Abstract
Background
Cerebral venous thrombosis (CVT) is associated with intracranial hemorrhage.
Aim
To identify clinical and imaging features of CVT-associated intracranial hemorrhage. We hypothesized that higher clot burden would be associated with a higher risk of intracranial hemorrhage.
Methods
We performed a retrospective analysis of an international, multicenter cohort of patients with confirmed cerebral venous thrombosis who underwent computed tomography within 2 weeks of symptom onset. Clinical and imaging features were compared between patients with and without intracranial hemorrhage. Clot burden was assessed by counting the number of thrombosed venous sinuses and veins on confirmatory imaging.
Results
We enrolled 260 patients from 10 institutions in Europe and Mexico. The mean age was 42 years and 74% were female. Intracranial hemorrhage was found in 102 (39%). Among them parenchymal hemorrhage occurred in 64 (63%), in addition, small juxta-cortical hemorrhage was found in 30 (29%), subarachnoid hemorrhage in 24 (24%) and subdural hemorrhage in 11 (11%). Multiple concomitant types of hemorrhage occurred in 23 (23%). Older age and superior sagittal thrombosis involvement were associated with presence of hemorrhage. The number of thrombosed venous sinuses was not associated with intracranial hemorrhage (median number IQRInterquartile ratio] of sinuses/veins involved with hemorrhage 2 (1–3) vs. 2 (1–3) without hemorrhage, p = 0.4).
Conclusion
The high rate of intracranial hemorrhage in cerebral venous thrombosis is not explained by widespread involvement of the venous sinuses. Superior sagittal sinus involvement is associated with higher bleeding risk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cerebral venous thrombosis (CVT) is associated with intracranial hemorrhage in up to 40% of patients [1,2,3]. Intracranial hemorrhage is related to increased venous pressure, ischemia and subsequent blood brain barrier breakdown with erythrocyte diapedesis [4, 5]. In an experimental model, involvement of cortical vein thrombosis was strongly associated with hemorrhage [6]. Other studies suggest that their involvement is not required and that occlusion of major sinuses is sufficient [7, 8]. Subarachnoid hemorrhage may occur due to rupture of thin cortical veins [9, 10]. Presence of hemorrhage in the context of CVT is a poor prognostic factor, and is a challenge for physicians when antithrombotic therapy is considered, or when decompressive surgery is needed [11,12,13]. In patients with nontraumatic intracranial hemorrhage, a diagnosis of CVT should be considered as it is known to cause various types of intracranial hemorrhages including subarachnoid hemorrhage (SAH), subdural hemorrhage (SDH) and various types of intracerebral hemorrhage (ICH) [9, 10, 14,15,16]. In the large, prospective International Study on Cerebral Vein and Dural Sinus Thrombosis, it was found that patients with intracerebral hemorrhage were older, more often had a severe, acute clinical presentation with coma, focal neurological deficit and seizures. On imaging, patients more often had bilateral lesions and concomitant infarction. Right lateral sinus occlusion was a risk factor for ICH [16].
Aim and hypothesis:
The aim of this study is to evaluate the clinical and radiological features of hemorrhagic CVT and to test the hypothesis that hemorrhagic CVT is related to thrombus load as measured by involvement of a larger number of thrombosed venous channels.
Methods
Study population
We retrospectively enrolled 285 patients with cerebral venous thrombosis (CVT) from ten institutions in Europe and Mexico (between 2001 and 2015) as part of a study to identify CT imaging markers of cerebral venous thrombosis [17]. We asked sites to identify from their hospital records, subjects fulfilling the following criteria: patients were at least 18 years old, and a proven CVT diagnosis with MRI, MR venography, CT venography or intra-arterial digital subtraction angiography (IADSA). A non-contrast CT had to be available and performed within 2 weeks of symptom onset (headache, seizures, altered mental state and focal neurological signs).
Data collection
We collected demographic data and presenting symptoms, categorized as headache, focal neurological signs, seizures and altered mental state. We collected data on the presence of thrombophilia defined as protein C or S deficiency, antithrombin deficiency, activated protein C resistance or presence of factor V Leiden, PT20210 G-A mutation or the presence of antiphospholipid syndrome. We recorded transient risk factors for venous thrombosis (pregnancy, puerperium, medication use) and conditions associated with CVT (malignancies, hematological abnormalities, vasculitis and autoimmune diseases).
Imaging
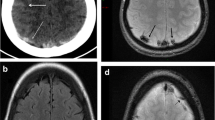
Non-contrast CT scans (NCCT) were centrally collected and were analyzed by a neuroradiologist (GB) and a neurologist (KA). Images were transferred by various means: portable media, cloud sharing services and via secure File Transfer Protocols (sFTP). The readers evaluated the presence of hemorrhage on non-contrast CT [intracerebral hemorrhage (ICH) including small juxta-cortical hemorrhage < 20 mm (JCH), subdural hemorrhage (SDH), subarachnoid hemorrhage (SAH)]. JCH was defined as a hemorrhage (diameter < 20 mm) located in the white matter just below the cortex. (Fig. 1) [14] Patients were divided into 2 groups (1) hemorrhagic CVT group and (2) Non-Hemorrhagic CVT group based on the presence of hemorrhage on NCCT.
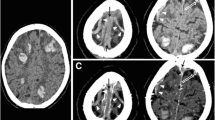
Types of hemorrhages in cerebral venous thrombosis (a) Parenchymal hemorrhage in patient with left transverse sinus thrombosis (b) Small, juxtacortical hemorrhage in patient with superior sagittal sinus thrombosis (c) Combination of subarachnoid hemorrhage and parenchymal hemorrhage in patient with superior sagittal sinus thrombosis (d) Left frontal subdural hematoma in patient with superior sagittal sinus thrombosis
We evaluated laterality (right, left or bilateral), multiplicity (single or multiple bleeds) as well as location of hemorrhage (frontal, parietal, temporal, occipital, cerebellum, brainstem, thalamus and basal ganglia). Subarachnoid hemorrhages were categorized either as convexity/cortical/sulcal or basal/deep. Hemorrhages were also categorized into isolated hemorrhage (with only one subtype of hemorrhage) or combined hemorrhage (any combination of ICH, JCH, SDH and SAH). We also identified direct signs of CVT (dense triangle, dense superior sagittal sinus, cord sign, dense deep veins, dense transverse, sigmoid & straight sinuses and dense internal jugular vein IJV) [18].
A neuroradiologist (GB) reviewed the MRI or CT-venography or DSA of all CVT cases to confirm the diagnosis and to identify the involved venous channels. All available MRI sequences were used to make a diagnosis of CVT and to rule out congenital hypoplasia by analyzing the following sequences: T1, T2, Fluid Attenuated Inversion Recovery (FLAIR), gradient recalled echo or susceptibility weighted imaging, diffusion weighted images, post -contrast T1 and MR venograms. On DSA, the venous phase was used to identify thrombosis. We recorded the side and location of the occluded venous channels (superior sagittal sinus/inferior sagittal sinus/straight sinus/lateral sinus/cortical vein/ deep venous system). Superior sagittal sinus thrombosis was divided into two groups (partial and total thrombosis). We defined the lateral sinus as involvement of transverse or sigmoid sinuses or both. Involvement of the deep venous system was defined as thrombosis of internal cerebral veins, vein of Galen and vein of Rosenthal. Thrombosed venous segments were scored as follows: superior sagittal sinus thrombosis (1 point), inferior sagittal sinus thrombosis (1 point), straight sinus thrombosis (1 point), lateral (transverse or sigmoid sinus or both) sinus thrombosis (1 point for unilateral and 2 points for bilateral), internal jugular vein thrombosis (1 point for unilateral and 2 points for bilateral), deep venous system thrombosis (1 point) and cortical vein thrombosis (1 point regardless of the number of the thrombosed veins), with a total score of 9.
Statistical analysis
The results are presented as means (SD) or counts (percentage). Student’s t-test was used to compare continuous variables, and Pearson’s Chi square test was used to compare categorical variables. The Mann–Whitney U test was used to compare differences between the distribution of involved venous channels in hemorrhagic and non-hemorrhagic groups. Logistic regression was performed with the variables that were associated with hemorrhagic venous thrombosis in univariate analysis (p < 0.10). All statistical analyses were performed using SPSS software (version 22, IBM Corp.), and p < 0.05 was considered statistically significant.
Results
Of the 285 patients with CVT, confirmatory images were not available in 17 and venous thrombosis could not be confirmed in eight. This left 260 CVT patients for this analysis with a mean age of 42 years, of whom 192 (73.8%) were female. Hemorrhagic CVT was present in 102 (39.2%). The baseline characteristics of patients with hemorrhagic CVT are shown in (Table 1). Age and the presence of hematologic disease or a family history of venous thromboembolism were associated with hemorrhage. A presentation with isolated headache was less common with hemorrhage.
Types of hemorrhage
Figure 2 shows the types of hemorrhages in the 102 patients with bleeding events. ICH occurred in 64 (62.7%), small JCH < 20 mm in 30 (29.4%), SAH in 24 (23.5%) and SDH in 11 (10.7%). Isolated hemorrhage (77%) was more frequent than combined hemorrhage (23%). SAH was present in 20/23 (86.9%) of combined hemorrhagic cases. Most cases of SAH (83.3%) were distributed over cerebral.
Single lobe involvement occurred in 69 (67.6%) of patients. The parietal lobe was involved in 42 (41%) of hemorrhagic CVT patients. The frontal and temporal lobes were involved in 34 (33.3%) and 35 (34.3%) respectively. The occipital lobe was involved in 21 (20.5%), basal ganglia in 6 (5.8%) and cerebellum in 3 (2.9%). There were no brainstem hemorrhages. Bilateral hemorrhagic CVT was present in 8 (7.8%) of all hemorrhagic cases, combined in 5/8.
Hemorrhage in association with presence of direct signs, number of involved sinuses and location
There was no difference in the presence of direct signs of CVT with hemorrhagic CVT [77 (76%) versus 128 (82%), p = 0.28]. The delay between the first imaging and the confirmatory imaging for thrombus load measurement was 0 days (25th percentile-75th percentile 0–1 days). On ancillary imaging, the distribution of thrombosed venous segments was also similar between hemorrhagic CVT and non-hemorrhagic CVT (Table 2). The number of involved venous segments was not different between isolated versus combined hemorrhagic cases, between unilateral and bilateral hemorrhages and between single versus multiple hemorrhages.
The thrombosed venous segments in the hemorrhagic and then non-hemorrhagic group are shown in (Table 3). The superior sagittal sinus was the only sinus which was associated with hemorrhage. Interestingly, hemorrhage was more common with partial thrombosis (38% rate of hemorrhage) of the superior sagittal sinus than with complete superior sagittal sinus thrombosis (16% rate of hemorrhage). ICH was not more common with simultaneous involvement of the superior sagittal sinus and cortical veins (44%) compared to either isolated cortical vein thrombosis or isolated superior sagittal sinus thrombosis (37%, p = 0.39). Frontal ICH was strongly associated with superior sagittal sinus involvement (p < 0.001). Temporal ICH was strongly associated with transverse sinus involvement (p = 0.005).
Multivariable analysis
In multivariable analysis, the following factors were associated with hemorrhagic CVT (age 1.02, 95% CI 1.01–1.04, superior sagittal sinus involvement, 95% CI 1.15–3.49, presentation other than isolated headache 6.7, 95% CI 3.05–14.88, and presence of a family history of venous thromboembolism 6.4, 95% CI 1.45–28.5).
Discussion
We did not confirm our hypothesis that intracranial bleeding is more frequent with more widespread thrombosis. We also did not observe more widespread thrombosis in patients with multiple hemorrhages, hemorrhages occurring in bilateral locations or multiple types of hemorrhage. Traditionally, bleeding in CVT is explained by severe venous hypertension (due to lack of collateral venous drainage) and resultant blood product extravasation [5,6,7]. The variability of the collateral venous drainage may explain the lack of association, although a previous study found no association between collateral patterns and type of parenchymal lesion in CVT [19]. Alternatively, involvement of multiple sinuses may reflect a slower thrombotic process allowing collateralization. Involvement of cortical veins and lateral sinus involvement have previously been associated with more bleeding [7, 10]. We did not observe this pattern, but instead showed that involvement of the superior sagittal sinus was, associated with bleeding. The lack of association with cortical vein involvement may be due to the difficulty in assessing cortical vein involvement. Alternatively, as suggested by animal studies, their involvement may not be a requirement for bleeding [8]. Higher peak and mean velocities in CVT patients with hemorrhage/edema and partially thrombosed transverse sinuses were recently reported using 4D-flow MRI [20]. Further studies using these novel techniques are needed.
Hemorrhagic CVT was present in 39% of patients, a rate similar to other cohorts [16, 21]. Higher age has previously been observed in hemorrhagic CVT [14]. With ageing, increased atrophy may lead to enlargement of the extracerebral space and increased stretching of the thin- walled, fragile, valve-less bridging veins making them more liable to bleed in a similar way to what occurs in subdural bleeding. Subdural hematomas were however observed in only 10% of hemorrhages. Older patients may use antiplatelets or anticoagulants more frequently. We did not record medication intake at onset to assess this association.
Hematological disease (polycythemia vera, essential thrombocythosis and thrombocytopenia) was more common in hemorrhagic CVT. Acquired von Willebrand disease is commonly found in patients with polycythemia vera and essential thrombocytosis and may explain increased bleeding [22,23,24]. We do not have an obvious explanation for the observed higher rate of hemorrhagic CVT in patients with a family history of venous thrombosis. This was not observed in a larger cohort [16].
We confirm the findings of previous studies which showed that patients with hemorrhage have a more severe presentation with increased seizures, focal neurological signs and altered mental state, and less isolated headache [16, 25, 26].
From a diagnostic standpoint, we suggest that a combination of hemorrhage subtypes, especially a combination of parenchymal bleed with SAH or the presence of sulcal SAH or small JCH should raise suspicion about CVT as the cause of intracranial hemorrhage. These findings are similar to previous studies of hemorrhagic CVT [11]. Sites that are typically involved in hypertension related ICH were not commonly involved in our cases; we note the absence of brain stem hemorrhages and very few basal ganglia, thalamic or cerebellar hemorrhages. This may help in distinguishing hemorrhage of CVT from ICH due to hypertensive arteriopathy.
In previous studies, the superior sagittal sinus and the transverse sinus were the most frequently affected locations in CVT [25, 26]. In our cohort the lateral sinus (transverse/sigmoid) was the most affected venous channel in both hemorrhagic CVT and non-hemorrhagic CVT. One study of 68 patients with CVT suggested that venous infarction and/or hemorrhage was more common in CVT patients with straight sinus thrombosis compared to other locations [19].
The strengths of our study include its large sample size, the multicenter nature and the independent review of the source images by experts. Most previous studies of hemorrhagic CVT were case reports or case series or emphasized specific hemorrhage subtypes [27,28,29,30,31].There are also some limitations to our study. The study was retrospective, and we lacked some clinical information, e.g., we did not collect detailed clinical information and we did not collect patient outcomes. Platelet counts and antithrombotic use on admission were alos not available. We did not evaluate non-hemorrhagic lesions (venous infarction and/or edema). The age (acute, subacute, chronic) and the actual length of the venous clot and the volume of the hemorrhagic lesions were not evaluated.
Conclusion
Widespread sinus thrombosis does not seem to be a major factor in the development of hemorrhage in cerebral venous thrombosis. Venous thrombosis involving superior sagittal sinus is associated with hemorrhagic thrombosis.
Change history
12 August 2020
The original version of this article unfortunately contained mistakes. The correct information is given below.
References
Ferro JM, Bousser MG, Canhao P et al (2017) European stroke o. european stroke organization guideline for the diagnosis and treatment of cerebral venous thrombosis - endorsed by the european academy of neurology. Eur J Neurol 24:1203–1213
Saposnik G, Barinagarrementeria F, Brown RD et al (2011) American heart association stroke c, the council on e, prevention diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the american heart association/american stroke association. Stroke 42:1158–1192
Silvis SM, De Sousa DA, Ferro JM et al (2017) Cerebral venous thrombosis. Nat Rev Neurol 13:555–565
Rother J, Waggie K, van Bruggen N et al (1996) Experimental cerebral venous thrombosis: evaluation using magnetic resonance imaging. J Cereb Blood Flow Metab 16:1353–1361
Sato S, Miyahara Y, Dohmoto Y, Kawase T, Toya S (1983) Cerebral Microcirculation in experimental sagittal sinus occlusion in dogs. In: Auer LM, Loew F (eds) The Cerebral Veins. Springer, Vienna
Gotoh M, Ohmoto T, Kuyama H (1993) Experimental study of venous circulatory disturbance by dural sinus occlusion. Acta Neurochir (Wien) 124:120–126
Schaller B, Graf R, Sanada Y et al (2003) Hemodynamic changes after occlusion of the posterior superior sagittal sinus: an experimental PET study in cats. AJNR Am J Neuroradiol 24:1876–1880
Kurokawa Y, Hashi K, Okuyama T et al (1990) Regional ischemia in cerebral venous hypertension due to embolic occlusion of the superior sagittal sinus in the rat. Surg Neurol 34:390–395
Oppenheim C, Domigo V, Gauvrit JY et al (2005) Subarachnoid hemorrhage as the initial presentation of dural sinus thrombosis. AJNR Am J Neuroradiol 26:614–617
Sztajzel R, Coeytaux A, Dehdashti A et al (2001) Subarachnoid hemorrhage: a rare presentation of cerebral venous thrombosis Headache. J Head Face Pain 41:889–892
Ferro JM, Canhao P, Stam J et al (2004) Investigators I Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke 35:664–670
Fink JN, McAuley DL (2001) Safety of anticoagulation for cerebral venous thrombosis associated with intracerebral hematoma. Neurology 57:1138–1139
Pizzi MA, Alejos DA, Siegel JL et al (2016) Cerebral venous thrombosis associated with intracranial hemorrhage and timing of anticoagulation after hemicraniectomy. J Stroke Cerebrovasc Dis 25:2312–2316
Coutinho JM, van den Berg R, Zuurbier SM et al (2014) Small juxtacortical hemorrhages in cerebral venous thrombosis. Ann Neurol 75:908–916
Takahashi S, Shinoda J, Hayashi T (2012) Cerebral venous sinus thrombosis in an adult patient presenting as headache and acute subdural hematoma. J Stroke Cerebrovasc Dis 21:338–340
Girot M, Ferro JM, Canhao P et al (2007) Investigators I Predictors of outcome in patients with cerebral venous thrombosis and intracerebral hemorrhage. Stroke 38:337–342
Buyck P (2019) Diagnostic accuracy of non-contrast ct imaging markers in cerebral venous thrombosis. Neurology 1:6
Dmytriw AA, Song JSA, Yu E et al (2018) Cerebral venous thrombosis: state of the art diagnosis and management. Neuroradiology 1:97
Barboza MA, Mejias C, Colin-Luna J et al (2015) Intracranial venous collaterals in cerebral venous thrombosis: clinical and imaging impact. J Neurol Neurosurg Psychiatry 86:1314–1318
Schuchardt F, Hennemuth A, Schroeder L et al (2017) Acute Cerebral venous thrombosis: three-dimensional visualization and quantification of hemodynamic alterations using 4-dimensional flow magnetic resonance imaging. Stroke 48:671–677
Zhang S, Zhao H, Li H et al (2017) Decompressive craniectomy in hemorrhagic cerebral venous thrombosis: clinicoradiological features and risk factors. J Neurosurg 127:709–715
Sahin N, Solak A, Genc B et al (2014) Cerebral venous thrombosis as a rare cause of subarachnoid hemorrhage: case report and literature review. Clin Imaging 38:373–379
Mohri H (1987) Acquired von Willebrand disease in patients with polycythemia rubra vera. Am J Hematol 26:135–146
Murakawa M, Okamura T, Tsutsumi K et al (1992) Acquired von Willebrand's disease in association with essential thrombocythemia: regression following treatment. Acta Haematol 87:83–87
Ferro JM, Lopes MG, Rosas MJ et al (2002) Cerebral venous thrombosis portugese collaborative study G. Long-term prognosis of cerebral vein and dural sinus thrombosis results of the VENOPORT study. Cerebrovasc Dis 13:272–278
Damak M, Crassard I, Wolff V et al (2009) Isolated lateral sinus thrombosis: a series of 62 patients. Stroke 40:476–481
Kumral E, Polat F, Uzunkopru C et al (2012) The clinical spectrum of intracerebral hematoma, hemorrhagic infarct, non-hemorrhagic infarct, and non-lesional venous stroke in patients with cerebral sinus-venous thrombosis. Eur J Neurol 19:537–543
Bansal H, Chaudhary A, Mahajan A et al (2016) Acute subdural hematoma secondary to cerebral venous sinus thrombosis: case report and review of literature. Asian J Neurosurg 11:177
Azeemuddin M, Awais M, Mubarak F et al (2018) Prevalence of subarachnoid haemorrhage among patients with cranial venous sinus thrombosis in the presence and absence of venous infarcts. Neuroradiol J 31:496–503
Arnoux A, Triquenot-Bagan A, Andriuta D et al (2017) Imaging characteristics of venous parenchymal abnormalities. Stroke 48:3258–3265
Zhou G, Li M, Zhu Y et al (2016) Cerebral venous sinus thrombosis involving the straight sinus may result in infarction and/or hemorrhage. Eur Neurol 75:257–262
Acknowledgments
The Florey Institute of Neuroscience and Mental Health acknowledges the strong support from the Victorian Government and in particular the funding from the Operational Infrastructure Support Grant.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
VT designed the manuscript and collected data. KA collected data and wrote the initial draft of the manuscript. GB analysed the images. The other authors (SZ, PB, CE, MB, PC, IE, DR, NH, RL, FF, JJC, ES, SH, AA, AP, JM, JP, CW, TG, TT, JC, PD, MS) collected data. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there is no conflict of interest.
Consent to participate
Informed consent was not sought for the present study because of the retrospective, anonymized nature of the study, in accordance with the participating center’s ethics committee’s policies.
Ethical approval
Specific approval of the project by an Ethics Committee (Leuven, Graz) or a waiver due to the anonymized, retrospective data and imaging collection (Amsterdam, Nîmes, Mexico City, Essen, Brescia, Sevilla, Barcelona, Helsinki) were obtained by each participating site, according to local regulations.
Rights and permissions
About this article
Cite this article
Afifi, K., Bellanger, G., Buyck, P.J. et al. Features of intracranial hemorrhage in cerebral venous thrombosis. J Neurol 267, 3292–3298 (2020). https://doi.org/10.1007/s00415-020-10008-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-10008-0