Abstract
Amyloid-β (Aβ) is the main component of senile plaques, one of the hallmarks of Alzheimer,s disease. Is been shown that Aβ25–35 decreased neuronal viability while it increased generation of reactive oxygen species (ROS), and albumin (BSA) prevented ROS production and neuronal death in a dose-and time-dependent manner. One of the major sources of ROS is mitochondrion, and is believed that Mitochondrial ATP-regulated potassium channels (mitoKATP) protect synapses and neurons against oxidative and metabolic stress by modulating inner membrane potential and ROS production. Here we investigate the possible participation of MitoKATP channels on toxic effect of Aβ and the protective effect of BSA, by studying the influence of diazoxide (DIAZ) and tolbutamide (TOLB) on the effect of Aβ25–35 in neuronal morphology, cell viability and ROS generation in presence and absence of BSA. DIAZ decreased ROS generation induced by Aβ25–35 in a concentration dependent manner, but increased with the addition of BSA. TOLB increased Aβ25–35 effect on ROS production in a concentration dependent manner, but only in presence of BSA. Neither DIAZ nor TOLB rescued neurons from morphological damage and cell death induced by Aβ25–35. Hence, it could be proposed that MitoKATP channels participate on toxic effects of Aβ25–35, but not in protective effect of BSA, which seems to go through an extraneuronal mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Alzheimer’s disease (AD) is the most prevalent dementia, especially among the elderly, striking about 60 million people worldwide (Alzheimer’s Association, 2012). AD is characterized by severe neurodegeneration and increasing cognitive impairment, together with the formation of senile plaques composed of amyloid-β-peptides (Aβ) as well as the presence of neurofibrillary tangles (NFTs) made of highly phosphorylated tau protein (PHF tau).
Aβ monomers play important functions such as: (1) inducing an enhanced expression of proteins related to insulin-like growth factor (IGF) function or transcription factor (TF) regulation (IGFBP3/5, and Lim only domain protein 4, respectively); (2) favoring adult neurogenesis in the subgranular zone of dentate gyrus; (3) modulating synaptic plasticity, long-term potentiation (LTP), and memories recording in the hippocampus; (4) sealing blood vessels to preserve blood-brain barrier (BBB) integrity; and (5) fine-tuning Ca2+ homeostasis by binding a α-nicotinic acetylcholine receptors (α-nAChRs) and enhancing intracellular Ca2+ signals without triggering intercellular Ca2+ waves in astrocytes. Thus, Aβ monomers assist in the mutual modulation of neuron-astrocyte signals promoting long-term potentiation (LTP) and memory storing [1–7].
However, Aβ peptide is considered a key molecule in AD pathogenesis, since when Aβ concentration is not kept at physiological levels, the resulting accumulation of Aβ triggers the assembling of Aβ monomers into an assortment of toxic Aβ oligomers of growing sizes eventually forming Aβ fibrils [8–11]. It has been informed that Aβ1–40 and Aβ1–42 peptides can be detached by a not enzymatic mechanism, generating different oligomers that can diffuse from it [12].
The shorter Aβ oligomer with toxic effect is Aβ25–35, a peptide of 11 amino acids that corresponds to a fragment of Aβ1–40 and Aβ1–42 and is an intermembrane domain of the amyloid-β precursor protein (APP) [13]. It has been demonstrated that Aβ25–35 administration caused cognitive impairment in rats [14, 15], and it has also been founded in AD patients, probably coming from Aβ1–40 cleavage [16]. Aβ25–35 has been proposed as the biologically active region of Aβ, and seems to be responsible for the toxic effect of Aβ that leads to brain damage [17].
Aβ25–35 retains both its physical and biological properties of the full-length peptide [18–22]. As an example, the toxic effect of Aβ25–35 in neurons in primary culture was investigated and compared with that induced by Aβ1–40, founding that decreased neuronal viability induced by both peptides was quite similar [23]. Because of the former, Aβ25–35 is often selected as a model for full length Aβ.
AD is considered a multifactorial pathogenesis [24], but one of the most widely described factors involved in AD is oxidative stress, defined as an imbalance between reactive oxygen species (ROS) and the antioxidant defenses [25]. Accordingly with different authors the mechanism of Aβ25–35 induced cytotoxicity involve the formation of oxygen radical species (ROS) [26–31]. An evidence of this is the protective effect of albumin against toxicity induced by Aβ25–35 in neurons in primary culture, attributed to its bind to the peptide in an equimolecular way, counteracting the ROS increase induced by the peptide [23].
Mitochondrial failure plays a key role in the generation of ROS [32], which leads in oxidative damage to different cellular compartments [33]. It has been hypothesized that oligomeric Aβ has the ability to permeabilize cellular membranes and lipid bilayers and thus to be imported in organelles such as mitochondrion [34–36]. Increasing evidence indicates that Aβ accumulation in mitochondrion is detrimental with Aβ1–42 oligomers susceptible to induce a massive entry of Ca2+ in neurons leading to mitochondrial Ca2+ overload [37], and Aβ1–40 and Aβ1–42 acting directly on mitochondrion by promoting mitochondrial dysfunctions and oxidative damages [38]. However, it is still unclear how Aβ causes mitochondrial structural and functional abnormalities.
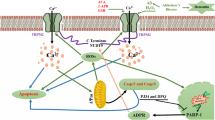
Mitochondrion plays also central roles in meeting the demands of synapses for ATP and in regulating calcium homeostasis, therefore mitochondrial damage can cause dysfunction and degeneration of synapses, which would lead to neuronal dead. Potassium channels similar to those identified at the plasma membrane, including ATP-regulated potassium channels (mitoKATP) have already been found in the inner mitochondrial membrane [39]. It has been proposed that mitochondrial potassium channels are involved in mitochondrial volume regulation, cytoprotection, acidification, apoptosis, calcium homeostasis and in the control of inner mitochondrial membrane integrity [39, 40].
It is believed that MitoKATP channels are one of the mitochondrial proteins that serve the function of protecting synapses and neurons against oxidative and metabolic stress, as the result of changing ion gradients and ATP usage, by modulating inner membrane potential and ROS production. As a matter of fact, the oxidation of potassium channels triggered by ROS in mammalian brain has equally appeared to contribute to different neuropathies [41]. Specifically, potassium channel dysfunctions have already been documented in fibroblasts [42] and platelets [43] from AD patients, and post mortem studies have also showed alterations of potassium channel expression in AD brains [44]. Besides, it has also been suggested that the composition of potassium channels could change after being treated with Aβ1–42 [45]. Because of the former, MitoKATP channels may participate in the toxic effect of Aβ and could represent a neuroprotection site.
In light of the above, here we investigate the possible participation of MitoKATP channels on deleterious effect of Aβ25–35 and the protective effect of albumin, by studying the influence of an opener and a blocker of these channels on the toxic effect of the peptide in presence as well as in absence of albumin.
MATERIALS AND METHODS
Reagents
Dulbecco,s modified Eagle,s medium (DMEM), penicillin, streptomycin, poly-L-lysine, cytosine arabinoside, bovine serum albumin (BSA; fatty acid-free and dialyzed before use) and 3-(4,5-dimetiltiazol-2-ilo)-2,5-difeniltetrazol (MTT), diazoxide (DIAZ) and tolbutamide (TOLB) were purchased from Sigma-Aldrich Chemical Co (Madrid, Spain). Aβ25–35 was obtained from Bachem (Torrance, California, USA) and dissolved in deionized water. Fetal calf serum (FCS) was obtained from Serva Boehringer Ingelheim (Heidelberg, Germany). 2,7-dichlorodihydrofluorescein-diacetate (DCFH-DA) was obtained from Molecular Probes, Eugene, OR, USA). Standard analytical grade laboratory reagents were obtained from Merck (Dramstad, Germany) or Sigma-Aldrich Chemical Co.
Animals
All experiments were performed according with the Helsinki Guide for the Care and Use of Laboratory Animals as adopted in the European Union and approved by the institutional committee of INCyL.
Albino Wistar rats (Rattus norvegicus), fed ad libitum on a stock laboratory diet (49.8% carbohydrates, 23.5% protein, 3.7% fat, 5.5% minerals and added vitamins and amino acids; w/w) were used for the experiments. The animals were maintained on a 12-h light-dark cycle. Females with a mean weight of 250 g were caged with males overnight and conception was considered to occur at 01:00 h; this was verified the following morning by the presence of spermatozoa in the vaginal smears. To prepare neurons in primary culture, fetuses at 17.5 days of gestation were delivered by rapid hysterectomy after cervical dislocation of the mother.
Neuronal Cultures
Cells were cultured essentially as described by Ta-bernero et al. [46]. Briefly, animals were decapitated and the brains immediately excised. After removing the meninges and blood vessels, the forebrain was placed in Earle’s balanced solution (EBS) containing 20 µg/mL DNase and 0.3% (w/v) BSA. The tissue was minced, washed, centrifuged at 500g for 2 minutes and incubated in 0.025% trypsin (type III) and 60 µg/mL DNase I for 15 min at 37°C. Trypsinization was halted by the addition of DMEM supplemented with 10% FCS. The tissue was then dissociated by passing-it gently 4 to 8 times through a siliconized Pasteur pipette and the supernatant was recovered. This operation was repeated and the resulting cell suspension was centrifuged at 500g for 5 minutes. The cells were then resuspended in DMEM supplemented with 10% FCS and counted in with a Neubauer chamber (test for the exclusion of trypan blue dye was used to indicate cell viability). Once diluted in DMEM supplemented with 10% FCS, 50 U/mL penicillin, 37.5 U/mL streptomycin, and 25 mM KCl, cells were plated on 3.5 cm or 6.0 cm diameter Petri dishes coated with 10 µg/mL of poly-L-Lysine at a density of 1.5 × 105 cells/cm2. One day after plating, 10 µM cytosine arabinoside was added to avoid glial cell proliferation.
Neuronal survival
For survival experiments, three days after plating cultured neurons were maintained in a serum-free medium (Hank’s medium, pH 7.4), and after the treatment neuronal survival was determined by the MTT reduction assay [47]. This assay is based on the ability of active mitochondrial dehydrogenases to convert MTT (dissolved in PBS, 5 mg/mL) to water insoluble purple formazan crystals. MTT was diluted 1 : 10 in DMEM and added to the cells. At the end of the incubation period (75 min in darkness), the medium with MTT was replaced by dimethyl sulfoxide (DMSO) and gently shaken for 10 min in darkness. Then, 100 µL of lysate was transferred to a 96-well plate and the absorbance of the dye was measured at a wavelength of 570 nm. Data are presented as percentages of absorbance compared with control cells. All experiments were performed at least 6 times with 4 reruns per condition.
Quantification of Reactive Oxygen Species
Production of reactive oxygen species (ROS) was measured using the fluorogenic DCFH-DA probe. DCFH-DA is intracellularly deacetylated to 2,7-dichlorodihydrofluorescein (DCFH) and then converted to the fluorescent oxidized compound 2,7-dichlorofluorescein (DCF) by hydrogen peroxide [48]. All experiments were performed at least 6 times with 4 reruns per condition.
Influence of MitoKATP Channels on the Toxic Effect of Aβ25–35 and the Protective Effect of Albumin in Neurons in Primary Culture
Influence of diazoxide and tolbutamide on the toxic effect of Aβ25–35 and the protective effect of albumin in neurons in primary culture. Neurons were cultured in DMEM supplemented with FCS 10% (v/v) for three days. Then cells were deprived from serum and maintained in Hank,s medium pH 7.4. Then they were exposed to 30 µM Aβ25–35 together with DIAZ (150 µM) or TOLB (150 µM), in presence as well as in absence of BSA 30 µM for 24 hours.
After the corresponding treatment, phase contrast photographs were taken and cellular viability and ROS generation were determined by the MTT reduction and DCFH-DA methods respectively, as described above.
Influence of different concentrations of diazoxide and tolbutamide on the toxic effect of Aβ25–35 and the protective effect of albumin in neurons in primary culture. Neurons cultured in DMEM with FCS 10% (v/v) for three days were deprived from serum and maintained in Hank,s medium pH 7.4. Then they were exposed to 30 µM Aβ25–35 simultaneously with different concentration of DIAZ (125, 250, 500 and 750 µM) or TOLB (30, 150, 300 and 450 µM), in presence as well as in absence of BSA 30 µM for 24 hours.
After the corresponding treatment, phase contrast photographs were taken and cellular viability and ROS generation were determined by the MTT reduction and DCFH-DA methods respectively, as described above.
Influence of diazoxide, alone or in combination with tolbutamide, on Aβ25–35 toxicity and the protective effect of albumin in neurons in primary culture. Neurons were cultured in DMEM with FCS 10% (v/v) for three days. Then cells were maintained in Hank’s medium pH 7.4 and were exposed to 30 µM Aβ25–35 together with DIAZ 150 µM alone or in combination with 150 µM of TOLB, for 24 hours. Described treatments were performed in presence as well as in absence of BSA 30 µM.
After the corresponding treatment, cellular viability and ROS generation were determined by the MTT reduction and DCFH-DA methods respectively, as described above.
Influence of different times of treatment with tolbutamide on Aβ25–35 toxicity and the protective effect of albumin in neurons in primary culture. Neurons cultured in DMEM with FCS 10% (v/v) for three days were deprived from serum and maintained in Hank,s medium pH 7.4. Then they were exposed to 30 µM Aβ25–35 with and without BSA 30 µM, in presence as well as in absence of tolbutamide 150 µM which acted during 1,2,3 or 6 hours.
After the corresponding time of treatment, cellular viability was determined by the MTT reduction method as described above.
RESULTS
Influence of Diazoxide and Tolbutamide on Effect of Aβ25–35, with and without Albumin, on Neuronal Morphology
In Fig. 1a but also on Fig. 4a, a severe damage on neuronal morphology caused by Aβ25–35 can be appreciated, which was partially reversed by BSA. Neither DIAZ (Fig. 1a) nor TOLB (Fig. 4a) protected cells from toxic effect of Aβ25–35. Protective effect of BSA on neuronal morphology was modified neither by DIAZ nor by TOLB (Figs. 1a and 4a).
Influence of diazoxide on albumin effect on neurons in primary culture with and without amyloid-β 25–35. Neurons were cultured in DMEM with fetal calf serum 10% (V/V) for three days. Then cells were serum-deprived and 30 µM of amyloid-β 25–35 (Aβ) was added for 24 hours with and without 30 µM of bovine serum albumin (BSA), both in the presence and absence of 150 µM of diazoxide (DIAZ). (a) Phase-contrast photographs were taken and labeled according to the culture conditions (bar = 100 µm). (b) Cellular viability was determined by the MTT reduction method. (c) ROS were quantified using 2,4-dihydro-dichloro-fluorescein. Results are presented as the mean ± SEM and were expressed as percentages of the respective controls. An ANOVA test was applied, and an asterisk (*) is used to indicate statistically different groups compared with the control (N ≥ 6, Observationseach experiment = 4, total degrees of freedom = 42, F = 3.057, p ≤ 0.05, Tukey’s test).
Different concentrations of DIAZ as well as TOLB were also tested, but no change was observed on effects of Aβ25–35 on cell morphology, regardless of whether the BSA is present or not (Figs. 2a and 5a).
Influence of increasing concentrations of diazoxide on albumin effect on neurons in primary culture with and without amyloid-β 25–35. Neurons were cultured in DMEM with 10% of fetal calf serum (V/V) for three days. Then cells were serum-deprived and 125, 250, 500 and 750 µM of diazoxide (DIAZ) was added for 24 hours with and without 30 µM of amyloid-β 25–35 (Aβ). (a) Phase-contrast photographs were taken and labeled according to the culture conditions. (b) Cellular viability was determined by the MTT reduction method. (c) ROS were quantified using 2,4-dihydro-dichloro-fluorescein. Results are presented as the mean ± SEM and were expressed as percentages of the respective controls. An ANOVA test was applied, and an asterisk (*) is used to indicate statistically different groups compared with the control (N ≥ 6, Observationseach experiment = 4, total degrees of freedom = 54, F = 2.836, p ≤ 0.05, Tukey’s test).
Influence of diazoxide on amyloid-β 25–35 effect on neurons in primary culture with and without albumin and tolbutamide. Neurons were cultured in DMEM with 10% of fetal calf serum (V/V) for three days. Then cells were serum-deprived and 30 µM of amyloid-β 25–35 (Aβ) was added for 24 hours with and without 30 µM of bovine serum albumin (BSA), both in the presence and absence of 150 µM of diazoxide (DIAZ), in combination or not with 150 µM of tolbutamide (TOLB). (a) Cellular viability was determined by the MTT reduction method. (b) ROS were quantified using 2,4-dihydro-dichloro-fluorescein. Results are presented as the mean ± SEM and were expressed as percentages of the respective controls. An ANOVA test was applied, and an asterisk (*) is used to indicate statistically different groups compared with the control (N ≥ 6, Observationseachexperiment = 4, total degrees of freedom = 42, F = 3.029, p ≤ 0.05, Tukey’s test).
Influence of tolbutamide on albumin effect on neurons in primary culture with and without amyloid-β 25–35. Neurons were cultured in DMEM with fetal calf serum 10% (V/V) for three days. Then cells were serum-deprived and 30 µM of amyloid-β 25–35 (Aβ) was added for 24 hours with and without 30 µM of bovine serum albumin (BSA), both in the presence and absence of 150 µM of tolbutamide (TOLB). (a) Phase-contrast photographs were taken and labeled according to the culture conditions (bar = 100 µm). (b) Cellular viability was determined by the MTT reduction method. (c) ROS were quantified using 2,4-dihydro-dichloro-fluorescein. Results are presented as the mean ± SEM and were expressed as percentages of the respective controls. An ANOVA test was applied, and an asterisk (*) is used to indicate statistically different groups compared with the control (N ≥ 6, Observationseach exeperiment = 4, total degrees of freedom = 42, F = 2.973, p ≤ 0.05, Tukey’s test).
Influence of Diazoxide and Tolbutamide on Effect of Aβ25–35, with and without Albumin, on Neuronal Viability
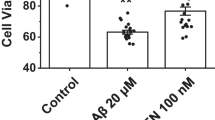
Results are presented on Figs. 1b and 4b, and indicate that Aβ25–35 decreased neuronal viability about 50%. The presence of BSA increased this parameter about 20%.
The addition of DIAZ itself did not modify cell survival, but did not rescue neurons from toxic effect of Aβ25–35 either, even in presence of BSA (Fig. 1b). The increase on DIAZ concentration had no effect on mortality induced by Aβ25–35 (Fig. 2b).
The addition of TOLB had no effect on observed cell viability when neurons are exposed to Aβ25–35, regardless if BSA is present or not (Fig. 4b). Enhancing TOLB concentration did not change the fact that this drug did not rescue neurons from toxic effect of Aβ25-35 or did not modify the protective effect of BSA (Fig. 5b). When different times of treatment were essayed, TOLB (150 µM) caused a light increase on cell viability, but did not influence BSA effect on mortality induced by Aβ25–35 (Fig. 6).
Influence of increasing concentrations of tolbutamide on albumin effect on neurons in primary culture with and without amyloid-β 25–35. Neurons were cultured in DMEM with 10% of fetal calf serum (V/V) for three days. Then cells were serum-deprived and 30, 150, 300 and 450 µM of tolbutamide (TOLB) was added for 24 hours with and without 30 µM of amyloid-β 25–35 (Aβ). (a) Phase-contrast photographs were taken and labeled according to the culture conditions (bar = 100 µm). (b) Cellular viability was determined by the MTT reduction method. (c) ROS were quantified using 2,4-dihydro-dichloro-fluorescein. Results are presented as the mean ± SEM and were expressed as percentages of the respective controls. An ANOVA test was applied, and an asterisk (*) is used to indicate statistically different groups compared with the control (N ≥ 6, Observationseach experiment = 4, total degrees of freedom = 114, F = 2.4967, p ≤ 0.05, Tukey’s test).
Influence of tolbutamide at different times of treatment on albumin effect on neuronal viability in neurons in primary culture without amyloid-β 25–35. Neurons were cultured in DMEM with fetal calf serum 10% (V/V) for three days. Then cells were serum-deprived and 30 µm of amyloid-β 25–35 (Aβ) was added for 1, 2, 3 and 6 hours with and without 30 µm of bovine serum albumin (BSA), both in the presence and absence of 150 µM of tolbutamide (TOLB). After the different times of treatment cellular viability was determined by the MTT reduction method. Results are presented as the mean ± SEM and were expressed as percentages of the respective controls. An ANOVA test was applied, and an asterisk (*) is used to indicate statistically different groups compared with the control (N ≥ 6, Observationseach experiment = 4, total degrees of freedom = 144, F = 2.002, p ≤ 0.05, Tukey’s test).
Figure 3 shows DIAZ effects when was administrated together with TOLB, where can be appreciated that, without BSA, TOLB decreased slightly neuronal viability respect to those cells added only with DIAZ, when they received Aβ25–35. In presence of BSA but in absence of Aβ25–35, TOLB increased neuronal survival.
Influence of Diazoxide and Tolbutamide on Effect of Aβ25–35, with and without Albumin, on Reactive Oxygen Spicies (ROS) Generation
According to Fig. 1c, Aβ25–35 increased ROS about 80%, which was completely reversed in presence of BSA. DIAZ diminished slightly ROS respect to the control (12%) and diminished 30% ROS generation induced by Aβ25–35, since it felled down from 180% to 150% respect to the control group. Such ROS level (150%) is similar to the observed in presence of BSA when cells were treated with Aβ25–35 and DIAZ.
The described decrease on ROS generation caused by DIAZ when neurons are exposed to Aβ25–35 (from 180 to 150%) is a concentration dependent effect, diminishing gradually until the concentration 250 µM was reached. Subsequently ROS levels remained basically constant even with high concentrations (Fig. 2c).
TOLB itself did not modified ROS generation, whether or not there Aβ25–35 in the culture medium. However, when BSA was added TOLB increased ROS generation about 55%, with or without Aβ25–35 (Fig. 4c). The above mentioned increase induced by TOLB when BSA was added is concentration dependent, and no difference between neurons treated or not with Aβ25–35 was observed (Fig. 5c).
In absence of BSA, TOLB did not modify DIAZ effect, since there is no difference between cells which received TOLB from those that did not. When BSA was added, TOLB caused an increase on ROS generation, even in cells which were no exposed to Aβ25–35 (Fig. 3b).
DISCUSSION
Potassium channels are essentials for both, excitable and non excitable cells, in the control and regulation of different processes like membrane potential, cellular volume and secretion of different ions, hormones and neurotransmitters.
In brain mitochondrial membrane MitoKATP channels are 7 folds higher than in heart or in liver, indicating its important functions. It has been suggested that the activation of MitoKATP channels can potentially reduce cell death during hypoxia; however, the mechanism by which this occurs is still unclear [49–51]. It is supposed that MitoKATP channels of neurons are closed in the presence of physiological levels of intracellular ATP and open when ATP is depleted during hypoxia or metabolic damage [52].
Is been reported that AD neurons generate free radicals in mitochondrion, but is not clear yet how Aß induce ROS production [38]. Particularly, it has been described that Aβ25–35 can reach mitochondrion where it may cause the disarrangement of respiratory machinery. This can be accomplished by the occlusion of TIM23/TOM40 protein permease system resulting in the inhibition of protein import to mitochondrion [53]. In addition, Aβ interaction with the mitochondrion permeability transition pore may lead to the increase mitochondrial permeability and subsequent apoptosis [54]. Because of the former, MitoKATP channels may participate on toxic effect of Aβ25–35 on neurons in primary culture attributed to the increase in ROS levels.
The strategy to demonstrate this hypothesis was the use of substances which act on MitoKATP channels, such as DIAZ and TOLB. It is been demonstrated that DIAZ has a direct effect on mitochondrial membrane potential, Ca2+ transport, oxygen consumption and ATP generation in pancreatic β-cells and liver mitochondria, and is considered as one of the most potent openers of MitoKATP channels [55]. On the other hand, the union of TOLB with sulphonylureas receptor causes the close of MitoKATP channels, with the subsequent opening of calcium channels. Specifically in the brain a receptor for sulphonylureas has been described, which make brain MitoKATP channels sensitive to this kind of drugs.
To get started, it must be mentioned that the concentrations of Aβ25–35 and BSA employed in this experiments (30 µM) were selected after previous studies that showed the maximal toxic effect of Aβ25–35 as well as the maximal protective effect of BSA in our experimental conditions with those concentrations [23]. The morphological damage and the neuronal mortality caused by Aβ25–35, as well as the improvement on both parameters induced by BSA shown in Fig. 1a and/or Fig. 4a verify the former.
Neither DIAZ (Fig. 1a) nor TOLB (Fig. 4a) affected neuronal morphology or cellular viability, which means that those drugs had not toxic effects themselves. However, neither the toxic effect of Aβ25–35 nor the protective effect of BSA, were modified by these drugs either (Figs. 1a, 4a).
Opening MitoKATP channels by using DIAZ reduced ROS levels caused by the treatment with Aβ25–35, in a concentration dependent manner (Figs. 1c and 2c). It may be explained considering that Aß impairs ion-motive ATPase activities, such as sodium and calcium ATPases, causing membrane depolarization and calcium influx through activated NMDA-gated channels [24, 56–58]. Thus, activation of MitoKATP channels induced by DIAZ would decrease membrane depolarization and calcium influx to mitochondrion, thus ROS generation.
On the contrary, when BSA is added an increase in ROS levels was produced, in a very similar magnitude to the observed in neurons exposed only to Aβ25–35, meaning without BSA in the culture medium. It has been proposed that BSA form an equimolecular complex with Aβ25–35, which prevents the internalization of the peptide into the neurons, counteracting the ROS increase caused by the Aβ25–35 [23]. Since DIAZ bind highly to BSA [59], it may displace Aβ25–35 from the complex described above, which would result in the inability of DIAZ to open Mito KATP channels (since it is bounded to BSA) but also in free Aβ25–35, which may exert its toxic effect despite of BSA treatment. That could explain the opposite effects caused by DIAZ with and without BSA addition.
Besides its effects as MitoKATP opener, DIAZ also has antioxidant properties [60, 61]. Thus, described effects of DIAZ could be a consequence not only of its actions on MitoKATP channels but to its antioxidants effects. To explore this alternative, TOLB was used simultaneously with DIAZ. If observed DIAZ effects are result of its interaction with MitoKATP channels, it would be expected that DIAZ effects would be counteracted by TOLB.
According to Fig. 3b, without BSA TOLB did not reverse DIAZ effect on ROS, even when Aβ25–35 was added; nevertheless, if there is BSA in the culture medium, TOLB increased ROS generation. Such ROS increase would be the result of closing MitoKATP channels, which lead to higher intracellular calcium levels. Hence, at least in presence of BSA, TOLB reversed DIAZ effects, and could be assumed that described actions of DIAZ are the result of its effects on MitoKATP channels. However, the different ability of DIAZ and TOLB to cross the cell membrane because of its polarity and their different affinity by BSA must be considered, and did not allow clarify if DIAZ effects on ROS generation are a consequence of its antioxidant properties or to its interaction on MitoKATP channels when they are supplied together.
It must be realized that TOLB increased ROS in a concentration dependent manner only when BSA is added to the culture medium, with or without Aβ25–35 (Fig. 5c). It is considered as a consequence to the affinity of TOLB for BSA, so that TOLB can get into the neurons only if it is carried by BSA, at least enough concentration to close MitoKATP channels. That doesn’t seem to be the case with DIAZ, maybe because it is highly lipophilic.
The decrease in ROS levels observed in the group treated with Aβ25–35 when DIAZ was added, as well as the largest increase in this parameter induced by the peptide when was administrated together with TOLB in presence of BSA, suggest the participation of MitoKATP channels in the ROS generation induced by Aβ25–35. However, described changes had any repercussion on neuronal morphology or viability. In other words, neither the diminished ROS levels caused by DIAZ rescued neurons form toxic effect of Aβ25–35, nor the enhanced ROS production induced by TOLB increased cell mortality. This apparent paradox may be explained considering that toxicity may be a function of not only the kinetics of ROS generation but also the stability of the radical and its efficiency of transfer to lipids and proteins. These characteristics of the ROS likely depend on intra and intermolecular interactions that differ among peptides [62]. For example, nitrogen- and oxygen-centered radicals are normally very reactive and biologically toxic; however, the presence of sterically bulky or resonance-active ligands adjacent to the radical center converts the radical into an essentially inert species. Hence, the presence of a radical center does not imply toxicity independent of other chemical considerations. Thus, while several peptides may generate radicals, the toxicity of a radicalizing peptide depends on several factors.
COLCLUSIONS
MitoKATP channels are proposed to participate in ROS generation induced by Aβ25–35, although they do not seem to be the only ROS source responsible of the toxic effects of the peptide in neurons in primary culture. However, they do not seem to participate in the protective effect of BSA, which reinforce the hypothesis that BSA protection goes through an extraneuronal mechanism.
REFERENCES
Plant, L.D., Boyle, J.P., Smith, I.F., Peers, C., and Pearson, H.A., J. Neurosci., 2003, vol. 23, no. 13, pp. 5531–5535.
Koudinov, A.R. and Berezov, T.T., Acta Neurobiologiae Experimentalis, 2004, vol. 64, no, 1, pp. 71–79.
Puzzo, D., Privitera, L., Leznik, E., Fa, M., Staniszewski, A., Palmeri, A., and Arancio, O., J. Neurosci., 2008, vol. 28, no. 53, pp. 14537–14545.
Garcia-Osta, A. and Alberini, C.M., Learning & Memory, 2009, vol. 16, no. 4, pp. 267–272.
Morley, J.E., Farr, S.A., Banks, W.A., Johnson, S. N., Yamada, K.A., and Xu, L., J. Alzheimers. Dis., 2010, vol. 19, no. 2, pp. 441–449.
Cardenas-Aguayo, M. del C., Gomez-Virgilio, L., DeRosa, S., and Meraz-Rios, M.A., ACS Chemical Neuroscience, 2014, vol 5, no. 12, pp. 1178–1191.
Storck, S.E., Meister, S., Nahrath, J., Meissner, J.N., Schubert, N., Di Spiezio, A., Baches, S., Vandenbroucke, R.E., Bouter, Y., Prikulis, I., Korth, C., Weggen, S., Heimann, A., Schwaninger, M., Bayer, T.A., and Pietrzik, C.U., J. Clin. Invest., 2016, vol. 126, no. 1, pp. 123–136.
Braak, H. and Braak, E., Brain. Pathol., 1991, vol. 1, no. 3, pp. 213–216.
Mawuenyega, K.G., Sigurdson, W., Ovod, V., Munsell, L., Kasten, T., Morris, J.C., Yarasheski, K.E., and Bateman, R.J., Science, 2010, vol. 330, no. 6012, pp. 1774.
Masters, C.L. and Selkoe, D.J., Cold Spring Harbor Perspectives in Medicine, 2012, vol. 2, no. 6, a006262.
Lesne, S.E., International Journal of Cell Biology, 2013, 950783
Lyons, B., Friedrich, M., Raftery, M., and Truscott R., Analytical Chemistry, 2016, vol 88, no. 5, pp. 2675–2684.
Kang, J., Lemaire, H.G., Unterbeck, A., Salbaum, J.M., Masters, C.L., Grzeschik, K.H., Multhaup, G., Beyreuther, K., and Müller-Hill, B., Nature, 1987, vol. 325, no. 6106, pp. 733–736.
Zhang, G.L., Zhang, J., Li, S.F., Lei, L., Xie, H.Y., Deng, F., Feng, J.C., and Qi, J.S., Physiol. Behav., 2015, vol. 149, pp. 95–100.
Bergin, D.H. and Liu, P., Neuroscience, 2010, vol. 169, no. 2, pp. 794–811.
Kaneko, I., Morimoto, K., and Kubo, T., Neuroscience, 2001, vol. 104, no. 4, pp. 1003–1011.
Varadarajan, S., Kanski, J., Aksenova, M., Lauderback, C., and Butterfield, D.A., J. Am. Chem. Soc., 2001, vol. 123, no. 24, pp. 5625–5631.
Iversen, L.L., Mortishire-Smith, R.J., Pollack, S.J., and Shearman, M.S., Biochem. J., 1995, vol. 311, no. Pt. 1, pp. 1–16.
Pike, C.J., Walencewicz-Wasserman, A.J., Kosmoski. J., Cribbs, D.H., Glabe, C.G., and Cotman, C. W., J. Neurochem., 1995, vol. 64, no. 1, pp. 253–265.
Hughes, E., Burke, R.M., and Doig, A.J., J. Biol. Chem., 2000, vol. 275, no. 33, pp. 25109–25215.
Shearman, M.S., Ragan, C.I., and Iversen, L.L., Proc. Natl. Acad. Sci. U.S.A., 1994, vol. 91, no. 4, pp. 1470–1474.
Terzi, E., Holzemann, G., and Seelig, J., Biochemistry, 1994, vol. 33, no. 6, pp. 1345–1350.
Vega, L., Arroyo, A.A., Tabernero, A., and Medina, J.M., J. Alzheimers. Dis., 2009, vol 17, no. 4, pp. 795–805.
Benzi, G. and Moretti, A., Neurobiol. Aging, 1995, vol. 16, no. 4, pp. 661–674.
Multhaup, G., Ruppert, T., Schlicksupp, A., Hesse, L., Beher, D., Masters, C.L. and Beyreuther, K., Biochem. Pharmacol., 1997, vol. 54, no. 5, pp. 533–539.
Behl, C., Davis, J.B., Lesley, R., and Schubert, D., Cell, 1994, vol. 77, no. 6, pp. 817–827.
Harris, M.E., Hensley, K., Butterfield, D.A., Leedle, R.A., and Carney, J.M., Exp. Neurol., 1995, vol. 131, no. 2, pp. 193–202.
Malyshev, I.Y., Wiegant, F.A., Mashina, S.Y., Torshin, V.I., Goryacheva, A.V., Khomenko, I.P., Kruglov, S.V., Pokidyshev, D.A., Popkova, E.V., Pshennikova, M.G., Vlasova, M.A., Zelenina, O.M., and Manukhina, E.B., Med. Sci. Monit., 2005, vol. 11, no. 8, pp. HY31–HY38.
Kaminsky, Y.G., Marlatt, M.W., Smith, M.A., and Kosenko, E.A., Exp. Neurol., 2010, vol. 221, no. 1, pp. 26–37.
Gulyaeva, N.V. and Stepanichev, M.Y., Exp. Neurol., 2010, vol. 222, no. 1, pp. 6–9.
Popova, M.S. and Stepanichev, M.Y., Neurochemical Journal, 2008, vol. 2, no. 3, pp. 146–152.
Cheah, K.S. and Chance, B., Biochim. Biophys. Acta., 1970, vol. 223, no. 1, pp. 55–60.
Aliev, G., Smith, M.A., De la Torre, J.C., and Perry, G., Mitochondrion, 2004, vol. 4, nos. 5–6, pp. 649–663.
Glabe, C.G. and Kayed, R., Neurology, 2006, vol. 66, no. 2 Suppl 1, S74–S78.
Reddy, P.H. and Beal, M.F., Trends. Mol. Med., 2008, vol. 14, no. 2, pp. 45–53.
Reddy, P.H., Exp. Neurol., 2009, vol. 218, no. 2, pp. 286–292.
Sanz-Blasco, S., Valero, R.A., Rodriguez-Crespo, I., Villalobos, C. and Nunez, L., PLoS One, 2008, vol. 3, no. 7, e2718.
Manczak, M., Anekonda, T.S., Henson, E., Park, B.S., Quinn, J., and Reddy, P.H., Hum. Mol. Genet., 2006, vol. 15, no. 9, pp. 1437–1449.
Szabo, I. and Zoratti, M., Physiol. Rev., 2014, vol. 94, no. 2, pp. 519–608.
Zoratti, M., De Marchi, U., Gulbins, E., and Szabo, I., Biochim. Biophys. Acta, 2009, vol. 1787, no. 5, pp. 351–363.
Cotella, D., Hernandez-Enriquez, B., Wu, X., Li, R., Pan, Z., Leveille, J., Link, C.D., Oddo, S., and Sesti, F., J. Neurosci., 2012, vol. 32, no. 12, pp. 4133–4144.
Etcheberrigaray, R., Ito, E., Oka, K., Tofel-Grehl, B., Gibson, G.E., and Alkon, D. L., Proc. Natl. Acad. Sci. U.S.A., 1993, vol. 90, no. 17, pp 8209–8213.
De Silva, H.A., Aronson, J.K., Grahame-Smith, D.G., Jobst, K.A., and Smith, A.D., Lancet, 1998, vol. 352, no. 9140, pp. 1590–1593.
Ikeda, M., Dewar, D., and McCulloch, J., Brain. Res., 1991, vol. 567, no. 1, pp. 51–56.
Ma, G., Fu, Q., Zhang, Y., Gao, J., Jiang, J., Bi, A., Liu, K., Du, Y., Chen, C., Cui, Y. and Lu, L., Neurochem. Res., 2008, vol. 33, no. 7, pp. 1419–1424.
Tabernero, A., Bolaños, J.P., and Medina, J.M., Biochem. J., 1993, vol. 294, pp. 635–638.
Denizot, F. and Lang, R., J. Immunol. Methods, 1986, vol. 89, no. 2, pp. 271–277.
Rosenkranz, A.R., Schmaldienst, S., Stuhlmeier, K.M., Chen, W., Knapp, W., and Zlabinger, G. J., J. Immunol. Methods, 1992, vol. 156, no. 1, pp. 39–45.
Ballanyi, K., J. Exp. Biol., 2004, vol. 207, Pt. 18, pp. 3201–3212.
Fujimura, N., Tanaka, E., Yamamoto, S., Shigemori, M. and Higashi, H., Journal of Neurophysiology, 1997, vol. 77, no. 1, pp. 378–385.
Heron-Milhavet, L., Xue-Jun, Y., Vannucci, S.J., Wood, T. L., Willing, L. B., Stannard, B., Hernandez-Sanchez, C., Mobbs, C., Virsolvy, A., and LeRoith, D., Mol. Cell. Neurosci., 2004, vol. 25, no. 4, pp. 585–593.
Pissarek, M., Reichelt, C., Krauss, G.J, and Illes, P., Brain. Res., 1998, vol. 812, nos. 1–2, pp. 164–171.
Devi, L., Prabhu, B.M., Galati, D.F., Avadhani, N.G. and Anandatheerthavarada, H.K., J. Neurosci., 2006, vol. 26, no. 35, pp. 9057–9068.
Du, H. and Yan, S.S., Biochim. Biophys. Acta, 2010, vol. 1802, no. 1, pp. 198–204.
Grimmsmann, T. and Rustenbeck, I., Br. J. Pharmacol., 1998, vol. 123, no. 5, pp. 781–788.
Mattson, M.P., Tomaselli, K.J., and Rydel, R.E., Brain. Res., 1993, vol. 621, no. 1, pp. 35–49.
Weiss, J.H., Pike, C.J., and Cotman, C.W., J. Neurochem., 1994, vol. 62, no. 1, pp. 372–375.
Mark, R.J., Hensley, K., Butterfield, D.A., and Mattson, M.P., J. Neurosci., 1995, vol. 15, no. 9, pp. 6239–6249.
Sellers, E.M. and Koch-Weser, J., Biochem. Pharmacol., 1974, vol. 23, no. 3, pp. 553–566.
Quast, U., Fundam. Clin. Pharmacol., 1992, vol. 6, no. 7, pp. 279–293.
Alarcon, S., Hernandez, J., and Laorden, M.L., Gen. Pharmacol., 1995, vol. 26, no. 3, pp. 589–592.
Hensley, K., Carney, J.M., Mattson, M.P., Aksenova, M., Harris, M., Wu, J.F., Floyd, R.A. and Butterfield, D.A., Proc. Natl. Acad. Sci. U.S.A., 1994, vol. 91, no. 8, pp. 3270–3274.
ACKNOWLEDGMENTS
This work was supported by the Junta de Castilla y León, Spain, and by the Section of Postgraduate Studies and Research, Academic Secretary of National Polytechnic Institute, México.
Funding
No external funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interest.
Ethical approval. All applicable international, national and institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Lourdes A. Vega Rasgado, Urbieta, A.T. & Medina Jiménez, J.M. Influence of Mitochondrial ATP-Sensitive Potassium Channels on Toxic Effect of Amyloid-β 25–35. Neurochem. J. 14, 90–100 (2020). https://doi.org/10.1134/S181971242001016X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S181971242001016X