Abstract
Introduction
The main objectives of the present study are to assess the incidence of cerebral venous thrombosis (CVT) presenting as isolated subarachnoid hemorrhage (SAH) and to determine the occurrence of cortical venous thrombosis (CoVT).
Methods
Among 332 patients with CVT, investigated with the same CT and MR standardized protocol, 33 (10 %) presented with SAH, associated in 11 cases with hemorrhagic infarct or intracerebral hemorrhage. This study is based on 22 cases of CVT presenting as SAH in the absence of hemorrhagic brain lesion. Diagnosis of sinus thrombosis was established on T2* and magnetic resonance venography and that of CoVT on T2* sequence. Diagnostic of SAH was based on fluid-attenuated inversion recovery (FLAIR) sequence.
Results
CVT involved lateral sinus in 18 patients, superior sagittal sinus in 16, and straight sinus in 1. Cortical veins were involved in all patients, in continuity with dural sinus thrombosis when present. SAH was circumscribed to few sulci in all cases and mainly localized at the convexity (21 cases). CoVT implied different areas on the same side in four patients and was bilateral in seven. There was no perimesencephalic or basal cisterns hemorrhage. Cortical swelling was present in 12 cases, associated with localized edema. All patients except one had a favorable outcome.
Conclusion
This report shows that the incidence of CVT presenting as isolated SAH is evaluated to 6.4 % and that SAH is, in all cases, in the vicinity of CoVT and when dural thrombosis is present in continuity with it.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cerebral venous thrombosis (CVT) has long been known as a cause of parenchymal hemorrhage. Subarachnoid hemorrhage (SAH), isolated or associated with parenchymal hemorrhages, is a rare presentation of CVT, only reported as isolated case reports or in small series. Up to now, 41 patients with complete radiologic data have been reported with CVT-related SAH out of which 32 presented isolated SAH and 26 have been evaluated by MRI examination [1–23]. Systematic studies investigating SAH occurring in CVT and using T2* sequence are missing, and the incidence of this association is not known. Therefore, the main objective of the present study was to evaluate the incidence and the radiological findings, using T2*, of SAH associated with CVT, with attention to the presence, location, and distribution of associated cortical venous thrombosis (CoVT).
This study is based on 22 patients seen in our institution from 2003 to 2013 who had CVT presenting as SAH without associated parenchymal hemorrhage.
Patients and methods
In our prospective registry of consecutive CVT patients, we retrospectively analyzed the data of all subjects (22 patients) with SAH (in the absence of parenchymal hemorrhage)-associated CVT between January 2003 and December 2013.
Non-contrast brain CT and MRI were obtained within 24 h of admission and at 3 months of follow-up.
Brain CT was performed on a CT 64-slice multi-detector row CT scan. CT imaging was obtained with 2.5-mm section thickness through the posterior fossa with 5-mm section thickness through the supratentorial hemispheres.
MRI was performed on a 1.5-T unit (GE Medical System) using a standardized protocol for brain examination, including sagittal T1, axial fluid-attenuated inversion recovery (FLAIR), coronal T2SE, T2*, and diffusion-weighted imaging (DWI) sequences, apparent diffusion coefficient (ADC) map and 2D time-of-flight (TOF) MR venography (MRV).
The diagnosis of both CVT and SAH was based on MRI using the following criteria: (1) for CVT, presence of intraluminal thrombosis in sinuses or veins on T1 (isointense or hyperintensity) or T2 (isointensity or hyperintensity) and on T2* (hypointensity), associated with a filling defect in venous structures on 2D TOF MRV; CoVT was recognized as a well-delineated, tubular, serpentine, or rounded hyposignal on T2*, and (2) for SAH, presence of hyperintense subarachnoid signals on FLAIR sequence, without associated hemorrhagic parenchymal lesions on T2* sequence.
Clinical signs and etiological workup were recorded in detail for all patients.
Non-hemorrhagic intraparenchymal lesions were classified as cortical swelling when there was a sulcus effacement around the cortical SAH on FLAIR sequence, as localized parenchymal edema when there was an area of hypodensity on CT or of hyperintensity on FLAIR and DWI, and as cortical infarction when the DWI hypersignal was associated with ADC restriction [5].
Results
Among 332 patients with CVT, investigated during the same period in our institution with the same CT and MR standardized protocol, 33 (10 %) presented with SAH, associated in 11 cases with hemorrhagic infarct or intracerebral hemorrhage.
Thus, our study is based on the cohort of 22 patients (6.4 %) who had CVT presenting as SAH without associated parenchymal hemorrhage: 18 women and 4 men, mean age 39 years old (range 24–54).
CT and MR images were reviewed independently by a neuroradiologist (MB) and a neurologist (IC) experienced in CVT and blinded to the clinical and imaging findings. There was a complete agreement in the assessment of neuroradiological findings.
Baseline clinical, etiological, and radiological data are summarized in Table 1.
Headache was present in 21 patients (95 %), and in all, it was the initial symptom. Headache was of the thunderclap type in only three patients (Table 1). All patients received heparin (UFH or LMWH). One patient (no. 2) died of pulmonary embolism at day 9. The 21 surviving patients all had an excellent 3-month functional outcome.
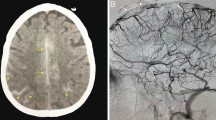
SAH was visualized on non-enhanced CT in 7/22 cases. It was limited to a few sulci, appearing very slightly hyperdense in two cases (Fig. 1a). On FLAIR sequence, SAH was localized unilaterally and in most cases circumscribed to a few sulci at the convexities (21/22 cases) (Fig. 1b) (Table 1). In addition to convexity location, SAH was present in the frontal parasagittal sulci (Fig. 2) and in the distal sylvian fissure in respectively one case each.
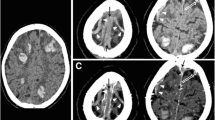
Case no. 3 non-enhanced CT scan (a, short arrow) and FLAIR images show cortical subarachnoid hemorrhage (SAH) at high right frontal convexity with cortical swelling (b, short arrow). FLAIR images show additional SAH hyperintensities at high parietal convexity (b, long arrow). SSS thrombosis and bilateral cortical vein thrombosis (CoVT) are seen on CT (a, long arrow). On T2*, CoVT appears as tubular hypointense structures converging to the SSS, adjacent to the areas of SAH (c). Additional thrombosis of left anterior frontal veins is depicted (d, arrowhead). Furthermore, an hypointense signal in the right fronto-parietal sulci, compared to the left one, is consistent with blood in the subarachnoid space (hemosiderin deposition) (d, thin arrow)
Case no. 1. FLAIR images show two locations of SAH: in the fronto-parietal right sulci (short arrows) and in the left parasagittal sulci (long arrow) (a). T2*, in the same plane, show the cortical thrombosed vein adjacent to the area of SAH (b, short arrow) and hemosiderin deposition in the left paragittal sulci) (b, long arrow)
Twelve patients had a localized cortical swelling adjacent to SAH, including five with additional localized subcortical edema.
Sinus thrombosis was present in 21/22 cases. Thrombosis affected both superior sagittal sinus (SSS) and lateral sinus (LS) in 12 patients.
CoVT was present in all patients. It appeared in all cases as a tubular, serpiginous strongly hypointense structure on T2*, hyperintense on T1 in five cases, and hyperintense on FLAIR and DWI in only one case. CoVT was visibly adjacent to the area of SAH in all cases (Figs. 1 and 2). When there was a sinus thrombosis, the cortical thrombus was always in continuity with it (Fig. 2).
The vein most frequently involved was the vein of Labbé (14 patients), always in association with ipsilateral LS thrombosis. Frontal veins were involved in 11 patients, in association with SSS thrombosis in 10. There was frequently an association of several CoVT (Table 1) (Figs. 1 and 2). In the two cases presenting with bilateral frontal vein thrombosis, SAH was observed bilaterally in the frontal areas. In two other cases, limited SAH was observed in multiple areas. CoVT was isolated in one case, involving the frontal and parietal veins on the same side, and SAH was localized at the ipsilateral fronto-parietal convexity.
Discussion
We report a series of 22 patients with CVT presenting as isolated SAH, i.e., in the absence of brain hemorrhage. This is the largest series so far. The incidence of concomitant CVT and SAH, in the absence of cerebral bleeding, was of 6.4 % in our experience.
SAH was observed at the brain convexity in 21 patients, and it was always localized, involving only a few sulci. The diagnosis of SAH was overlooked on CT scan in 15 patients because of the small amount of blood. The diagnosis was based on MRI [24–26] which is well established as superior to CT scan for presumptive diagnosis and localization of acute and subacute low-grade SAH. The typical pattern is the presence on FLAIR MRI of hyperintensities in subarachnoid spaces, sometimes associated with hypointensities at the same location on T2*.
In our series, all patients had CoVT and SAH was invariably located adjacent to the thrombosis. CVT is a well established but rare cause of SAH. The diagnosis of CVT in a patient with SAH is crucial, because of the need to treat CVT with heparin whereas, by contrast, heparin is contraindicated in all other causes of SAH. In patients presenting with isolated SAH, the diagnosis of CVT is easy when there is a sinus thrombosis as in 21 of our patients. By contrast, the diagnosis is extremely difficult when there is an isolated cortical vein thrombosis. Firstly, even in the absence of SAH, CoVT is seriously difficult to diagnose, because cortical veins are variable in number and location and because only the largest veins are detectable on MRV or CT venography [24–26]. Secondly, when there is a SAH localized at the convexity, it is sometimes impossible on FLAIR sequence to differentiate it from a CoVT. T2* sequence is then crucial, showing CoVT as an hypointense tubular structure while SAH appears as a slight hemosiderin deposit [24–26].
Furthermore, as related in our series, SAH localized at the fronto-parietal areas, even bilaterally, and/or at the paragittal sulci should rise the possibility of thrombosis of the SSS and/or of the frontal and parietal veins, while SAH confined to the posterior temporal or temporo-occipital convexity or at the distal sylvian fissure suggests searching for signs of lateral sinus and Labbé vein thrombosis. Thus, the localization of convexal SAH appears to be a good indicator of the involved venous structure.
Localized non-traumatic convexity SAH has diverse causes that need to be ruled out, most commonly amyloid angiopathy and reversible cerebral vasoconstriction syndrome (RCVS), but the pattern of clinical presentation is different, and other imaging signs are present such as micro-bleeds in amyloid angiopathy and segmental arterial vasoconstriction in RCVS [27–29]. Another important radiological clue in favor of CVT-related SAH is the location purely at the convexity, spearing the basal cisterns (2, 5–7, 10–12).
Different hypothesis have been suggested to explain the occurrence of SAH in patients with CVT. A first hypothesis is that sinus thrombosis increases the venous pressure, thereby causing the dilatation and rupture of the adjacent fragile, thin-walled cortical veins. This could lead to the localized hemorrhage entering the subarachnoid space [2, 11, 17, 30, 31]. Another hypothesis is that SAH could be due to the secondary rupture of parenchymal hemorrhagic lesions into the subarachnoid space [2, 4, 17]. This was however not the case in our patients since patients with brain hemorrhages were excluded. A third possibility is that the extension of sinus thrombosis into cortical or cerebellar veins causes a localized venous hyperpressure with subsequent dilatation and eventually rupture of these veins. Such a mechanism has been demonstrated in experimental studies [32–34]. Our findings are in favor of this last hypothesis, since there was in all cases a high concordance between the location of SAH and CoVT, which was in continuity with sinus thrombosis in 21 cases. Thrombosed cortical veins thus seem to be the cause of isolated SAH in patients with CVT.
Conclusion
SAH in the presence of CVT is uncommon, occurring in 6.4 % of CVT in our experience.
This report shows that in CVT presenting as isolated SAH, SAH is, in all cases, circumscribed and closely adjacent to the area of cortical vein thrombosis and when dural thrombosis is present in continuity with it. The presence of acute and limited SAH of the convexity, especially when basal cisterns are spared, should prompt dedicated vascular imaging, including the venous system along with T2* sequence.
Abbreviations
- CVT:
-
Cerebral venous thrombosis
- CoVT:
-
Cortical vein thrombosis
- SAH:
-
Subarachnoid hemorrhage
- SSS:
-
Superior sagittal sinus
References
Ciccone A, Citterio A, Santilli I, Sterzi R (2000) Subarachnoid haemorrhage treated with anticoagulants. Lancet 356:1818
Sztajzel R, Coeytaux A, Dehdashati AR, Delavelle J, Sinnreich M (2001) Subarachnoid hemorrhage: a rare presentation of cerebral venous thrombosis. Headache 41:899–901
Selim M, Fink J, Linfante I, Kumar S, Schlaug G, Caplan LR (2002) Diagnosis of cerebral venous thrombosis with echoplanar T2*-weighted magnetic resonance imaging. Arch Neurol 59:1021–1026
Widjaja E, Romanowski CA, Sinanan AR, Hodgson TJ, Griffiths PD (2003) Thunderclap headache: presentation of intracranial sinus thrombosis? Clin Radiol 58:648–652
Chang R, Friedman DP (2004) Isolated cortical venous thrombosis presenting as subarachnoid hemorrhage: a report of three cases. AJNR Am J Neuroradiol 25:1676–1679
Oppenheim C, Domingo V, Gauvrit J-Y, Lamy C, Mackowiak-Cordoliani M-A, Pruvo J-P, Meder J-F (2005) Subarachnoid hemorrhage as the initial presentation of dural sinus thrombosis. AJNR Am J Neuroradiol 26:614–617
Spitzer C, Mull M, Rohde V, Kosinski CM (2005) Non-traumatic cortical subarachnoid haemorrhage: diagnostic work-up and aetiological background. Neuroradiology 47:525–531
Adaletti I, Sirikei A, Kara B, Kurugoglu S, Ozer H, Bayram M (2005) Cerebral venous sinus thrombosis presenting with excessive subarchnoid hemorrhage in a 14-year-old boy. Emerg Radiol 12:575–579
Rice H, Tang YM (2006) Acute subarchnoid hemorrhage: a rare presentation of cerebral dural sinus thrombosis. Austr Radiol 50:241–245
Lin JH, Kwan SY, Wu D (2006) Cerebral venous thrombosis initially presenting with acute subarachnoid hemorrhage. J Chin Med Assoc 69:282–285
Pradhan S, Yadav R, Diwakar H, Phadke RV (2007) Subarachnoid hemorrhage following chronic dural venous sinus thrombosis. Angiology 58:498–501
Wang YF, Fuh JL, Lirng JF, Chang FC, Wang SJ (2007) Spontaneous intracranial hypotension with isolated cortical vein thrombosis and subarachnoid haemorrhage. Cephalalgia 27:1413–1417
Tang PH, Chai J, Chan YH, Chng SM, Lim CC (2008) Superior sagittal sinus thrombosis: subtle signs on neuroimaging. Ann Acad Med Singapore 37:397–401
Jaiser SR, Raman A, Maddison P (2008) Cerebral venous sinus thrombosis as a rare cause of thunderclap headache and nonaneurysmal subarachnoid haemorrhage. J Neurol 255:448–449
Lai NK, Hui JW, Wong GK, Yu SC, Sun DT, Poon WS (2008) Cerebral venous thrombosis presenting as subarachnoid haemorrhage. Hong Kong Med J 14:499–500
Benabu Y, Mark L, Daniel S, Glikstein R (2009) Cerebral venous thrombosis presenting with subarachnoid hemorrhage. Case report and review. Am J Emerg Med 27:96–106
Kato Y, Takeda H, Furuya D, Nagoya H, Deguchi I, Fukuoka T, Tanahashi N (2010) Subarachnoid hemorrhage as the initial presentation of cerebral venous thrombosis. Intern Med 49:467–470
Morris JG, Fisher M, Carandang RA (2010) Cortical vein thrombosis as a mimic for isolated cortical subarachnoid hemorrhage and transient ischemic attack. Case Rep Neurol 2:63–68
Panda S, Prashantha DK, Shankar SR, Nagaraja D (2010) Localized convexity subarachnoid haemorrhage—a sign of early cerebral venous sinus thrombosis. Eur J Neurol 17:1249–1258
Oda S, Shimoda M, Hoshikawa K, Osada T, Yoshiyama M, Matsumae M (2011) Cortical subarachnoid hemorrhage caused by cerebral venous thrombosis. Neurol Med Chir 51:30–36
Sayadnasiri M, Taheraghdam AA, Talebi M (2012) Cerebral venous thrombosis presenting as subarachnoid hemorrhage: report of two cases. Clin Neurol Neurosurg 114:1099–1101
Yamamoto J, Kakeda S, Takahashi M, Idei M, Nakano Y, Soejima Y, Saito T, Akiba D, Shibata E, Korogi Y, Nishizawa S (2013) Severe subarachnoid hemorrhage associated with cerebral venous thrombosis in early pregnancy: a case report. J Emerg Med 45:849–855
Sahin N, Solak A, Genc B, Bilgic N (2014) Cerebral venous thrombosis as a rare cause of subarachnoid hemorrhage: case report and literature review. Clin Imaging 38:373–379
Idbaih A, Boukobza M, Crassard I, Porcher R, Bousser MG, Chabriat H (2006) MRI of clot in cerebral venous thrombosis: high diagnostic value of susceptibility-weighted images. Stroke 37:991–995
Leach JL, Strub WM, Gaskill-Shipley MF (2007) Cerebral venous thrombus signal intensity and susceptibility effects on gradient recalled-echo MR imaging. AJNR Am J Neuroradiol 28:940–945
Boukobza M, Crassard I, Bousser MG, Chabriat H (2009) MRI features of isolated cortical vein thrombosis: diagnosis and follow-up. AJNR Am J Neuroradiol 30:344–348
Khurram A, Kleinig T, Leyden J (2014) Clinical associations and causes of convexity subarachnoid hemorrhage. Stroke 45:1151–1153
Ducros A, Boukobza M, Porcher R, Sarov M, Valade D, Bousser MG (2007) The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain 130:3091–3101
Katoh M, Yoshino M, Katsuyuki A, Aoki T, Imamura H, Kashiwazaki D, Takano K, Aida T (2007) A restricted subarachnoid haemorrhage in the cortical sulcus in cerebral amyloid angiopathy: could it be a warning sign? Surg Neurol 68:457–460
Ameri A, Bousser MG (1992) Cerebral venous thrombosis. Neurol Clin 10:87–111
Villringer A, Mehraein S, Einhäupl KM (1994) Pathophysiological aspects of cerebral sinus venous thrombosis (SVT). J Neuroradiol 21:72–80
Nakase H, Heimann A, Kempski O (1996) Local cerebral blood flow in a rat cortical vein occlusion model. J Cereb Blood Flow Metab 16:720–728
Nakase H, Takeshima T, Sakaki T, Heimann A, Kempski O (1998) Superior sagittal sinus thrombosis: a clinical and experimental study. Skull Base Surg 8:169–174
Stracke CP, Spuentrup E, Katoh M, Günther RW, Spangenberg P (2006) New experimental model of sinus and cortical vein thrombosis in pigs for MR imaging studies. Neuroradiology 48:721–729
Ethical standards and patient consent
We declare that all human studies have been approved by the Ethics Committee of our Institution and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boukobza, M., Crassard, I., Bousser, MG. et al. Radiological findings in cerebral venous thrombosis presenting as subarachnoid hemorrhage: a series of 22 cases. Neuroradiology 58, 11–16 (2016). https://doi.org/10.1007/s00234-015-1594-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-015-1594-5