Abstract
Amyloid-β peptide (Aβ) has been shown to cause synaptic dysfunction and can render neurons vulnerable to excitotoxicity and oxidative stress. Na,K-ATPase plays an important role to maintain cell ionic equilibrium and it can be modulated by N-methyl-d-aspartate (NMDA)–nitric oxide (NO)–cyclic GMP pathway. Disruption of NO synthase (NOS) activity and reactive oxygen species (ROS) production could lead to changes in Na,K-ATPase isoforms’ activities that may be detrimental to the cells. Our aim was to evaluate the signaling pathways of Aβ in relation to NMDA–NOS–cyclic GMP versus oxidative stress on α1-/α2,3-Na,K-ATPase activities in rat hippocampal slices. Aβ1–40 induced a concentration-dependent increase of NOS activity and increased cyclic guanosine monophosphate (cGMP), TBARS (thiobarbituric acid reactive substances), and 3-Nitrotyrosine (3-NT)-modified protein levels in rat hippocampal slices. The increase in NOS activity and cyclic GMP levels induced by Aβ1–40 was completely blocked by MK-801 (inhibitor of NMDA receptor) and L-NAME (inhibitor of NOS) pre-treatment but changes in TBARS levels were only partially blocked by both compounds. The Aβ treatment also decreased Na,K-ATPase activity which was reverted by N-nitro-l-arginine methyl ester hydrochloride (L-NAME) but not by MK-801 pre-treatment. The decrease in enzyme activity induced by Aβ was isoform-specific since only α1-Na,K-ATPase was affected. These findings suggest that the activation of NMDA–NOS signaling cascade linked to α2,3-Na,K-ATPase activity may mediate an adaptive, neuroprotective response to Aβ in rat hippocampus.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by extensive synaptic and neuronal loss that leads to impaired memory and cognitive decline (Braak and Braak 1994; Katzman and Saitoh 1991). The accumulation of toxic amyloid-β (Aβ) peptide oligomers and aggregates as well as neurofibrillary tangles formed by hyperphosphorylated tau protein in AD brain have been proposed to be a hallmark of the pathology of the disease (Arriagada et al. 1992; Selkoe 1994).
Findings from studies in mouse models of AD (Holcomb et al. 1998) and AD patients (Morris et al. 1996; Naslund et al. 2000) suggest that cognitive decline occurs prior to amyloid plaque deposition and neurodegeneration. Thus, although Aβ may ultimately kill neurons (Yankner 1996), it likely causes alterations in cellular signaling pathways that account for synaptic dysfunction well prior to degeneration of neurons. Disturbances in neuronal Ca2+ signaling, oxidative stress and changes in Na,K-ATPase activity are probably linked to the occurrence of impaired neuronal function observed in animal models of familial AD and in studies of postmortem brain samples from sporadic AD patients (Ahmed 2012; Dickey et al. 2005; Hattori et al. 1998; Liguri et al. 1990; Mark et al. 1995). In fact, Na,K-ATPase is one of the protein complexes in the plasma membrane that plays key roles in the regulation of intracellular Ca2+ concentration, working together with Ca2+-ATPase and the Na+/Ca2+ exchanger. The mutations in α2 and α3 isoform genes are involved in neurological disorders, such as familial hemiplegic migraine type-2 (De Fusco et al. 2003) and rapid-onset dystonia Parkinson (de Carvalho Aguiar et al. 2004), and genetic variations in Na,K-ATPase are associated with bipolar disorders (Goldstein et al. 2009), suggesting a role of this enzyme in the etiology of this disease. It is interesting that mouse models targeting a specific α-isoform cause different, although still comparable, phenotypes consistent with classical symptoms and other manifestations observed in these patients (Bøttger et al. 2011). In addition, the deficiency in Na,K-ATPase α2 and α3 isoform genes induced spatial learning and memory deficits in mice (Moseley et al. 2007) and Na,K-ATPase expression is reduced in patients with AD by postmortem examination and in a transgenic mouse model of AD (Albensi and Mattson 2000; Dickey et al. 2005; Hattori et al. 1998). Therefore, Na,K-ATPase α isoform plays a critical role in the modulation of learning and memory in AD (Zhang et al. 2013).
Nitric oxide (NO•) is synthesized from its precursor l-arginine by a highly regulated family of enzymes called NO synthases (NOS) that utilize O2 and NADPH as co-substrates and generate citrulline as co-product. Three isoforms of NOS (types 1–3) encoded by distinct genes have been identified. Two of these isoforms, NOS-1 and NOS-3, first identified in neurons and endothelial cells, respectively, are expressed constitutively and both are stimulated by agonists that increase intracellular Ca2+. In contrast, NOS-2 is regulated by transcriptional induction and does not require Ca2+ increases for activity (Knowles and Moncada 1994). NO• in the presence of superoxide anion (O2•−) forms peroxynitrite anion (ONOO−) leading to the formation of hydroxyl radical (OH•), one of the responsible factors for cellular death. However, NO• produced by Ca2+–calmodulin-dependent activation of NOS in neurons can also activate soluble guanylyl cyclase (sGC) with the formation of the second messenger cyclic GMP (Lipton et al. 1993).
Energy deficiency and dysfunction of the Na,K-ATPase are common consequences of many pathological insults in central nervous system (CNS). Glutamate through N-methyl-d-aspartate (NMDA)–NOS–cyclic GMP has been shown to stimulate α2/3-Na,K-ATPase activity in CNS (Munhoz et al. 2005; Nathanson et al. 1995), and NMDA signaling cascade receptor activation can mediate an adaptive, neuroprotective response to Aβ (Kawamoto et al. 2008a). The aim of the present work was to evaluate the signaling pathways of Aβ in relation to NMDA–NOS–cyclic GMP versus oxidative stress and α1-/α2/3-Na,K-ATPase activities in rat hippocampal slices.
Materials and Methods
Animal and Tissue Preparation and Viability Test
Male Wistar rats (240–300 g) (Biomedical Sciences Institute, University of São Paulo) were euthanized by decapitation following procedure approved by the National Council of Animal Experimentation Control (CONCEA). All procedures were also approved by the Ethical Committee for Animal Research (CEEA) of the Biomedical Sciences Institute of the University of São Paulo. For each replicate, ten animals per group were used and a total of 120 animals were used for all experiments.
The brain was immediately removed and the hippocampus (approximately 100 mg/rat) was isolated and prepared on a Mcllwain tissue chopper (The Mickle Laboratory Engineering Co. LTD.), washed extensively to remove small particles, and rapidly resuspended in the same cold (4ºC) modified artificial cerebrospinal fluid (aCSF) aerated (95% O2/5% CO2) buffer containing 126 mM NaCl, 3.5 mM KCl, 1.2 mM NaH2PO4, 2.0 mM CaCl2, 1.3 mM MgCl2, 11 mM glucose, and 25 mM NaHCO3 (adjusted to pH 7.4 at 37 °C). Hippocampus slice suspensions were pre-incubated for 60 min at 37 °C in the same buffer prior to starting drug incubation. At the end of this pre-incubation process, slices were transferred to microtubes (five replicates/condition) and were pre-incubated again for 3 min at 37 °C. Aβ1–40 obtained from Bachem (San Carlos, CA) was diluted in distilled water to 1 mM and incubated at 37 °C for 7 days to allow peptide aggregation (Troy et al. 2000). Before use, the peptide was diluted in PBS to 0.2 and 2.0 µM. PBS or Aβ1–40 (0.2 and 2.0 µM) were added to these tubes that contained 1.0 mL aliquots of the slice suspension, incubated for 60 min at 37 °C, and then rapidly transferred to an ice bath to terminate the reaction and store at − 80 °C. The microtubes containing hippocampus slice suspensions were pre-incubated (15 min at 37 °C) with saline, or MK-801 (1 μM) or LNAME (100 μM) (six replicates for each pre-treatment), before the addition of saline or Aβ1–40 in half of the microtubes of each pre-treatment (three replicates for each treatment). The tubes were thawed in ice water, centrifuged (1700×g for 5 min at 4 °C), and the supernatant (containing the drug) was removed.
Tissue viability was determined by a modified version of the tetrazolium dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Connely et al. 2000). The MTT assay is based on the conversion of pale yellow MTT into formazan, the reduced form of MTT, which has a purple color. The reaction is performed by mitochondrial dehydrogenase enzymes, and so the MTT assay provides an index of mitochondrial activity (Liu et al. 1997). Tissue viability from each group was assayed before and after drug incubation using 0.5 mg/ml MTT. The dye was diluted in double-distilled water and then 100 μl was added to the slice preparation (900 μl buffer pH 7.4 at 37 °C—95% O2/5% CO2) followed by 5 min incubation at room temperature. The supernatants were removed and slices were incubated in water–ethanol solution (1:2) for 15 min room temperature to dissolve all blue crystals. After brief sonication, aliquots (200 μl) of centrifuged samples were transferred to plates and read spectrophotometrically at 570 nm.
Measurement of Na,K-ATPase Activity
Na,K-ATPase activity was determined by assaying Pi (inorganic phosphate) released from ATP hydrolysis. This inorganic compound forms a complex with molybdate which can be read spectrophotometrically at 700 nm (Esmann 1988). For this colorimetric ATP assay, the tissue slice pellets were resuspended in a buffer containing 0.32 M sucrose, 20 mM HEPES, 1 mM EDTA, 1 mM DTT, and 1 mM PMSF, pH 7.4 and homogenized in a Dounce (glass/glass) homogenizer. The particulate fraction (12,000×g for 15 min at 4 °C) was resuspended in the same buffer and protein content was determined in both supernatant and particulate samples by colorimetric assay (Bradford 1976). Na,K-ATPase activity was tested by adding 10 μg of the particulate fraction (in 40 μl of buffer) to 360 μl of buffer containing 3 mM ATP, 120 mM NaCl, 2 mM KCl, 3 mM MgCl2, and 30 mM histidine (pH 7.2), with or without ouabain (3 μM or 3 mM). The Na,K-ATPase activity was measured at 20 min (37 °C). The reaction was terminated by the addition of a quenching solution (0.6 ml) containing 1 N H2SO4 and 0.5% ammonium molybdate. Formation of a phosphomolybdate complex was determined spectrophotometrically at 700 nm (Esmann 1988). The total-ATPase, Mg-ATPase, and α1- and α2/3-Na,K-ATPase activities were linearly related up to 20 min. Total Na,K-ATPase activity was measured as the difference between ouabain-untreated and ouabain-treated samples. To distinguish between α1 and α2,3 activities, ouabain in two different concentrations were used according to ouabain-affinity of the isoforms as described in our previous publications (Kawamoto et al. 2008b; Kinoshita et al. 2014). The fraction of the high-affinity α2,3 isoform was calculated by subtracting the activity obtained with 3 μM ouabain from total-ATPase activity. To determine the α1 isoform-associated Na,K-ATPase activity (low-affinity fraction), the Na,K-ATPase activity measured with 3 μM ouabain was subtracted from the activity obtained with 3 mM ouabain. The Na,K-ATPase activity was expressed as nmol/mg protein x minute.
Measurement of NOS Activity
NOS activity in hippocampal slices was determined by the conversion of [3H]arginine to [3H]citrulline, employing an radioassay previously described by our group and others with minor modifications (Bredt and Snyder 1989; Mckee et al. 1994).Briefly, the tissue slice pellets were resuspended and homogenized in ice-cold 0.32 M sucrose/20 mM HEPES buffer (pH 7.4) containing 1 mM dithiothreitol (DTT) in an ice bath for 1 min using a Teflon homogenizer. Each homogenate was centrifuged at 10,000×g for 30 min at 4 °C. The supernatant was passed through a Dowex AG 50 Wx-8 (Na+ form) column to remove the endogenous arginine. The arginine-free eluent was used to assay the NOS activity. To evaluate NOS activity, the assay reaction medium of 200 μl, containing 100 mM HEPES, pH 7.4; 1 mM NADPH; FMN, FAD, tetrahydrobiopterin 0.45 mM CaCl2; 80 units of calmodulin, 100 µM l-arginine, and 1 μM L-[2, 3-3H]-arginine (0.5 μCi), or with no addition of CaCl2 and calmodulin (in the presence of 0.425 mM EDTA), and 100 μl of hippocampus cytosolic protein (0.2 μg/μl). The reaction mixture was incubated for 30 min at 37 °C and stopped by the addition of stop buffer containing 20 mM HEPES at pH 5.5. The entire reaction mixture was passed through a column packed with Na+ form of Dowex AG 50 Wx-8 resin. The flow through fraction containing [3H]-citrulline was counted for radioactivity using a Beckman 6000 liquid scintillation counter. The NOS activity was expressed as picomoles citrulline per milligram protein per minute. Samples of rat cerebellum were analyzed simultaneously as a positive control. Inhibition of the enzyme was evaluated in all tissues using N-nitro-l-arginine methyl ester hydrochloride (L-NAME: 10−6 to 10−4 M).
Measurement of Cyclic GMP Levels
Cyclic GMP levels in slice hippocampus were determined in triplicate by direct, competitive enzyme immunoassay kits (Stressgen, Enzo Life Sciences, Inc., USA). Hippocampal slices were homogenized in 0.5 ml 50 mM Tris–HCl buffer (pH 8.0) containing 1 mM theophylline and 0.1 mM 3-isobutyl-1-methylxanthine (IBMX) at 4 °C, followed by heating at 90 °C for 5 min and centrifuged at 10,000×g for 5 min at room temperature. The supernatant was acetylated to improve signal detection and samples were run in duplicate. The concentration of cyclic GMP is expressed as fmol/ml.
TBARS Determination
Measurement of TBARS was employed to determine lipid peroxidation as previously described (Vasconcelos et al. 2015). Thiobarbituric acid reacts with products of lipid peroxidation, mainly malondialdehyde, producing a colored compound. The tissue slice pellets were resuspended and homogenized in saline buffer and precipitated proteins were removed by centrifugation at 12,000×g for 10 min. The supernatant was mixed with thiobarbituric acid (1% in NaOH 50 mM) and HCl 25%. The samples were then heated in a boiling water bath for 10 min and, after cooling, were extracted with 1.5 ml of butanol. The mixture was centrifuged at 12,000×g for 10 min and the absorbance of the supernatant was determined.
Immunoblotting
Electrophoresis was performed using a 10% polyacrylamide gel and the Bio-Rad mini-Protean III apparatus. In brief, the proteins present in the hippocampal slice extract (15 μg) were size separated in 10% SDS-PAGE (90 V). The proteins were blotted onto a nitrocellulose membrane (Bio-Rad Laboratories, Richmond, CA, USA) and incubated with specific antibody against 3-Nitrotyrosine (sc-32731; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The Ponceau method of immunoblotting was used to ensure equal protein loading. Proteins recognized by antibodies were revealed by the ECL technique, following the instructions of the manufacturer (Amersham, Buckinghamshire, England). The membranes were exposed to X-ray film. To quantify the immunoblots, we used the NIH ImageJ software. Several exposure times were analyzed to ensure the linearity of the band intensities. β-actin antibody (sc-1616; Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as an internal control for the experiments. Results were expressed in relation to the intensity of β-actin band.
Chemicals and Kits
L-[2, 3, 4, 5-3H]Arginine monohydrochloride (sp. act. 63.0 Ci/mmol) was purchased from Amersham International (Buckinghamshire, England). Cyclic GMP Enzyme Immunoassay kits were purchased from Enzo Life Sciences Inc. (Farmingdale, NY, USA). Resin Dowex AG50WX-8 (H+ form converted to Na+ form using 1 N NaOH), sucrose, HEPES, EDTA, DTT, TET, CaCl2, calmodulin, FMN, FAD, NADPH, l-arginine, Tris–HCl, and L-NAME were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Dizocilpine (MK-801) maleate was obtained from Research Biochemicals International (Natick, MA, USA). Protein assay dye reagent from Bio-Rad Laboratories (Richmond, CA, USA). All solutions were prepared immediately before use.
Statistics
The data expressed as mean ± standard error of the mean (S.D.) were obtained from three independent experiments. In each experiment, three replicate samples were quantified. Statistical comparisons were made by one-way analysis of variance (ANOVA), followed by the Newman–Keuls test. All P values < 0.05 were considered to reflect a statistically significant difference.
Results
After 60 min of incubation with Aβ1–40 (0.2 and 2.0 µM), rat hippocampal slices demonstrated a concentration-dependent increase of Ca2+-dependent NOS activity [F (2, 27) = 49,91, P < 0.0001; P < 0.05 CTR vs Aβ 0.2/2 and Aβ 0.2 vs Aβ 2] (Fig. 1A) but only the low concentration of Aβ induced an increase in cyclic GMP levels [F (2, 21) = 4.368, P = 0.0259; P < 0.05 CTR vs Aβ 0.2] (Fig. 1B). The pre-incubation of slices with MK-801 (1 μM) induced a decrease in both NOS activity [F (5, 28) = 88, P < 0.0001; P < 0.05 MK vs CTR, Aβ] and cyclic GMP levels [F (5, 40) = 9.463, P < 0.0001; P < 0.05 CTR vs MK] by itself and it completely blocked the increase in NOS activity induced by Aβ1–40 (2.0 µM) (P < 0.05 Aβ vs MK + Aβ). In addition, pre-incubation of slices with L-NAME (100 μM) in the absence (P < 0.05 LN vs CTR, Aβ) or presence (P < 0.05 Aβ vs LN + Aβ) of Aβ1–40 (2.0 µM) induced a decrease in both NOS activity and cyclic GMP levels when compared to PBS-treated slices (control) (Fig. 2A and B).
Effect of Aβ 1–40 (0.2 or 2.0 µM) on A constitutive NOS activity (pmol/mg/min), B cyclic GMP (fmol/mg protein), C TBARS (nmol/mg protein), and D 3-Nitrotyrosine levels (relative density of western blot autoradiographies bands normalized by β-actin) in rat hippocampus. Values are the mean ± S.D. of three individual experiments. Statistical analysis: one-way ANOVA followed by Newman–Keuls post-test. (a) P < 0.05 vs CTR, (b) P < 0.05 vs Aβ 1–40 (0.2 µM). A: CTR (n = 13), Aβ 0.2 (n = 9), Aβ 2 (n = 8); B, C: CTR, Aβ 0.2, Aβ 2 (n = 8); D: CTR (n = 3), Aβ 0.2 (n = 4), Aβ 2 (n = 3)
Effects of MK-801 (MK-1 μM), an antagonist of the NMDA receptor, and L-NAME (LN-100 μM) an antagonist of NOS on Aβ 1–40 (2.0 µM)-induced activation of A constitutive neuronal NOS activity (pmol/mg/min) and increase in B cyclic GMP (fmol/mg protein) and C TBARS (nmol/mg protein) levels in rat hippocampus. Values are the mean ± S.D. of three individual experiments. Statistical analysis: one-way ANOVA followed by Newman–Keuls post-test. A: (a) P < 0.05 vs CTR, (b) P < 0.05 vs Aβ, (c) P < 0.05 vs MK and MK + Aβ, (d) P < 0.05 vs LN and LN + Aβ. B: (a) P < 0.05 vs CTR, (b) P < 0.05 vs Aβ. C: (a) P < 0.05 vs CTR, MK and LN, (b) P < 0.05 vs MK + Aβ, (c) P < 0.05 vs LN + Aβ. A: CTR (n = 9), Aβ (n = 7), MK (n = 4), MK + Aβ (n = 3), LN (n = 6), LN + Aβ (n = 5); B: CTR (n = 6), Aβ (n = 8), MK (n = 8), MK + Aβ (n = 8), LN (n = 8), LN + Aβ (n = 8); C: CTR (n = 8), Aβ (n = 8), MK (n = 8), MK + Aβ (n = 8), LN (n = 8), LN + Aβ (n = 8)
TBARS and 3-NT determinations showed a similar concentration-dependent increase in products of lipid peroxidation and nitrotyrosine-modified proteins in hippocampal slices after 60 min of incubation with Aβ1–40 (0.2 and 2.0 µM) [for TBARS: F (2, 21) = 15,26, P < 0.0001; P < 0.05 CTR vs Aβ 0.2/2 and Aβ 0.2 vs Aβ 2. For 3-NT: F (2, 7) = 19.25, P = 0.0014; P < 0.05 CTR vs Aβ 0.2/2 and Aβ 0.2 vs Aβ 2] (Fig. 1C and D). However, the increase in TBARS levels induced by Aβ1–40 (2.0 µM) was only partially blocked by pre-incubation of slices with L-NAME (100 μM) and MK-801 (1 μM) [F (5, 42) = 26.67, P < 0.0001; P < 0.05 (Aβ vs MK + Aβ, Aβ vs LN + Aβ)] (Fig. 2C).
The incubation of slices with Aβ1–40 (2.0 µM) caused a decrease of the total-ATPase activity [F (5, 18) = 4.413, P = 0.0085; P < 0.05 CTR vs Aβ] (Fig. 3A). This effect induced by Aβ1–40 (2.0 µM) was specific for ouabain-sensitive activity (Na,K-ATPase) [F (5, 18) = 3.728, P = 0.0172; P < 0.05 CTR vs Aβ], as ouabain-insensitive activity was not affected [F (5, 18) = 0.07340, P = 0.9955] (Fig. 3B and C). Furthermore, the decrease of both total- and Na,K-ATPase induced by Aβ1–40 (2.0 µM) incubation of hippocampal slices were not blocked by the pre-treatment of the slices with L-NAME (100 μM) and by MK-801 (1 μM) either (Fig. 3A–C).
Effect of Aβ 1–40 (2.0 µM) in the presence or absence of MK-801 (MK-1 μM) or L-NAME (LN-100 μM) in A Total-ATPase, B Mg-ATPase, and C Na,K-ATPase activities (µmol/mg/min) in rat hippocampus. Values are the mean ± S.D. of three individual experiments. Statistical analysis: one-way ANOVA followed by Newman–Keuls post-test. (a) P < 0.05 vs CTR. A–C: CTR (n = 4), Aβ (n = 4), MK (n = 4), MK + Aβ (n = 4), LN (n = 4), LN + Aβ (n = 4)
The results showed that changes in Na,K-ATPase activity (P = 0.0011) in hippocampus slice induced by Aβ1–40 (2.0 µM) were specific for the α1-Na,K-ATPase (P = 0.0091) with no changes for the α2,3-Na,K-ATPase activity (P = 0.8841) (Fig. 4A, C, and D). Again, the effect of Aβ1–40 (2.0 µM) was specific to Na,K-ATPase because no change was observed in ouabain-insensitive (Mg-ATPase) activity (P = 0.9674) (Fig. 4B).
Effect of Aβ 1–40 (2.0 µM) on A Na,K-ATPase and Mg-ATPase, B α1-Na,K-ATPase and α2,3-Na,K-ATPase activities in rat hippocampus. Values are the mean ± S.E.M. of three individual experiments. α2/3-Na,K-ATPase activity was measured by subtracting the activity obtained with 3 μM ouabain from total Na,K-ATPase activity. To determine the α1 subunit-associated Na,K-ATPase activity, the Na,K-ATPase activity measured with 3 μM ouabain was subtracted from the activity obtained with 3 mM ouabain (Mg-ATPase). Results are expressed as µmol of Pi released/mg/min protein (mean ± S.D.) from three individual experiments. Statistical analysis: Student T test. (a) P < 0.05 vs CTR. A, B: CTR (n = 6), Aβ (n = 4)
Slice viability (% purple MTT precipitate/slice) was similar after 60 min of incubation with Aβ1–40 (0.2 and 2.0 µM) or after MK-801 and L-NAME pre-treatment in the presence or absence of Aβ (2.0 µM) when compared to control group (PBS) [F (8, 48) = 0.4218, P = 0.9023] (Fig. 5). Therefore, Aβ effect in the presence or absence of MK-801 and L-NAME was not due to a tissue death associated with slice preparation and/or drug incubation.
Effect of Aβ 1–40 (2.0 µM) in the presence or absence of MK-801 (MK-1 μM) or L-NAME (LN-100 μM) on tissue viability. MTT reduction assay data are expressed as percentage of the mean value of control (vehicle alone). Values are the mean ± S.D. of five separate experiments. Statistical analysis: one-way ANOVA followed by Newman–Keuls post-test
Discussion
Although the basic etiology of AD remains unknown, much of the progress in elucidating the pathogenesis of this disorder has centered on the apparent role of the 25–35, 1–40, and 1–42 residues of Aβ in neurodegenerative process. In the present study, we further evaluated the signaling pathways of Aβ in relation to NMDA–NOS–cyclic GMP versus oxidative stress on α1-/α2,3-Na,K-ATPase activities in rat hippocampus slices.
In this report, we demonstrated that 60 min of incubation of Aβ1–40 induces a decrease in Na,K-ATPase activity in rat hippocampal slices. Several studies have already shown that different fragments of Aβ, such as 1–40, 1–42, and 25–35, can affect Na,K-ATPase in cortical cultures and in hippocampal slices, but they may involve different mechanisms since this effect can occur in some situations prior to cell death as well as they behave differently in various tissue preparations (Bores et al. 1998; Mark et al. 1995). Our data confirmed that Aβ1–40 effect on Na,K-ATPase occurs without tissue death based on MTT studies and it is not linked to a direct action on Na,K-ATPase because enzyme activity was not inhibited when membranes were prepared first and then challenged to Aβ (data not shown) (Bores et al. 1998).
Several lines of evidence show that dysfunction in Ca2+ homeostasis is associated with AD. In fact, studies performed in different neuronal culture models revealed that Aβ treatment causes progressive intracellular Ca2+ increase and elevated response to excitatory amino acids (George-Hyslop et al. 1987; Koh et al. 1997; Mattson et al. 1992, 1991). Early effects of Ca2+ influx include the activation of Ca2+-dependent enzymes and generation of reactive oxygen species (ROS) (Varadarajan et al. 2000; Wang and Robinson 1997). The increase in O2• production would lead to an accumulation of intracellular Ca2+ and activation of Ca2+-dependent enzymes, like neuronal NOS, which is responsible for the formation of NO• (Harman 1995). In fact, in the CNS, NO synthesis seems predominantly regulated by the influx of Ca2+ through receptor-dependent channels, in particular following postsynaptic stimulation of NMDA receptors by the excitatory neurotransmitter, glutamate (Bredt and Snyder 1989; Garthwaite 1991).
Our data confirmed that Aβ1–40 activates this pathway increasing Ca2+-dependent NOS activity, as well as cyclic GMP and TBARS levels. Aβ-induced NOS activation and increase in cyclic GMP levels were linked to NMDA receptor as pre-incubation of slices with MK-801 (1 μM) completely blocked these effects.
Although increased glutamate levels are not observed in AD (Carlson et al. 1993; Hyman et al. 1987), such mechanism of excitotoxicity can occur in the presence of additional pathological factors, particularly in highly susceptible areas such as the parahippocampal gyrus and the entorhinal cortex (Arias et al. 1998; Guo et al. 1999). Recent evidence showed that Aβ can promote abnormal release of astrocytic glutamate in hippocampal neurons, which may contribute to dysregulation of extra synaptic NMDA receptors signaling in neurons (Brito-Moreira et al. 2011; Talantova et al. 2013).
The involvement of NMDA receptor in the Aβ-effect just confirmed previous evidence of glutamate-mediated cell death in the presence of this peptide (Mattson 1994). However, part of this effect can also be mediated by Aβ interaction with a cell surface receptor for advanced glycation end (RAGE) products as well as by its reaction with molecular oxygen to produce superoxide radical and hydrogen peroxide (Behl et al. 1994). Recent studies also suggest that Aβ peptides induce chloride channel activity in isolated microglia and increase the production of ROS (Milton et al. 2008).
Our data showed that the decrease of Na, K-ATPase activity induced by Aβ1–40 (2.0 µM) in hippocampal slices seem not to be mediated by ROS produced by activation of NOS since it was not blocked by pre-treatment of the slices with L-NAME. Moreover, it was not mediated by NMDA receptor either as MK-801 (1 μM) was unable to revert the Aβ1–40 effect on Na, K-ATPase activity. In fact, NMDA receptor blocker pre-treatment only partially reverted the Aβ1–40 -induced ROS production in hippocampal slices confirming that ROS non-mediated by NMDA receptor can also act on Na,K-ATPase activity.
Several studies showed that NO• protector or deleterious effect is based on its redox status. NO• donors or synthesized endogenously after NMDA receptor activation can lead to neurotoxicity (Yu 1996). The NOS–ROS metabolism is much more complex than addressed in the present work. Although O2•− and NO• play important roles in several aspects of cellular signaling and metabolic regulation, in the inflammatory environment, the concentrations of these radicals can drastically increase, and the antioxidant defenses may become overwhelmed. A complex interplay also exists between iron metabolism and NOS–ROS since iron deficiency (anemia) is associated with oxidative stress, but its role in the induction of nitrosative stress is largely unclear (Koskenkorva-Frank et al. 2013).
Evidence suggests that part of the physiological NO• effects occurs mainly through stimulation of soluble guanylate cyclase (GC), with a consequent increase in levels of cyclic GMP (Chiavegatto et al. 1998). The cyclic GMP, in turn, may act on ion channels or activate protein kinases (Garthwaite and Boulton 1995) mediating their intracellular [Ca2+] lowering and excitoprotective effects on target neurons (Barger et al. 1995) and increasing α2,3-Na,K-ATPase which plays an important role in this process (Nathanson et al. 1995). In addition, the interaction of NO• and NO•-derived metabolites to form S-nitrosothiols (RSNOs), N-nitrosamines (RNNOs), and nitrosyl-heme, respectively, also are important physiologically as the activation of cyclic GMP by NO• in signaling functions in physiological and pathophysiological processes (Ahern et al. 2002; Bogdan 2001).
Thus, we further investigated the signaling pathways of Aβ in relation to NMDA–NOS–cyclic GMP versus oxidative stress on α1-/α2,3-Na,K-ATPase isoforms activities in rat hippocampal slices. Results demonstrated the first evidence that Aβ1–40 induced an isoform-specific decrease in the enzyme activity since only α1-Na,K-ATPase was affected. It is interesting that Aβ1–40 increased cyclic GMP levels, which can balance the increase in ROS production and avoid the decrease in α2,3-Na,K-ATPase activity induced by amyloid peptide effect on α2,3-Na,K-ATPase.
These results therefore suggest that NO• produced in excess after Aβ treatment is not activating only the soluble GC, which may be due to the increase in ONOO− production. Previous data from our laboratory also showed an age-related decrease in cyclic GMP contents and an increase in both NOS activity in platelets and TBARS levels in erythrocytes as well as in hippocampus and frontal cortex of rats (Kawamoto et al. 2013). In fact, previous studies have already shown an age-related reduction in the levels of cyclic GMP in the cerebral cortex, striatum, cerebellum, hippocampus, and hypothalamus of rats (Chalimoniuk and Strosznajder 1998; Kawamoto et al. 2008b; Puri and Volicer 1981; Scavone et al. 2005; Schmidt and Thornberry 1978).
Changes induced by Aβ in the presence or absence of MK-801 and L-NAME were not linked to any tissue death. The relevance of impaired NO•-dependent cyclic GMP production to long-term amyloid exposure is unknown at present but it might predispose or potentiate an effect of specific factor(s) in the manifestation of age-related degenerative disorders. The aberrant NO•–cyclic GMP cascade is not only observed in neurodegenerative diseases but also in several other disease states, such as hypertension, atherosclerosis, and diabetes, which have been linked to cognitive deficits. Experimental data suggest that the hippocampal-prefrontal network can participate in the formation and consolidation of memories (Dégenètais et al. 2003; Laroche et al. 1990). Therefore, changes observed in NOS activity and TBARS/cyclic GMP levels during aging process and the characteristics of the excitatory synaptic transmission in the hippocampal-prefrontal cortex pathway support the existence of a cooperative relationship between two structures known to be involved in higher cognitive processes.
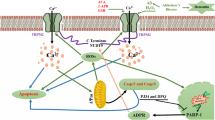
Taken together, the results suggest that Aβ’s inhibition of Na,K-ATPase by ROS and 3-NT-modified protein production may initiate a cascade of detrimental effects that lead to cell death. However, the activation of NMDA–cyclic GMP signaling cascade linked to α2,3-Na,K-ATPase activity may mediate an adaptive, neuroprotective response to Aβ in rat hippocampus (Fig. 6).
Schematic representation of the hypothetical molecular mechanism model for Aβ-induced toxicity. Aβ can bind to RAGE, which in turn produces hydrogen peroxide, reacts with molecular oxygen to produce ROS such as superoxide radical and hydrogen peroxide and also can cause damage do lipid bilayer increasing ion permeability such as Ca2+. Aβ can induce NO production by disrupting Ca2+ homeostasis and subsequent increase in intracellular Ca2+ (nNOS and eNOS-mediated NO release). NO is a free radical and can produce peroxynitrite. These ROS can induce a variety of neurotoxic mechanisms, including DNA/protein alterations, lipid peroxidation, and impairment of α1-Na,K-ATPase activity. Aβ induced-NMDA activation can also activate cyclic GMP pathway which in turn can emerge as a neuroprotective signaling involved in modulating α2,3-Na,K-ATPase
Abbreviations
- Aβ:
-
Amyloid-β peptide
- AD:
-
Alzheimer’s disease
- NMDA:
-
N-methyl-d-aspartate
- NOS:
-
Nitric oxide synthase Sodium
- Na,K-ATPase:
-
Potassium pump
- ROS:
-
Reactive oxygen species
- TBARS:
-
Thiobarbituric acid reactive substances
- 3-NT:
-
3-Nitrotyrosine
- O2 • :
-
Superoxide anion
- ONOO− :
-
Peroxynitrite anion
- OH• :
-
Hydroxyl radical
- sGC:
-
Soluble guanylyl cyclase
- RAGE:
-
Receptor for advanced glycation end
- RSNOs:
-
S-nitrosothiols
- RNNOs:
-
N-nitrosamines
- PDE:
-
Phosphodiesterase
References
Ahern G, Klyachko V, Jackson M (2002) cGMP and S-nitrosylation: two routes for modulation of neuronal excitability by NO. Trends Neurosci 25:510–517
Ahmed HH (2012) Modulatory effects of vitamin E, acetyl-L-carnitine and α-lipoic acid on new potential biomarkers for Alzheimer’s disease in rat model. Exp Toxicol Pathol 64:549–556
Albensi BC, Mattson MP (2000) Evidence for the involvement of TNF and NF-kappaB in hippocampal synaptic plasticity. Synapse 35:151–159
Arias C, Becerra-García F, Tapia R (1998) Glutamic acid and Alzheimer’s disease. Neurobiology 6:33–43
Arriagada P, Growdon J, Hedley-White T, Hyman B (1992) Neurofibrillary tangles but not senile plaques parallel duration and severity in Alzheimer’s disease. Neurology 42:631–639
Barger SW, Fiscus RR, Ruth P, Hofmann F, Mattson MP (1995) Role of cyclic GMP in the regulation of neuronal calcium and survival by secreted form of b-amyloid precursor. J Neurochem 64:2087–2096
Behl C, Davis J, Lesley R, Schubert D (1994) Hydrogen peroxide mediates amyloid beta protein toxicity. Cell 77:817–827
Bogdan C (2001) Nitric oxide and the regulation of gene expression. Trends Cell Biol 11:66–75
Bores G, Smith C, Wirtz-Brugger F, Giovanni A (1998) Amyloid beta-peptides inhibit Na+/K+-ATPase: tissue slices versus primary cultures. Brain Res Bull 46:423–427
Bøttger P, Doğanlı C, Lykke-Hartmann K (2011) Migraine- and dystonia-related disease-mutations of Na+/K+-ATPases: relevance of behavioral studies in mice to disease symptoms and neurological manifestations in humans. Neurosci Biobehav Rev 36:855–871
Braak H, Braak E (1994) Morphological criteria for the recognition of Alzheimer’s disease and the distribution pattern of cortical changes related to this disorder. Neurobiol Aging 15:355–356
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bredt DS, Snyder SH (1989) Nitric oxide mediates glutamate-linked enhancement of cGMP levels in cerebellum. Proc Natl Acad Sci USA 86:9030–9033
Brito-Moreira J, Paula-Lima A, Bomfim T, Oliveira F, Sepúlveda F, De Mello F, Aguayo L, Panizzutti R, Ferreira S (2011) Aβ oligomers induce glutamate release from hippocampal neurons. Curr Alzheimer Res 8:552–562
Carlson M, Penney JJ, Young A (1993) NMDA, AMPA, and benzodiazepine binding site changes in Alzheimer’s disease visual cortex. Neurobiol Aging 14:343–352
Chalimoniuk M, Strosznajder JB (1998) Aging modulates nitric oxide synthesis and cGMP levels in hippocampus and cerebellum. Effects of amyloid beta peptide. Mol Chem Neuropathol 35:77–95
Chiavegatto S, Scavone C, Canteras NS (1998) Nitric oxide synthase activity in the dorsal periaqueductal gray of rats expressing innate fear response. NeuroReport 9:571–576
Connely CA, Chen LC, Colquhoun SD (2000) Metabolic activity of cultured rat brainstem, hippocampal and spinal cord slices. J Neurosci Methods 99:1–7
de Carvalho Aguiar P, Sweadner K, Penniston J, Zaremba J, Liu L, Caton M, Linazasoro G, Borg M, Tijssen M, Bressman S, Dobyns W, Brashear A, Ozelius L (2004) Mutations in the Na+/K+ -ATPase alpha3 gene ATP1A3 are associated with rapid-onset dystonia parkinsonism. Neuron 43:169–175
De Fusco M, Marconi R, Silvestri L, Atorino L, Rampoldi L, Morgante L, Ballabio A, Aridon P, Casari G (2003) Haploinsufficiency of ATP1A2 encoding the Na+/K+ pump alpha2 subunit associated with familial hemiplegic migraine type 2. Nat Genet 33:192–196
Dégenètais E, Thierry A-M, Glowinski J, Gioanni Y (2003) Synaptic influence of hippocampus on pyramidal cells of the rat prefrontal cortex: an in vivo intracellular recording study. Cereb Cortex 13:782–792
Dickey C, Gordon M, Wilcock D, Herber D, Freeman M, Morgan D (2005) Dysregulation of Na+/K+ ATPase by amyloid in APP+PS1 transgenic mice. BMC Neurosci 6:1–11
Esmann M (1988) ATPase and phosphatase activity of Na+, K+-ATPase: molar and specific activity, protein determination. Methods Enzymol 156:105–115
Garthwaite J (1991) Glutamate, nitric oxide and cell-cell signaling in the nervous system. Trends Neurosci 14:60–67
Garthwaite J, Boulton CL (1995) Nitric oxide signaling in the central nervous system. Annu Rev Physiol 57:683–706
George-Hyslop P, Tanzi R, Polinsky R, Haines J, Nee L, Watkins P, Myers R, Feldman R, Pollen D, Drachman D et al (1987) The genetic defect causing familial Alzheimer’s disease maps on chromosome 21. Science 235:885–890
Goldstein I, Lerer E, Laiba E, Mallet J, Mujaheed M, Laurent C, Rosen H, Ebstein R, Lichtstein D (2009) Association between sodium- and potassium-activated adenosine triphosphatase alpha isoforms and bipolar disorders. Biol Psychiatry 65:985–991
Guo Q, Sebastian L, Sopher B, Miller M, Ware C, Martin G, Mattson M (1999) Increased vulnerability of hippocampal neurons from presenilin-1 mutant knock-in mice to amyloid beta-peptide toxicity: central roles of superoxide production and caspase activation. J Neurochem 72:1019–1029
Harman D (1995) Free radical theory of aging: Alzheimer’s disease pathogenesis. Age (dordr) 18:97–119
Hattori N, Kitagawa K, Higashida T, Yagyu K, Shimohama S, Wataya T, Perry G, Smith M, Inagaki C (1998) CI-ATPase and Na+/K(+)-ATPase activities in Alzheimer’s disease brains. Neurosci Lett 254:141–144
Holcomb L, Gordon M, Mcgowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O’Campo K, Hardy J, Prada C, Eckman C, Younkin S, Hsiao K, Duff K (1998) Accelerated Alzheimer phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med 4:97–100
Hyman B, Van Hoesen G, Damasio A (1987) Alzheimer’s disease: glutamate depletion in the hippocampal perforant pathway zone. Ann Neurol 22:37–40
Katzman R, Saitoh T (1991) Advances in Alzheimer’s disease. FASEB J 4:278–286
Kawamoto EM, Lepsch LB, Boaventura MF, Munhoz CD, Lima LS, Yshii LM, Avellar MC, Curi R, Mattson MP, Scavone C (2008a) Amyloid beta-peptide activates nuclear factor-kappaB through an N-methyl-D-aspartate signaling pathway in cultured cerebellar cells. J Neurosci Res 86:845–860
Kawamoto EM, Munhoz C, Lepsch L, de Sá Lima L, Glezer I, Markus R, de Silva C, Camarini R, Marcourakis T, Scavone C (2008b) Age-related changes in cerebellar phosphatase-1 reduce Na, K-ATPase activity. Neurobiol Aging 29:1712–1720
Kawamoto E, Vasconcelos A, Degaspari S, Böhmer A, Scavone C, Marcourakis T (2013) Age-related changes in nitric oxide activity, cyclic GMP, and TBARS levels in platelets and erythrocytes reflect the oxidative status in central nervous system. Age (dordr) 35:331–342
Kinoshita PF, Yshii LM, Vasconcelos AR, Orellana AMM, Lima LdS, Davel APC, Rossoni LV, Kawamoto EM, Scavone C (2014) Signaling function of Na, K-ATPase induced by ouabain against LPS as an inflammation model in hippocampus. J Neuroinflamm 11:218
Knowles RG, Moncada S (1994) Nitric oxide synthases in mammals. Biochem J 298:249–258
Koh WS, Jeon YJ, Herring AC, Kaminski NE (1997) Transient CRE-and kappa B site-binding is cross-regulated by cAMP-dependent protein kinase and a protein phosphatase in mouse splenocytes. Life Sci 60:425–432
Koskenkorva-Frank T, Weiss G, Koppenol W, Burckhardt S (2013) The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: Insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic Biol Med 65:1174–1194
Laroche S, Jay TM, Thierry AM (1990) Long-term potentiation in the prefrontal cortex following stimulation of the hippocampal CA1/subicular region. Neurosci Lett 114:184–190
Liguri G, Taddei N, Nassi P, Latorraca S, Nediani C, Sorbi S (1990) Changes in Na+, K(+)-ATPase, Ca2(+)-ATPase and some soluble enzymes related to energy metabolism in brains of patients with Alzheimer’s disease. Neurosci Lett 112:338–342
Lipton S, Choi Y, Pan Z, Lei S, Chen H, Sucher N, Loscalzo J, Singel D, Stamler J (1993) A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature 12:626–632
Liu Y, Peterson DA, Kimura R, Schubert D (1997) Mechanism of cellular 3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J Neurochem 69:581–593
Mark R, Hensley K, Butterfield D, Mattson M (1995) Amyloid beta-peptide impairs ion-motive ATPase activities: evidence for a role in loss of neuronal Ca2+ homeostasis and cell death. J Neurosci 15:6239–6249
Mattson MP (1994) Calcium and neuronal injury in Alzheimer’s disease. Contributions of beta-amyloid precursor protein mismetabolism, free radicals, and metabolic compromise. Ann N Y Acad Sci 747:50–76
Mattson MP, Rychlik B, Chu C, Christakos S (1991) Evidence for calcium-reducing and excito-protective roles for the calcium-binding protein calbindin-D28k in cultured hippocampal neurons. Neuron 6:41–51
Mattson M, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel R (1992) beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci 12:376–389
Mckee M, Scavone C, Nathanson JA (1994) Nitric oxide, cGMP, and hormone regulation of active sodium transport. Proc Natl Acad Sci USA 91:12056–12060
Milton R, Abeti R, Averaimo S, De Biasi S, Vitellaro L, Jiang L, Curmi PM, Breit SN, Duchen MR, Mazzanti M (2008) CLIC1 function is required for beta-amyloid-induced generation of reactive oxygen species by microglia. J Neurosci 28:11488–11499
Morris J, Storandt M, Mckeel JD, Rubin E, Price J, Grant E, Berg L (1996) Cerebral amyloid deposition and diffuse plaques in “normal” aging: evidence for presymptomatic and very mild Alzheimer’s disease. Neurology 46:707–719
Moseley A, Williams M, Schaefer T, Bohanan C, Neumann J, Behbehani M, Vorhees C, Lingrel J (2007) Deficiency in Na, K-ATPase alpha isoform genes alters spatial learning, motor activity, and anxiety in mice. J Neurosci 27:616–626
Munhoz CD, Kawamoto EM, de Sa Lima L, Lepsch LB, Glezer I, Marcourakis T, Scavone C (2005) Glutamate modulates sodium-potassium-ATPase through cyclic GMP and cyclic GMP-dependent protein kinase in rat striatum. Cell Biochem Funct 23:115–123
Naslund J, Haroutunian V, Mohs R, Davis K, Davies P, Greengard P, Buxbaum J (2000) Correlation between elevated levels of amyloid β-peptide in the brain and cognitive decline. JAMA 283:1571–1577
Nathanson JA, Scavone C, Scanlon C, Mckee M (1995) The cellular Na+ pump as a site of action for carbon monoxide and glutamate: a mechanism for long-term modulation of cellular activity. Neuron 14:781–794
Puri SK, Volicer L (1981) Age-related changes of cyclic nucleotide levels in rat brain regions. Mech Ageing Dev 15:239–242
Scavone C, Munhoz C, Kawamoto E, Glezer I, de Sa Lima L, Marcourakis T, Markus R (2005) Age-related changes in cyclic GMP and PKG-stimulated cerebellar Na, K-ATPase activity. Neurobiol Aging 26:907–916
Schmidt MJ, Thornberry JF (1978) Cyclic AMP and cyclic GMP accumulation in vitro in brain regions of young, old and aged rats. Brain Res 139:169–177
Selkoe D (1994) Alzheimer’s disease: a central role for amyloid. J Neuropathol Exp Neurol 53:438–447
Talantova M, Sanz-Blasco S, Zhang X, Xia P, Akhtar M, Okamoto S, Dziewczapolski G, Nakamura T, Cao G, Pratt A, Kang Y, Tu S, Molokanova E, McKercher S, Hires S, Sason H, Stouffer D, Buczynski M, Solomon J, Michael S, Powers E, Kelly J, Roberts A, Tong G, Fang-Newmeyer T, Parker J, Holland E, Zhang D, Nakanishi N, Chen H, Wolosker H, Wang Y, Parsons L, Ambasudhan R, Masliah E, Heinemann S, Piña-Crespo J, Lipton S (2013) Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc Natl Acad Sci USA 110:E2518–E2527
Troy C, Rabacchi S, Friedman W, Frappier T, Brown K, Shelanski M (2000) Caspase-2 mediates neuronal cell death induced by beta-amyloid. J Neurosci 20:1386–1392
Varadarajan S, Yatin S, Aksenova M, Butterfield D (2000) Review: Alzheimer’s amyloid beta-peptide-associated free radical oxidative stress and neurotoxicity. J Struct Biol 130:184–208
Vasconcelos A, Kinoshita P, Yshii L, Marques Orellana A, Böhmer A, de Sá Lima L, Alves R, Andreotti D, Marcourakis T, Scavone C, Kawamoto E (2015) Effects of intermittent fasting on age-related changes on Na, K-ATPase activity and oxidative status induced by lipopolysaccharide in rat hippocampus. Neurobiol Aging 36:1914–1923
Wang X, Robinson PJ (1997) Cyclic GMP-dependent protein kinase and cellular signaling in the nervous system. J Neurochem 68:433–456
Yankner B (1996) Mechanisms of neuronal degeneration in Alzheimer’s disease. Neuron 16:921–932
Yu BP (1996) Aging and oxidative stress: modulation by dietary restriction. Free Radic Biol Med 21:651–668
Zhang L, Sun Y, Pan S, Li J, Qu Y, Li Y, Wang Y, Gao Z (2013) Na+-K+-ATPase, a potent neuroprotective modulator against Alzheimer disease. Fundam Clin Pharmacol 27:96–103
Funding
M.M.C.L. was financially supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (2014/14199-6) and P.F.K. is supported by postdoc fellowship from FAPESP (2018/14289-6). L.S.L. and D.Z.A. are supported by University of São Paulo. This publication was made possible by grants from FAPESP to CS (2016/07427-8) and E.M.K. (2016/22996-9; 2019/12974-6); CNPq 405089/2018-0; CAPES—STINT program 88887.125409/2016-00 (Joint Brazilian-Swedish Research Collaboration) and USP Neuroscience Research Support Centres (NAPNA) to C.S. Grant. E.M.K., L.E.M.Q., and C.S. are research fellows of CNPq. The authors report no biomedical financial interests or potential conflicts of interest.
Author information
Authors and Affiliations
Contributions
EMK, MMCL, PFK, LEMQ, LSS, DZA, and CS: participated in all the experiments; have made substantial contributions to conception and design, acquisition of data, analysis, and interpretation of data; have been involved in drafting the manuscript or revising it critically for important intellectual content; and have written the manuscript to be published.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there is no actual or potential conflict of interest including any financial, personal or other relationships with other people or organizations within 3 years of beginning the work submitted that could inappropriately influence (bias) their work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kawamoto, E.M., Cararo-Lopes, M.M., Kinoshita, P.F. et al. Influence of Nitric Oxide–Cyclic GMP and Oxidative STRESS on Amyloid-β Peptide Induced Decrease of Na,K-ATPase Activity in Rat Hippocampal Slices. J Membrane Biol 254, 463–473 (2021). https://doi.org/10.1007/s00232-021-00196-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-021-00196-9