Abstract
Extracranial-intracranial (EC/IC) arterial bypass surgery is a valuable therapeutic modality in the field of cerebrovascular surgery. The assessment of bypass patency and its functional parameters are of utmost importance in the postoperative course. The present study examined the potential role of quantitative MR-based volume flow measurement techniques for the investigation of bypass patency. Forty-one patients with steno-occlusive cerebrovascular disease treated with EC/IC bypass surgery underwent conventional angiographic (CA) and two-dimensional cine-phase MR-based angiographic assessment (MRA) of bypass function. CA bypass function was evaluated as poor (grade I), moderate (grade II), or extensive (grade III) and was compared with quantitative volume flow measurements (BVF) obtained in MRA studies. Bypass filling was classified as grade I in 15% of the cases, grade II and grade III in 36% and 49% of the studies, respectively. Mean BVF differed significantly in the different grades: 31.9±9.8 ml/min in grade I, 73.6±16.7 ml/min in grade II, and 97.2±26.6 ml/min in grade III. BVF values of48 ml/min or lower ( n =6) were specific for grade I bypass function, while only BVF values higher than 111 ml/min (3/20, 15%) are specific for extensive angiographic bypass function. The assessment of EC/IC bypass patency with quantitative BVF measurements provides exact, investigator-independent information under physiological conditions. MRA is well correlated with the angiographic bypass grading system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Extracranial-Intracranial (EC/IC) arterial bypass surgery is a well known microsurgical procedure for augmenting distal cerebral circulation. Indications include the prevention of recurrent stroke in hemodynamic insufficiency due to occlusive cerebrovascular disease and its use as an adjunct in the treatment of complex cerebral aneurysms and skull base tumors [1, 2, 3, 4, 5, 6, 7]. The most frequent surgical modification of the technique consists in establishing a direct end-to-side anastomosis between either the frontal or parietal branch of the superficial temporal artery (STA) and a distal segment of the middle cerebral artery (MCA).
In the past various approaches were used to assure STA-MCA bypass function. Quantitative and qualitative ultrasound based techniques are applied during surgery, while conventional catheter angiography (CA) is used routinely for the assessment of bypass patency in the early postoperative course [8, 9, 10]. While intraoperative techniques can assess bypass functioning within a narrow time window only, CA allows time-independent investigation of the established anastomosis. In addition, CA enables grading of bypass function to some extent [3, 11, 12] for estimating the extent of blood supply via the EC/IC anastomosis. The information obtained by CA, however, appears to be investigator dependent and provides qualitative information only. Furthermore, this method carries some serious procedure-related risks, such as arterial embolism, arterial dissection, and groin hematoma and infection [13]. Thus, noninvasive, quantitative, and investigator-independent imaging techniques are desired for the serial assessment of EC/IC bypass patency and measurement of blood supply through the anastomosis in the postoperative course. The introduction of new magnetic resonance (MR) based imaging techniques such two-dimensional cine-phase contrast MR makes possible the noninvasive qualitative [14, 15, 16, 17] and quantitative [18, 19, 20, 21] investigation of blood flow within cerebral vessels. These techniques permit the examination of cerebral vessels without the need for intra-arterial contrast agents. Due to their technical principles [20] and their robustness these approaches allow the direct assessment of flow direction and measurement of volume flow even in small arterial vessels under physiological conditions.
The aim of this study was (a) to quantify an MR-based angiographic (MRA) grading system for the standard STA-MCA anastomosis function used at present, (b) to determine its value in measuring cerebral blood supply via the EC/IC bypass, and (c) to characterize the potential role of quantitative MR-based techniques for the investigation of bypass patency in the postoperative course.
Materials and methods
The study enrolled 41 patients (33 men, 8 women; mean age 57±9 years) with either transient or minor retinal ischemic attacks or minor stroke and confirmed occlusion of the ICA or MCA ( n =34) or stenosis of the MCA or ICA ( n =7) in CA, and without significant angiographic collateral flow via the ophthalmic artery, who fulfilled the criteria for application of EC/IC arterial bypass surgery.
Indication for EC/IC arterial bypass surgery and surgical procedure
The indication for EC/IC bypass surgery in our institution is, in addition to the necessity for symptomatic occlusive cerebrovascular disease, based upon the quantitative assessment of regional CBF (rCBF) and cerebrovascular reserve capacity (CVRC). Patients are considered candidates for surgical revascularization if CVRC is either less than 30%, or if a paradoxical decrease in rCBF (“steal phenomenon”) occurs after acetazolamide challenge (acetazolamide 15 mg/kg/body weight intravenously) [22]. Only patients with normal cranial computed tomography or evidence of border-zone infarction undergo evaluation procedures. Functional rCBF studies are performed after diagnostic CA by means of stable xenon-enhanced CT (4.5 min wash-in protocol, 30% Xe, 60% O2).
Patients meeting the inclusion criteria, i.e., in whom hemodynamic compromise is assured, undergo standard EC/IC bypass surgery. Neurosurgical procedure consists in the establishment of a direct anastomosis between either the frontal or the parietal branch of the superficial temporal artery (STA; donor vessel) and a cortical (recipient) vessel of the middle cerebral artery (MCA—M2 or M3 segment) via a standard temporal craniotomy [23, 24].
Conventional angiography
STA-MCA anastomosis were investigated by CA after the surgical procedure in all patients to assess bypass patency postoperatively. Bypass function and collateral circulation through the anastomosis were graded as follows extent [3, 11]: extensive (grade III), ante- and retrograde filling of the entire MCA system; moderate (grade II), filling of two or more MCA branches; and poor (grade I), filling of the anastomosed MCA branch only (Fig. 1). The bypass grade was assessed by two independent observers and finally rated using consensus among the observers.
MRI and MRA studies
MRI, MRA, and blood volume flow (BVF) studies were performed using a 1.5-T MR unit (Magnetom Vision, Siemens, Erlangen, Germany). All patients underwent identical MR protocols using a circular polarized head coil and a Helmholtz neck coil. After performing T1-weighted scout images (TR: 545 ms; TE: 15 ms slice thickness: 4 mm; slice gap: 0.6 mm) to gain anatomical reference information, BVF was assessed quantitatively within the established STA-MCA anastomosis. Sufficient angiographic and MRA studies were obtained in all patients; both investigations were carried out within 10 days after surgery.
Dynamic two-dimensional cine-phase contrast MR technique was applied using electrocardiography-triggered fast radiofrequency spoiled gradient-echo sequence with following sequence parameters: TR: 28 ms, TE: 5 ms, flip angle: 30°, FOV: 220 mm, matrix: 192×256, 1 acquisition. Velocity encoding was set between 40 and 250 cm/s depending on blood flow velocity of the measured vessel. Depending on the patient’s heart rate, 25–35 single two-dimensional phase-contrast images were acquired over the cardiac cycle (time resolution 28 ms). All BVF measurements were made perpendicular to the course of the arteries, within the straight segment of the distal STA (Fig. 2). Values for group BVF are presented as mean and standard deviation. Statistical evaluation of data used the paired t test for normally distributed data samples.
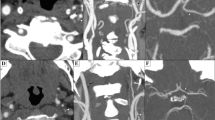
Two-dimensional cine-phase MR technique. A Coronal T2-weighted reference slice with measurement plane placed over straight segment of the superficial temporal artery. B Original registration curve obtained within the superficial temporal artery. C, D Postoperative MRA in a patient with unilateral carotid artery occlusion: coronal ( C) and transversal ( D) views
Results
Selective external carotid artery injection investigating the EC/IC anastomosis led to the following results. According to the diagnostic criteria applied [3], bypass filling was classified as grade I in 6 cases (15%), grade II in 15 (36%), and grade III in 20 (49%; Table 1). BVF obtained by means of two-dimensional cine-phase contrast MRI ranged between 13.8 and 177.6 ml/min in the EC/IC anastomosis, with a mean of 79.4±30.4 ml/min. Mean BVF in patients with grade I bypass filling was 31.9±9.8 ml/min, ranging from 13.8 to 48.0 ml/min. Significantly increased BVF was observed in grade II and grade III patients, in whom mean BVF reached 73.6±16.7 ml/min ( P <0.001 vs. grade I; range: 48.3–111.0 ml/min) and 97.2±26.6 ml/min ( P <0.001 vs. grade I; range: 49.2–177.6 ml/min), respectively.
All patients with BVF values of48 ml/min or lower ( n =6) showed grade I bypass function in angiography. Patients with grade II or III bypass demonstrated higher BVF values, with only BVF higher than 111 ml/min (3/20 studies, 15%) being specific for grade III bypass function. In 17 (85%) investigations with angiographic grade III bypass BVF values were within the range obtained in patients with grade II bypass. Thus despite a statistically significant difference between the patient groups with grade II and grade III bypass function (grade II vs. grade III, P <0.001) no specific lower cutoff BVF value was found representative for a grade III bypass that would allow differentiation between grade II and III by means of BVF.
Discussion
The main findings of the present study are that (a) angiographic grading of bypass function compares well with the BVF through the anastomosis in the early postoperative course, (b) considerable brain blood supply is provided via angiographically “poor” functioning bypasses, and (c) noninvasive MRI techniques are more effective for assessing bypass function under physiological conditions.
EC/IC arterial bypass surgery was used extensively for a variety of ischemic and latent ischemic disorders of the brain before the results of the international cooperative study of EC/IC anastomosis were published in 1985 [25]. The study failed to show a reduction in stroke and stroke-related deaths in patients with stenotic or occlusive cerebrovascular disease undergoing EC/IC bypass surgery. The patient selection criteria applied in this study were criticized extensively, leading to the development of the concept of hemodynamic insufficiency in occlusive and stenotic cerebrovascular disease over the following years [26, 27, 28, 29, 30, 31, 32, 33]. Based on this theory, patients with assured hemodynamic compromise are most likely to benefit from EC/IC bypass surgery since the risk of subsequent stroke in these patients is known to be increased in the natural course [32, 34].
The efficacy of this surgical procedure in terms of stroke prevention, which has been demonstrated in various studies [2, 3, 35, 36, 37, 38, 39], depends fundamentally upon graft patency and the capability of the EC/IC bypass to deliver blood into the dependent vascular territory. CA, which is widely available, is currently used to assess graft patency. CA allows reliable detection of primary bypass failure and furthermore enables the qualitative assessment of intracranial filling via the established anastomosis.
In the past, several angiographic grading systems have been introduced to measure blood supply through the bypass [3, 11, 12, 40]. These systems analyze the extent of intracranial vascular filling after selective external carotid artery injection using either a three-point [3, 11] or eight-point [12, 40] grading scale. The systems in use at present distinguish only between “low,” “medium,” and “high” or “poor,” “moderate,” and, “extensive” bypass function. Single studies have demonstrated a correlation between angiographic bypass filling and clinical course [40, 41] and intraoperative changes in rCBF [42]. However, criticism has been directed at the procedure itself. Since the manual injection of a contrast dye under variable pressure is necessary for angiographic evaluation, nonphysiological conditions are assumed to be present during CA. Consequently, alternative imaging techniques are required which allow the noninvasive assessment of graft patency and its function under physiological conditions in the postoperative course.
MRA, which permit the noninvasive investigation of the cerebral circulation, has therefore been to study EC/IC bypass patency [14, 15, 16, 17]. These investigations, however, have focused primarily on the capability of MRA technique to confirm bypass graft patency when shown with CA. The results demonstrate good agreement between the two techniques, with MRA providing additional information concerning flow direction within the bypass [16]. Since the introduction of two-dimensional cine-phase contrast MRI technique, which allows the quantitative measurement of blood volume flow within blood vessels, the methodology has gained wide acceptance for various indications [18, 19, 20, 21]. This technique, which provides intravascular BVF rates in absolute values, blood flow velocity, and blood flow characteristics and hence permits the quantitative evaluation of EC/IC arterial bypass function.
The patency of a EC/IC anastomosis depends on factors related both to the patient and to the surgery. Patient-associated factors include size and compatibility of donor and recipient vessel, extent of atherosclerotic changes in the donor vessel, and the patient’s blood requirement distal to the arterial stenosis or occlusion. In addition, the microsurgical technique applied directly affects graft patency. All patients studied here suffered from hemodynamic compromise, i.e., exhausted CVRC in functional rCBF studies. The current requirement for additional blood allowing the restoration of cerebral perfusion was thus assured prior to operation for every patient. The angiographic investigation of bypass patency therefore reflects structural anastomosis properties rather than hemodynamic aspects within the revascularized territory after EC/IC bypass surgery. Nevertheless, there are certainly individual local differences in blood requirement. Their effect on both BVF and vessel opacification with CA is uncertain.
The present study shows the practicability of a three-point angiographic bypass grading scale for estimating blood flow via an EC/IC anastomosis in the early postoperative course. The obtained group BVF values, measured by both two-dimensional cine-phase contrast MRI compared well with those using the angiographic scale. Thus the extent of intracranial blood vessel filling after selective external carotid artery injection, as qualitative measure for bypass function, reflects the structural properties of the EC/IC anastomosis to some extent. This holds true especially for the grade I bypass group, where the lowest BVF values were obtained. The measured volume flow through the bypass nevertheless reached as much as 48 ml/min. Thus despite only “poor” intracranial opacification there is considerable volume flow via the anastomosis, which appears to be higher than that described earlier [43, 44]. Patients with grade II and grade III bypass patency showed, in addition to a significant mean difference between groups, a wide range of BVF values within the groups. Thus, in contrast to patients with grade I graft patency, direct prediction of angiographic bypass grade II and III was not possible by MRA. The wide range of BVF values within the groups clearly demonstrates that angiographic, i.e., qualitative, assessment of graft patency does not necessarily reflect blood flow through the EC/IC bypass. These findings could be related either to the limitation of angiography, i.e., the necessity of a contrast media and variable application pressure, the subjective nature of the angiographic grading scale, or differences in blood requirement within the affected vascular territory. The BVF through the EC/IC bypass in these patients was not related to the degree of CVRC impairment in the preoperative rCBF studies. Follow-up investigations are required to determine whether bypasses with relatively low BVF but “good” and “extensive” intracranial filling tend to “mature,” i.e., to deliver more blood to the brain, over time, or whether a secondary bypass failure is likely to occur in the latter course.
Since two-dimensional cine-phase MRI has been validated in both phantom as human studies, it is extensively used to determine BVF in cerebral vessels. In the present study this technique allowed reliable quantitative assessment of BVF in a established EC/IC anastomosis under physiological conditions. Based on its apparent advantages, our results demonstrate the potential of MRA-based imaging techniques to study even very small arterial blood vessels. In addition, the technique is superior to CA for physiological investigation of the bypass. However, as for angiography, the contribution of the additional BVF via the anastomosis to restoring rCBF within the revascularized territory cannot be determined by this method.
In conclusion, use of an angiographic three-point grading system to assess EC/IC bypass patency allows estimation of BVF reliably, while quantitative BVF measurements with MRA provide more exact, investigator-independent information. This warrants the application of MRA to study the effects of EC/IC bypass surgery on cerebral hemodynamics in both the short and the long term.
References
Peerless SJ (1986) Indications for the extracranial-intracranial arterial bypass in light of the EC-IC Bypass Study. Clin Neurosurg 33:307–326
Nussbaum ES, Erickson DL (2000) Extracranial-intracranial bypass for ischemic cerebrovascular disease refractory to maximal medical therapy. Neurosurgery 46:37–42
Schmiedek P, Piepgras A, Leinsinger G, Kirsch CM, Einhupl K (1994) Improvement of cerebrovascular reserve capacity by EC-IC arterial bypass surgery in patients with ICA occlusion and hemodynamic cerebral ischemia. J Neurosurg 81:236–244
Takagi Y, Hashimoto N, Iwama T, Hayashida K (1997) Improvement of oxygen metabolic reserve after extracranial-intracranial bypass surgery in patients with severe haemodynamic insufficiency. Acta Neurochir (Wien) 139:52–56
Yonas H (1997) Predictability of extracranial/intracranial bypass function: a retrospective study of patients with occlusive cerebrovascular disease. Neurosurgery 41:1447–1448
Weinstein PR, Rodriguez y Baena R, Chater NL (1984) Results of extracranial-intracranial arterial bypass for intracranial internal carotid artery stenosis: review of 105 cases. Neurosurgery 15:787–794
Batjer H,Samson D (1986) Use of extracranial-intracranial bypass in the management of symptomatic vasospasm. Neurosurgery 19:235–246
Moritake K, Handa H, Yonekawa Y, Nagata I (1980) Ultrasonic Doppler assessment of hemodynamics in superficial temporal artery-middle cerebral artery anastomosis. Surg Neurol 13:249–257
Ausman JI (1978) Correlation of noninvasive Doppler and angiographic evaluation of extracranial-intracranial anastomoses. Surg Forum 29:534–535
Benzel EC, Kessler CW (1987) Angiography following extracranial-intracranial bypass surgery. Surg Neurol 27:585–586
Iwama T, Hashimoto N, Takagi Y, Tsukahara T, Hayashida K (1997) Predictability of extracranial/intracranial bypass function: a retrospective study of patients with occlusive cerebrovascular disease. Neurosurgery 40:53–59
Latchaw RE, Ausman JI, Lee MC (1979) Superficial temporal-middle cerebral artery bypass. A detailed analysis of multiple pre- and postoperative angiograms in 40 consecutive patients. J Neurosurg 51:455–465
Hankey GJ, Warlow CP, Molyneux AJ (1990) Complications of cerebral angiography for patients with mild carotid territory ischaemia being considered for carotid endarterectomy. J Neurol Neurosurg Psychiatry 53:542–548
Kodama T, Ueda T, Suzuki Y, Yano T, Watanabe K (1993) MRA in the evaluation of EC-IC bypass patency. J Comput Assist Tomogr 17:922–926
Kodoma T, Suzuki Y, Yano T, Watanabe K, Ueda T, Asada K (1995) Phase-contrast MRA in the evaluation of EC-IC bypass patency. Clin Radiol 50:459–465
Praharaj SS, Coulthard A, Gholkar A, English P, Mendelow AD (1996) Magnetic resonance angiographic assessment after extracranial-intracranial bypass surgery. J Neurol Neurosurg Psychiatry 60:439–441
Macchi C, Catini C, Federico C, Gulisano M, Pacini P, Cecchi F, Corcos L, Brizzi E (1996) Magnetic resonance angiographic evaluation of circulus arteriosus cerebri (circle of Willis): a morphologic study in 100 human healthy subjects. Ital J Anat Embryol 101:115–123
Everdingen KJ van , Visser GH, Klijn CJ, Kappelle LJ, van der Grond J (1998) Role of collateral flow on cerebral hemodynamics in patients with unilateral internal carotid artery occlusion. Ann Neurol 44:167–176
Everdingen KJ van , Klijn CJ, Kappelle LJ, Mali WP, van der Grond J (1997) MRA flow quantification in patients with a symptomatic internal carotid artery occlusion. The Dutch EC-IC Bypass Study Group. Stroke 28:1595–1600
Everdingen KJ van, Visser GH, Klijn CJ, Kappelle LJ, van der Grond J (1998) Role of collateral flow on cerebral hemodynamics in patients with unilateral internal carotid artery occlusion. Ann Neurol 44:167–176
Rutgers DR, Blankensteijn JD, van Der Grond J (2000) Preoperative MRA flow quantification in CEA patients: flow differences between patients who develop cerebral Ischemia and patients who do not develop cerebral ischemia during cross-clamping of the carotid artery. Stroke 31:3021–3028
Vorstrup S, Brun B, Lassen NA (1986) Evaluation of the cerebral vasodilatory capacity by the acetazolamide test before EC-IC bypass surgery in patients with occlusion of the internal carotid artery. Stroke 17:1291–1298
Yasargil MG. Yonekawa Y (1977) Results of microsurgical extra-intracranial arterial bypass in the treatment of cerebral ischemia. Neurosurgery 1:22–24
Vajkoczy P, Hubner U, Horn P, Bauhuf C, Thome C, Schilling L, Schmiedek P, Quintel M, Thomas JE (2000) Intrathecal sodium nitroprusside improves cerebral blood flow and oxygenation in refractory cerebral vasospasm and ischemia in humans. Stroke 31:1195–1197
International Cooperative Study of Extracranial/Intracranial Arterial Anastomosis (EC/IC Bypass Study) (1985) Methodology and entry characteristics. The EC/IC Bypass Study Group. Stroke 16:397–406
Ausman JI, Diaz FG (1986) Critique of the extracranial-intracranial bypass study. Surg Neurol 26:218–221
Gibbs JM, Wise RJ, Thomas DJ, Mansfield AO, Russell RW (1987) Cerebral haemodynamic changes after extracranial-intracranial bypass surgery. J Neurol Neurosurg Psychiatry 50:140–150
Yamanaka R, Satoh S, and Kawasaki S (1988) Changes in cerebral hemodynamics after extracranial-intracranial bypass. Neurol Med Chir (Tokyo) 28:981–985
Caplan LR, Piepgras DG, Quest DO, Toole JF, Samson D, Futrell N, Millikan C, Flamm ES, Heros RC, Yonekawa Y, Eguchi T, Yonas H, Rothbart D, Spetzler RF (1996) EC-IC bypass 10 years later: is it valuable? Surg Neurol 46:416–4423
Schmiedek P (1989) EC-IC bypass in hemodynamic cerebrovascular disease. J Neurosurg 71:464–466
Vorstrup S, Haase J, Waldemar G, Andersen A, Schmidt J, Paulson OB (1996) EC-IC bypass in patients with chronic hemodynamic insufficiency. Acta Neurol Scand Suppl 166:79–81
Yonas H, Smith HA, Durham SR, Pentheny SL, Johnson DW (1993) Increased stroke risk predicted by compromised cerebral blood flow reactivity. J Neurosurg 79:483–489
Awad IA. Spetzler RF (1986) Extracranial-intracranial bypass surgery: a critical analysis in light of the International Cooperative Study. Neurosurgery 19:655–664
Webster MW, Makaroun MS, Steed DL, Smith HA, Johnson DW, Yonas H (1995) Compromised cerebral blood flow reactivity is a predictor of stroke in patients with symptomatic carotid artery occlusive disease. J Vasc Surg 21:338–344
Rhodes RS, Spetzler RF, Roski RA (1981) Improved neurologic function after cerebrovascular accident with extracranial-intracranial arterial bypass. Surgery 90:433–438
Piepgras A, Leinsinger G, Kirsch CM, Schmiedek P (1994) STA-MCA bypass in bilateral carotid artery occlusion: clinical results and long-term effect on cerebrovascular reserve capacity. Neurol Res 16:104–107
Muraishi K, Kameyama M, Sato K, Sirane R, Ogawa A, Yoshimoto T, Hatazawa J, Itoh M (1993) Cerebral circulatory and metabolic changes following EC/IC bypass surgery in cerebral occlusive diseases. Neurol Res 15:97–103
Tsuda Y, Kimura K, Iwata Y, Hayakawa T, Etani H, Fukunaga R, Yoneda S, Abe H (1984) Improvement of cerebral blood flow and/or CO2 reactivity after superficial temporal artery-middle cerebral artery bypass in patients with transient ischemic attacks and watershed-zone infarctions. Surg Neurol 22:595–604
Vorstrup S, Lassen NA, Henriksen L, Haase J, Lindewald H, Boysen G, Paulson OB (1985) CBF before and after extracranial-intracranial bypass surgery in patients with ischemic cerebrovascular disease studied with 133Xe-inhalation tomography. Stroke 16:616–626
Jack CR, Sundt TM, Fode NC, Gehring DG (1988) Superficial temporal-middle cerebral artery bypass: clinical pre- and postoperative angiographic correlation. J Neurosurg 69:46–51
Bradac GB, Schramm J, Kaernbach A, Oppel F (1980) Angiographic aspects of extra-intracranial arterial bypass (EIAB) for cerebral arterial occlusive disease. Neuroradiology 20:111–122
Little JR, Yamamoto YL, Feindel W, Meyer E, Hodge CP (1979) Superficial temporal artery to middle cerebral artery anastomosis. Intraoperative evaluation by fluorescein angiography and xenon-133 clearance. J Neurosurg 50:560–569
Spetzler R, Chater N (1976) Microvascular bypass surgery. II. Physiological studies. J Neurosurg 45:508–513
Sekhar LN, Bucur SD, Bank WO, Wright DC (1999) Venous and arterial bypass grafts for difficult tumors, aneurysms, and occlusive vascular lesions: evolution of surgical treatment and improved graft results. Neurosurgery 44:1207–1223
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Horn, P., Vajkoczy, P., Schmiedek, P. et al. Evaluation of extracranial-intracranial arterial bypass function with magnetic resonance angiography. Neuroradiology 46, 723–729 (2004). https://doi.org/10.1007/s00234-004-1249-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-004-1249-4