingenia, Philips Healthcare, The Netherlands) equipped with a 16-channel receiver head and neck coil. The MRI protocol was performed according to an early published guide [26] and included high-resolution T2-weighted imaging (T2-WI), T2 gradient echo (T2 GRE), magnetization transfer (MT) and diffusion tensor imaging (DTI). The magnetization transfer technique (acquired in the axial plane) had the following parameters: TR = 57 ms, TE = 2 ms, FOV = 220∗220 mm, matrix = 256∗256, MT on and off, number of slices = 22, and slice thickness = 5 mm. The T1-weighted gradient echo sequence required for calculating MTsat maps had the following specifications: acquired in the axial plane, TR = 15 ms, TE = 2 ms, FOV = 220∗220 mm, matrix = 256∗256, number of slices = 22, and slice thickness = 5 mm. The central slice was placed at the level of the intervertebral disk C3-4 in the sagittal plane, and the final coverage was from vertebra C2 to C5.
MRI data processing
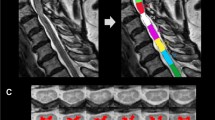
Spinal cord MRI processing was performed using the Spinal Cord Toolbox (https://spinalcordtoolbox.com/index.html) [27] after converting the DICOM files to the NIfTI format with dcm2niix software (https://github.com/rordenlab/dcm2niix). First, we performed spinal cord segmentation using high-resolution T2-WI with manual vertebral labelling. This was followed by registration on the PAM50 template [28]. After that, multimodal registration and spinal cord grey matter segmentation were performed using the T2 GRE sequence. The processing of magnetization transfer images involved generating magnetization transfer saturation (MTsat) maps, aligning them with T2-WI and T2 GRE images, and then performing atlas-based segmentation of the spinal cord across 36 distinct regions of interest (ROIs) in the axial plane [29]. For the analysis, the average values from the 2nd to 5th vertebrae were extracted, and the averages from the right and left sides were included. A certified neuroradiologist visually confirmed the results of the vertebrae labelling, multimodal registration, and spinal cord segmentation in all patients. Figure 1 illustrates data processing in the Spinal Cord Toolbox, including vertebrae labelling and grey matter segmentation. Figure 2 shows the atlas-based segmentation of MTsat maps. Figure 3 demonstrates an example of segmentation at the compression level in a patient with DCM.
a Sagittal T2-weighted image after manual vertebrae labelling and spinal cord segmentation; b Axial T2 GRE images at the level of compression; c Results of grey matter segmentation at the level of compression; d Results of white matter segmentation at the level of compression; e Axial magnetisation transfer images.
Statistical analysis
Statistical analysis was conducted using R software (www.r-project.org). Our data demonstrated a normal distribution based on the Shapiro‒Wilk test. Covariance analyses (ANCOVAs) were utilized to evaluate the variations in MTsat values, with group (DCM, radiculopathy) as the between-subject factor, ROI average signal intensity as the dependent variable, and patient age and sex as covariates. Pearson’s correlation test was employed to analyse correlations among different ROI MTsat data, disease severity (based on the JOA scale), and disease duration. Statistical significance was determined with a p value less than 0.05 after FDR correction.
Results
Clinical and demographic data
A total of 53 individuals with DCM (30 men and 23 women, 39–82 years old with an average of 56.4 years) and 41 individuals with cervical radiculopathy from disk extrusion (22 men and 19 women, 30–72 years old with an average of 48.3 years) were included in the study. Table 1 presents the clinical and demographic details of the participants. There were no significant differences in sex between the DCM and radiculopathy groups (p > 0.1), but there was a significant difference in age (p < 0.05). However, this age gap was accounted for by including age as a covariate in the subsequent ANCOVA. None of the patients in the radiculopathy group exhibited spinal cord compression or signal changes based on MRI findings or clinical symptoms of myelopathy. In the DCM group, 23 patients had compression in the upper cervical cord region (C3-C4 level), while 30 patients had compression in the lower cervical cord region (C5-C7 level). All patients in the DCM group exhibited spinal cord compression or signal changes based on MRI findings, as well as clinical symptoms of myelopathy. None of the DCM patients had undergone prior surgical interventions for their condition.
Cervical spinal cord MTsat values in patients with DCM and radiculopathy
A statistically significant reduction in the average MTsat values of the lateral funiculi of the spinal cord was revealed in the DCM group compared to the radiculopathy group (p < 0.01, FDR corrected; Fig. 4C). Additionally, a reduction in MTsat values in the ventral spinocerebellar tract was found in patients with DCM (p < 0.001, FDR corrected; Fig. 4A). Furthermore, a trend toward a decrease in MTsat values within the rubrospinal tract was found (p = 0.08, FDR corrected, Fig. 4B). All the results are provided in Supplementary Files 1 and 2.
Correlations between MTsat values and the JOA score
A trend towards a positive correlation was revealed between the JOA score and the values of MTsat within the ventral spinocerebellar tract (R = 0.33, adjusted p = 0.051; Fig. 5) was revealed. No statistically significant correlations were found between MTsat values within other ROIs and the JOA score (p > 0.1, not shown). All the results are provided in Supplementary Files 1 and 2.
Correlations between MTsat values and disease duration
No statistically significant correlations were found between the MTsat values within any of the analysed ROIs and disease duration (p > 0.1, not shown). All the results are provided in Supplementary File 1.
Discussion
The search for valuable neuroimaging markers to monitor disease progression and evaluate the extent of spinal cord injury in people with DCM is a crucial aspect of research in this field. The primary aim of this study was to compare myelin integrity (assessed as MTsat) in various spinal tracts between patients with DCM and individuals without DCM. Consistent with our hypothesis, a notable reduction in MTsat in several cervical spinal cord areas was observed in DCM patients compared to those with radiculopathy. Specifically, the most prominent decrease in MTsat was revealed within regions such as the ventral spinocerebellar tract, the rubrospinal tract, and the lateral funiculi of the spinal cord. Furthermore, alterations in MTsat values in the ventral spinocerebellar tract were associated with the severity of clinical symptoms, as indicated by the JOA score.
In general, these results are in alignment with our previous data [19], where a decrease in MTR values was shown through different regions of the cervical cord white matter. However, the differences revealed with the MTR technique involved more spinal tracts than those revealed with the MTsat technique. For example, MTR also showed myelin damage within regions such as the reticulospinal tracts and ventral funiculi of the spinal cord. One possible explanation is that the MTR technique is particularly dependent on cross-relaxation and longitudinal relaxation in tissues [20, 21], while MTsat is believed to be independent of these effects [21] and is likely more precise. It is important to note here that both the ventral spinocerebellar and rubrospinal tracts showed myelin damage with MTR as well as with MTsat metrics, which increases the reliability of these data.
Our findings generally support the existing evidence indicating that reduced MTsat levels in extrapyramidal spinal cord regions could serve as a valuable biomarker reflecting myelin damage associated with DCM. Long-term spinal cord compression is thought to result in demyelination and axonal damage [30]. Previous studies have demonstrated the effectiveness of magnetization transfer imaging in tracking the progression of symptoms in patients with DCM as part of a multiparametric advanced MRI assessment [18]. Another research group observed a decrease in MTR values, specifically in the anterior spinal cord region, among DCM patients, which was linked to hyperreflexia [15]. Moreover, alterations in MTR values were found to correlate with various clinical disability scales, such as the neck disability index, pain interference scale, and modified JOA scores, according to another study [14]. In addition, an association has also been demonstrated between a decrease in MTR and a decrease in DTI parameters within the fasciculus cuneatus and the lateral corticospinal tract [11].
To our knowledge, this study is the first attempt to evaluate differences in the vulnerability of different spinal tracts to myelin damage in patients with DCM using MTsat technique in a relatively large sample. The most prominent decrease in MTsat was observed in the ventral spinocerebellar tract and lateral funiculi of white matter. No differences in MTsat were detected within the posterior funiculi of the spinal cord between the groups, indicating that these areas are comparatively less prone to degenerative demyelination. This selective involvement could be explained by the pathobiology of the disease, where the degenerative process usually affects the anterior part of the vertebral canal and results in chronic ischemia affecting the circulation of the anterior spinal artery [15]. In fact, structural impairments were more prominent within regions that belong to the circulation of the anterior spinal artery in our study [31]. A recently published study that used spinal cord perfusion MRI in different white matter tracts also suggested perfusion impairment predominantly in the ventral areas of the spinal cord [32].
Therefore, long-term mechanical impact on white matter in the circulation of the anterior spinal artery can lead to demyelination, indicated by a reduction in MTsat values, probably caused by a combination of direct compression damage, chronic ischemia, and Wallerian degeneration mechanisms [30]. Interestingly, the primary involvement of the components of the extrapyramidal system, particularly the spinocerebellar and rubrospinal tracts, in the demyelination process explains the typical clinical manifestations of DCM [33]. Early signs of DCM often include impaired sensory perception, difficulties in movement, and clumsiness [33]. The ventral spinocerebellar tract is thought to play a role in initiating and sustaining locomotor behaviour [34]. On the other hand, the rubrospinal system is crucial for motor control, influencing muscle tone, motor responses, motor skill development, and sensory perception [35]. It is important to note that these white matter tracts are quite thin, making them challenging to visualize even with 3 T MRI systems [29]. Therefore, further research utilizing higher-field MRI systems could provide valuable insights into these mechanisms [7].
In our study, a trend towards a positive correlation between the severity of clinical symptoms and the values of MTsat within the ventral spinocerebellar tract was also revealed. These data generally confirm previously published results [11, 14, 15, 19]. Nevertheless, the duration of the disease did not correlate with the decrease in MTsat in any spinal cord region. We assume that the diverse clinical trajectories and medical histories often observed in DCM patients [1], along with the subjective nature of this measure, might account for this lack of association.
Together, the results of our study provide additional evidence supporting the occurrence of structural rearrangement in the white matter of the cervical cord in patients with DCM that appears secondary to chronic compression. Specifically, the ventral spinocerebellar and rubrospinal spinal tracts appear to be particularly susceptible to myelin damage in DCM patients. Furthermore, our findings suggest that this restructuring could be linked to the severity of the disease and thus may reflect the progression of DCM. The majority of patients in our study had a moderate or severe myelopathy stage according to JOA (Table 1), and both motor and sensory functions were involved. Thus, it is difficult to hypothesise an exact reason for such selectivity in the involvement of white matter tracts based on our data. More studies with patients in the early stages of the disease should be conducted to clarify this point.
This study has several limitations. First, the sample size was relatively small (n = 53 in the DCM group), which restricted our ability to draw definitive conclusions. Second, the absence of a healthy control group limited our comparisons to only patients with DCM and cervical radiculopathy. Additionally, our study lacked follow-up data. Finally, clinical evaluation was limited by the JOA scale in our study, which is not precise enough and allows us to only perform a general evaluation of the patient’s disability. It should also be noted that the present study does not include comparisons of MTsat with other quantitative techniques, such as DTI and T2 GRE. Future research with larger sample sizes, comprehensive follow-up assessments, and the use of additional quantitative neuroimaging methods is necessary to confirm our results and assess their relevance in clinical practice.
Conclusion
We found a notable decrease in MTsat values in the white matter of the spinal cord in patients with DCM compared to individuals with radiculopathy. This indicates that long-term spinal cord compression injury leads to myelin damage, particularly affecting the ventral spinocerebellar tract and lateral funiculi. Our results generally align with existing data, suggesting that magnetization-transfer MRI could serve as an early biomarker for myelopathy in this group of patients and may also indicate the ongoing progression of DCM.
Data availability
The raw data were generated at the Federal Neurosurgical Centre Novosibirsk. Derived data supporting the findings of this study are available from the corresponding author upon request.
References
Yurac R, Matamala JM, Zamorano JJ, Harrop JS, Davies BM, Nouri A, et al. Degenerative cervical myelopathy. Rev Med Chil. 2022;150:339–52. Available from: https://pubmed.ncbi.nlm.nih.gov/36156719/.
Nouri A, Tessitore E, Molliqaj G, Meling T, Schaller K, Nakashima H, et al. Degenerative Cervical Myelopathy: Development and Natural History [AO Spine RECODE-DCM Research Priority Number 2]. Global Spine J. 2022;12:39S.
Fehlings MG, Tetreault LA, Wilson JR, Skelly AC. Cervical spondylotic myelopathy: current state of the art and future directions. Spine. 2013;38. Available from: https://pubmed.ncbi.nlm.nih.gov/23962994/.
Fehlings MG, Smith JS, Kopjar B, Arnold PM, Yoon ST, Vaccaro AR, et al. Perioperative and delayed complications associated with the surgical treatment of cervical spondylotic myelopathy based on 302 patients from the AOSpine North America Cervical Spondylotic Myelopathy Study: Presented at the 2011 Spine Section Meeting. J Neurosurg Spine. 2012;16:425–32. https://thejns.org/spine/view/journals/j-neurosurg-spine/16/5/article-p425.xml.
Nouri A, Martin AR, Mikulis D, Fehlings MG. Magnetic resonance imaging assessment of degenerative cervical myelopathy: a review of structural changes and measurement techniques. Neurosurg Focus. 2016;40. Available from: https://pubmed.ncbi.nlm.nih.gov/27246488/.
Sasiadek MJ, Szewczyk P, Bladowska J. Application of diffusion tensor imaging (DTI) in pathological changes of the spinal cord. Med Sci Monit. 2012;18. Available from: https://pubmed.ncbi.nlm.nih.gov/22648262/.
Seif M, David G, Martin AR, Freund P. Conventional and advanced magnetic resonance imaging for degenerative cervical myelopathy. In Degenerative Cervical Myelopathy (pp. 101–111). Academic Press, 2023.
Liu H, MacMillian EL, Jutzeler CR, Ljungberg E, MacKay AL, Kolind SH, et al. Assessing structure and function of myelin in cervical spondylotic myelopathy: Evidence of demyelination. Neurology [Internet]. 2017;89:602. Aug 8 [cited 2022 Dec 1]. Available from: /pmc/articles/PMC5562959/.
Martin AR, De Leener B, Cohen-Adad J, Cadotte DW, Kalsi-Ryan S, Lange SF, et al. A Novel MRI Biomarker of Spinal Cord White Matter Injury: T2*-Weighted White Matter to Gray Matter Signal Intensity Ratio. AJNR Am J Neuroradiol. 2017;38:1266–73. https://pubmed.ncbi.nlm.nih.gov/28428212/.
He B, Sheldrick K, Das A, Diwan A. Clinical and Research MRI Techniques for Assessing Spinal Cord Integrity in Degenerative Cervical Myelopathy—A Scoping Review. Biomedicines. 2022;10:2621. https://www.mdpi.com/2227-9059/10/10/2621/htm.
Yang HE, Kim WT, Kim DH, Kim SW, Yoo WK, Yang HE, et al. Utility of Diffusion and Magnetization Transfer MRI in Cervical Spondylotic Myelopathy: A Pilot Study. Diagnostics. 2022;12:2090. https://www.mdpi.com/2075-4418/12/9/2090/htm.
Vallotton K, David G, Hupp M, Pfender N, Cohen-Adad J, Fehlings MG, et al. Tracking white and gray matter degeneration along the spinal cord axis in degenerative cervical myelopathy. J Neurotrauma. 2021;38:2978–87. https://www.liebertpub.com/doi/10.1089/neu.2021.0148.
Seif M, David G, Huber E, Vallotton K, Curt A, Freund P. Cervical Cord Neurodegeneration in Traumatic and Non-Traumatic Spinal Cord Injury. J Neurotrauma. 2020;37:860–7.
Paliwal M, Weber KA, Hopkins BS, Cantrell DR, Hoggarth MA, Elliott JM, et al. Magnetization Transfer Ratio and Morphometrics of the Spinal Cord Associates with Surgical Recovery in Patients with Degenerative Cervical Myelopathy. World Neurosurg. 2020;144:e939.
Cloney MB, Smith ZA, Weber KA, Parrish TB. Quantitative magnetization transfer MRI measurements of the anterior spinal cord region are associated with clinical outcomes in cervical spondylotic myelopathy. Spine. 2018;43:675.
Schading S, David G, Max Emmenegger T, Achim C, Thompson A, Weiskopf N, et al. Dynamics of progressive degeneration of major spinal pathways following spinal cord injury: A longitudinal study. Neuroimage Clin. 2023;37:103339.
Morris S, Swift-LaPointe T, Yung A, Prevost V, George S, Bauman A, et al. Advanced Magnetic Resonance Imaging Biomarkers of the Injured Spinal Cord: A Comparative Study of Imaging and Histology in Human Traumatic Spinal Cord Injury. J Neurotrauma. 2024;41:1223–39. https://pubmed.ncbi.nlm.nih.gov/38318802/.
Martin AR, De Leener B, Cohen-Adad J, Kalsi-Ryan S, Cadotte DW, Wilson JR, et al. Monitoring for myelopathic progression with multiparametric quantitative MRI. PLoS One. 2018;13. Available from: https://pubmed.ncbi.nlm.nih.gov/29664964/.
Filimonova E, Abdaev M, Vasilenko I, Kubetskij Y, Prokhorov O, Rzaev J. Evaluation of the structural integrity of different spinal cord tracts with magnetization transfer ratio in degenerative cervical myelopathy. Neuroradiology. 2024;66:839–46. https://springerlink.bibliotecabuap.elogim.com/article/10.1007/s00234-024-03327-w.
Smirnova LP, Yarnykh VL, Parshukova DA, Kornetova EG, Semke AV, Usova AV, et al. Global hypomyelination of the brain white and gray matter in schizophrenia: quantitative imaging using macromolecular proton fraction. Transl Psychiatry. 2021;11:365.
Franco Piredda G, Hilbert T, Thiran JP, Kober T. Probing myelin content of the human brain with MRI: A review. 2020. Available from: https://onlinelibrary.wiley.com/doi/10.1002/mrm.28509.
Hori M, Hagiwara A, Fukunaga I, Ueda R, Kamiya K, Suzuki Y, et al. Application of Quantitative Microstructural MR Imaging with Atlas-based Analysis for the Spinal Cord in Cervical Spondylotic Myelopathy. Sci Rep. 2018;8. Available from: https://pubmed.ncbi.nlm.nih.gov/29581458/.
Martin AR, Tetreault L, Nouri A, Curt A, Freund P, Rahimi-Movaghar V, et al. Imaging and Electrophysiology for Degenerative Cervical Myelopathy [AO Spine RECODE-DCM Research Priority Number 9]. Global Spine J. 2022;12:130S–146S. https://journals.sagepub.com/doi/full/10.1177/21925682211057484.
Hilton B, Gardner EL, Jiang Z, Tetreault L, Wilson JRF, Zipser CM, et al. Establishing Diagnostic Criteria for Degenerative Cervical Myelopathy [AO Spine RECODE-DCM Research Priority Number 3]. Global Spine J. 2022;12:55S.
Kato S, Oshima Y, Matsubayashi Y, Taniguchi Y, Tanaka S, Takeshita K. Minimum Clinically Important Difference and Patient Acceptable Symptom State of Japanese Orthopaedic Association Score in Degenerative Cervical Myelopathy Patients. Spine. 2019;44:691–7. https://pubmed.ncbi.nlm.nih.gov/30395093/.
Cohen-Adad J, Alonso-Ortiz E, Abramovic M, Arneitz C, Atcheson N, Barlow L, et al. Open-access quantitative MRI data of the spinal cord and reproducibility across participants, sites and manufacturers. Scientific Data. 2021;8:1–17. https://www.nature.com/articles/s41597-021-00941-8.
De Leener B, Lévy S, Dupont SM, Fonov VS, Stikov N, Louis Collins D, et al. SCT: Spinal Cord Toolbox, an open-source software for processing spinal cord MRI data. Neuroimage. 2017;145:24–43.
De Leener B, Fonov VS, Collins DL, Callot V, Stikov N, Cohen-Adad J. PAM50: Unbiased multimodal template of the brainstem and spinal cord aligned with the ICBM152 space. Neuroimage. 2018;165:170–9. https://pubmed.ncbi.nlm.nih.gov/29061527/.
Lévy S, Benhamou M, Naaman C, Rainville P, Callot V, Cohen-Adad J. White matter atlas of the human spinal cord with estimation of partial volume effect. Neuroimage. 2015;119:262–71. https://pubmed.ncbi.nlm.nih.gov/26099457/.
Akter F, Yu X, Qin X, Yao S, Nikrouz P, Syed YA, et al. The Pathophysiology of Degenerative Cervical Myelopathy and the Physiology of Recovery Following Decompression. Front Neurosci. 2020;14:138 www.frontiersin.org.
Hardy TA. Spinal Cord Anatomy and Localization. Continuum. 2021;27:12–29. https://journals.lww.com/continuum/fulltext/2021/02000/spinal_cord_anatomy_and_localization.4.aspx.
Lebret A, Lévy S, Pfender N, Farshad M, Altorfer FCS, Callot V, et al. Investigation of perfusion impairment in degenerative cervical myelopathy beyond the site of cord compression. Sci Rep. 2023;13:1–12. https://www.nature.com/articles/s41598-023-49896-3.
Milligan J, Ryan K, Fehlings M, Bauman C. Degenerative cervical myelopathy: Diagnosis and management in primary care. Canadian Family Phys. 2019;65:619.
Chalif JI, Martínez-Silva ML, Pagiazitis JG, Murray AJ, Mentis GZ. Control of mammalian locomotion by ventral spinocerebellar tract neurons. Cell. 2022;185:328–344.e26. https://pubmed.ncbi.nlm.nih.gov/35063074/.
Olivares-Moreno R, Rodriguez-Moreno P, Lopez-Virgen V, Macías M, Altamira-Camacho M, Rojas-Piloni G. Corticospinal vs Rubrospinal Revisited: An Evolutionary Perspective for Sensorimotor Integration. Front Neurosci. 2021;15:686481.
Acknowledgements
The authors thank Aria Nouri (Switzerland) for inspiration for this study and for providing valuable advice.
Author information
Authors and Affiliations
Contributions
Conceptualization, EF, MA, and JR; methodology, EF, MA, and OP; formal analysis, EF, OP, IV, and MA; data curation, YK and JR; investigation, EF, MA, and OP; resources, JR; writing—original draft preparation, EF; writing — review and editing, MA, OP, IV, JR, and YK; visualization – EF; supervision, YK and JR; project administration, JR. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The data were acquired and published in accordance with the principles outlined in the Declaration of Helsinki. The study was approved by the local Ethics Committee of the Federal Centre for Neurosurgery, Novosibirsk, Russia (protocol no. 7 dated 05-25-2021).
Consent for publication
All patients provided informed consent for publication.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Filimonova, E., Abdaev, M., Vasilenko, I. et al. White matter spinal tracts impairment in patients with degenerative cervical myelopathy evaluated with the magnetization transfer saturation MRI technique. Spinal Cord (2024). https://doi.org/10.1038/s41393-024-01025-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41393-024-01025-1
- Springer Nature Limited