Abstract
Alpha-dystrobrevin (α-DB) has been described primarily as a cytoplasmic component of the dystrophin-glycoprotein complex in skeletal muscle cells. Isoforms of α-DB show different localization in cells and tissues; at basolateral membranes in epithelial cells, dystrobrevins mediate contact with the extracellular matrix, peripheral and transmembrane proteins and the filamentous actin cytoskeleton. Beside their structural role, α-DBs are assumed to be important in cell signalling and cell differentiation. We have primarily assessed the role of α-DB in two epithelial cell lines (MDCK I, HT 29), which represent different developmental stages and exhibit distinct permeability characteristics. Using a polyclonal anti-α-DB antibody, we have investigated its expression, localization and association with tight junction (TJ)- associated proteins (ZO-1, occludin) before and after protein kinase C (PKC) activation with phorbol myristate acetate. Distinct subsets of α-DB isoforms were detected in the two cell lines by immunoblotting. In both cell lines there was submembranous localization of α-DB both apically and basolaterally, shown with confocal imaging. PKC activation caused a reorganization of TJ, which was parallel to increased localization of α-DB to TJ areas, most pronounced in MDCK I cells. Moreover, actin and ZO-1 co-immunoprecipitated with a-DB, as displayed with immunoblotting. Our findings suggest that a-dystrobrevin specifically is associated with the tight junctions during their reorganization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dystrobrevin (DB) has been described as a cytoplasmic component of the dystrophin-glycoprotein complex (DGC) in skeletal muscle cells (Carr, Fischbach & Cohen, 1989; Wagner, Cohen & Huganir, 1993; Nawrotzki et al., 1998), The complex forms a multimolecular, transmembrane link between the intracellular cytoskeleton and the extracellular basal lamina, where it is believed to participate in the stabilization of the membrane during muscle contraction and relaxation (Koenig & Kunkel, 1990; Yoshida & Ozawa, 1990; Ibraghimov-Beskrovnaya et al., 1992; Sadoulet-Puccio et al., 1996). The DGC can be divided into three subcomplexes, i.e.the dystroglycan, the sarcoglycan, and the cytoplasmic complexes, the latter comprising dystrophin or utrophin, and DB (Roberts, 2001; Blake et al., 2002). Dystrophin, a flexible rod-like protein, is linked to extracellular laminin through the dystroglycan complex. Dystrophin also interacts with actin, thereby providing a link between extracellular matrix and the cytoskeleton. The sarcoglycan complex consists of four transmembrane glycoproteins, and sarcospans (Blake et al., 2002; Grady et al., 2003), which associate laterally with the dystroglycan complex and probably also with the cytoplasmic components dystrophin and DB (Metzinger et al, 1997; Yoshida et al., 1994, 2000).
Beside its structural role, DGC is assumed to play a role in signal transduction through association with the syntrophins, a family of adapter proteins. Some syntrophin domains are often found in cytoskeletal and signalling proteins, e.g., a PDZ-domain that binds, for instance, to voltage-gated sodium channels (Gee et al., 1998) and to neuronal nitric oxide synthase (nNOS) (Brenman et al., 1996). Several of the DGC proteins can also be tyrosine-phosphorylated (Peters et al., 1998; James et al., 2000).
DB was originally identified in the Torpedo californica electric organ as an 87-kDa phosphoprotein (Carr et al., 1989). In mammalian cells, the DB family of proteins is encoded by two different genes, coding for α-DB and β-DB, respectively. Both the α-DB and the β-DB genes encode different protein isoforms, generated by alternative splicing (Blake et al., 1996, 1998; Sadoulet-Puccio et al., 1996; Peters et al., 1997). DBs, except for α-DB 3, bind to dystrophin and utrophin via homologous domains at their C-termini (Blake et al., 1995; Loh et al., 2000). Furthermore, recent experiments suggest that the N-terminal region of DB binds to sarcoglycans (Yoshida et al., 2000).
The dystrophin-associated proteins, like DB, are highly expressed in brain and muscle but have also been found in other tissues, such as lung, kidney, testis, and intestine (Blake et al., 2002). Moreover, the individual isoforms of DB display distinct expression patterns in different tissues (Blake et al., 1996; Holzfeind et al., 1999; Yoshida et al., 2000). Studies of muscle cells have, for instance, shown that the alternatively spliced variants of DB are developmentally regulated, and it has been suggested that a multipromotor system was originally developed to allow the regulation of the DB gene in different cell types and/or in different developmental stages (Enigk & Maimone, 1999; Holzfeind et al., 1999).
The precise role of the DBs in relation to the role of the DGC is not known. Alpha-DB knock-out mice exhibit muscular defects, abnormal neuromuscular junctions, and malformed myotendinous junctions (Grady et al., 1999; Akaaboune et al., 2002), and it has been suggested that α-DBs play multiple roles in muscle cells as a consequence of distinct signalling or structural properties of the isoforms.
Some mammalian splice variants of DB have a unique C-terminus containing tyrosine phosphorylation consensus sites (PYCT), while being absent in others (Wagner et al., 1993; Balasubramanian, Fung & Huganir, 1998). The presence of the PYCT motif thus makes DBs a candidate for regulation by tyrosine phosphorylation. Actually, it was recently shown that the enhanced efficacy of α-DB 1 to prevent synaptic and myotendinous defects depended in part on its tyrosine phosphorylation (Grady et al., 2003) and in the promyelytic HL 60 cell line an isoform of α-DB was found to undergo tyrosine phosphorylation during granulocytic differentiation (Kulyte et al., 2002).
Recent findings have demonstrated that DGC play a role also in non-muscle tissues, e.g., epithelia. Loh and co-workers (Loh et al., 2000) identified several different DGC-like complexes in the kidney, distributed in a cell-type specific manner. Alpha-DB and β-DB were found to be associated with different members of the dystrophin and syntrophin family of proteins, implying that there are differences in their functions in the different renal cell types and it was suggested that the DGC-like complexes act as scaffolds for signalling molecules. In addition, a syntrophin/utrophin/DB complex has been identified in polarized epithelial cells (Kachinsky, Froehner & Milgram, 1999), and it has been speculated that the complex serves a structural role but may also be involved in the development of polarity in epithelial cells.
In epithelial cells, the tight junctions (TJs) are multiprotein structures in which transmembrane proteins link adjacent cells together, thereby forming the epithelial barrier. The TJ structure has been extensively studied over the last 20 years (Stevenson et al., 1986; Gonzalez-Mariscal et al., 2003; Matter & Balda, 2003). The structure is complex, as is its regulation, but protein kinase C (PKC) plays an important role by being able to phosphorylate several of the TJ components (Balda et al., 1993; Clarke et al., 2000; Gonzalez-Mariscal, Betanzos & Avila-Flores, 2000). The TJ is associated with the cytoskeleton and a chain of reactions goes between the TJ and other structures such as the adherence junction and the nucleus. The TJ also participates in proliferation and differentiation (Cereijido, Shoshani & Contreras, 2000). Accordingly, the TJ and the DGC multiprotein complexes are both linked to the cytoskeleton and are involved in cell signalling and epithelial differentiation. To find out whether these two complexes are associated, we have studied expression and localization of the DGC protein α-DB in two epithelial cell lines, HT 29 and MDCK I. These cells are in different developmental stages and exhibit distinct permeability characteristics. In a recent study we have demonstrated that protein kinase C activation in not fully differentiated HT 29 cells improves the barrier function (Sjo, Magnusson & Peterson, 2003). By contrast, in MDCK I cells PKC activation causes increased permeability and opening of the TJs. The results of the present study show that α-DB is expressed as several isoforms in HT 29 and MDCK I with a submembranous localization in control cells, whereas in cell layers reorganizing after PKC activation, α-DB appeared as clusters in close vicinity to TJ. The TJ- protein ZO-1 and actin were demonstrated in immunoprecipitates of α-DB from MDCK I and HT 29. These findings suggest that α-DB specifically is associated with the reorganizing TJ.
Materials and Methods
CELL CULTURE
The Madin Darby canine kidney cell line I (MDCK I) and the human colon carcinoma cell line HT 29 were used primarily. For a comparison, normal porcine LLC-PK1 cells of kidney origin and another human carcinoma cell line, small intestine-like Caco-2 cells, were tested. Cells £ were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % fetal bovine serum, 100 U/ml penicillin, 100 jig/ml streptomycin, and 4 mM L-glutamine (all from Invitrogen, Paisley, UK). To Caco-2 cell medium, standard concentrations of non-essential amino acids were added. To promote an epithelial phenotype of HT 29 cells, a medium with 25 mM galactose (Sigma, St Louis, MO) instead of glucose was used. Cells were maintained in tissue culture flasks (NUNC, Roskilde, Denmark) at 37°C in a humidified 5 % CO2 atmosphere. For experiments, cells were plated on collagen-treated cover slips (Biocoat, Becton Dickinson, Franklin Lakes, NJ) or on tissue culture plates (NUNC). The culture medium was exchanged every second day and cells were used after 5 days for MDCK I and LLC-PK1, and after 7 days for HT 29 and Caco-2. Caco-2 cells spontaneously develop into small intestine-like cells in DMEM (Neutra & Louvard, 1989; Pinto et al., 1983), and LLC-PK1 cells behave like MDCK I when grown under standard conditions in DMEM (Hull, Cherry & Weaver, 1976).
Protein kinase C (PKC) activation was achieved using 100 nM phorbol 12-myristate 13-acetate (PMA; Sigma), diluted in culture medium.
ANTIBODIES
Polyclonal rabbit anti-ZO-1 antibodies and monoclonal mouse anti-occludin antibodies were purchased from ZYMED Laboratories Inc. (San Fransisco, CA), polyclonal goat anti-actin antibodies from Santa Cruz Biotechnology (Leiden, The Netherlands), and monoclonal mouse anti-phosphotyrosine antibodies (4G10) from Upstate Biotechnology (Lake Placid, NY). Horse radish peroxidase (HRP)-conjugated antibodies against rabbit IgG and mouse IgG, respectively, were obtained from Dako AS (Glostrup, Denmark), and Alexa 488-conjugated antibodies against rabbit IgG and Alexa 594-conjugated antibodies against mouse IgG, from Molecular Probes (Eugene, OR).
Polyclonal antibodies against human α-dystrobrevin (α-DB) were generated in rabbits by using a synthetic peptide with a terminal cysteine coupled to keyhole-limpet hemocyanin, according to standard methods by Agrisera (Vannas, Sweden). The synthetic peptide used was EHEQASQPTPEKAQQ, corresponding to amino acids 439–453 of the C-terminal of human α-DB. The antibodies were affinity-purified to obtain the total immunoglobulin fraction before use in experiments (Kulyte et al., 2002).
IMMUNEFLUORESCENCE LABELLING OF CELLS AND CONFOCAL LASER SCANNING MICROSCOPY
MDCK 1 and HT 29 cells grown on collagen-treated glass coverslips were treated with 100 nM PMA for 0, 2, or 18 h, respectively. They were rinsed in Krebs-Ringer glucose buffer (KRG; in mM: 120 NaCl, 4.9 KCl, 1.2 MgSO4, 1.7 KH2PO4, 8.3 Na2HPO4, 10 glucose, and 1.0 CaCl2) and fixed for 15 min in 2.5 % paraformaldehyde (Sigma) at room temperature. After washing in phosphate-buffered saline (PBS; in mM: 147 NaCl, 2.7 KCl, 1 .5 KH2PO4, and 6.7 Na2HPO4 ), the cells were permeabilized in 0.2 % Triton X-100 (Sigma) for 5 min at room temperature and washed in PBS. After blocking with 1 % bovine serum albumin (BSA; Roche, Mannheim, Germany), 1 mM glycine (Amersham Pharmacia Biotech, Uppsala, Sweden), and 10 % normal swine serum (Dako AS) in PBS, the cells were incubated with antibodies against α-DB for 1 h at 37°C washed in PBS and incubated with antibodies against ZO-1 or occludin for 1 h at 37°C. Thereafter the cover slips were washed in PBS and incubated with Alexa-labelled anti-rabbit and anti-mouse antibodies for 1 h at 37°C, washed in PBS and mounted upside down on a 3-well microscope slide in ProLong mounting medium (Molecular Probes, Eugene, OR).
Three-dimensional localization of α-DB, ZO-1, and occludin was performed with confocal laser scanning microscopy (Sarastro 2000, Molecular Dynamics, Sunnyvale, CA). The 488 and the 514 nm wavelength of the argon ion laser were used for simultaneous excitation of AlexaFluor 488 and AlexaFluor 594, A dichroic mirror with the cut-off wavelength of 535 nm was used to separate excitation light from emitted light. The green and red emitted light were separated using a 565 nm dichroic mirror, and for specific detection of the green signal, a 545DF30 nm band-pass filter was used and for detection of the red signal, a 600 long-pass emission filterB usedl Vertical sections and horizontal sections at the apical part of the cells were collected, using a 60 × oil immersion objective (NA 1.4).
IMMUNOPRECIPITATION AND IMMUNOBLOT ANALYSIS
Monolayers of MDCK I and HT 29, grown on tissue culture plates, were exposed to 100 nM PMA for 0, 2, or 1 8 h. Immediately after treatment, the plates were placed on ice and cells washed with ice-cold PBS containing 1 mM sodium orthovanadate (Sigma). The cell proteins were extracted using lysis buffer containing 1 % NP 40 (Roche), 0.15 M NaCl, 10 mM HEPES, 25 mM NaF, and 2 mM EDTA. Immediately before use the lysis buffer was supplemented with 0.01 % benzonase (Merck, Spanga, Sweden), 1 mM sodium orthovanadate and a mixture of protease inhibitors (Complete; Roche). After a 30-min extraction with vigorous shaking the lysate was transferred to microtubes and insoluble cell particles pelleted by centrifugation at 15,000 × g for 10 min. The supernatant was collected as the NP-40 soluble fraction (NP-sol). The pellet was further homogenized with a Kontes homogenizer (Vineland, NY) and solubilized in lysis buffer containing 1 % sodium dodecyl sulphate (SDS; Amersham Pharmacia Biotech), and 1 mM sodium orthovanadate. After centrifugation at 1,5000 × g for min the supernatant was collected as the NP 40-insolubIe fraction (NP-ins). For electrophoresis, the proteins were dissolved in sample buffer (1.25 M Tris-HCl pH 6.8, 2 % SDS, 0.1 M dithiothreitol, 10 % glycerol, and 0.01 % bromophenol blue) and heated for 5 min at 95°C. For immunoprecipitation, the NP-sol and NP-ins were precleared with protein-A agarose beads and normal rabbit serum (0.25 ug/ml lysate), incubated with antibodies for 1 h, and again with protein-A agarose beads. Finally, beads were collected by cenmiugation at 1000 × g for 5 min and washed four times in lysis buffer. Extraction, centrifugation and immunoprecipitation were carried out at 4°C. The precipitates were then dissolved in sample buffer and heated for 5 min at 95°C.
SDS polyacrylamide gel electrophoresis was performed according to the method of Laemmli (Laemmli, 1970) using either a 10 % or a 4–12 % gel (Invitrogen), and the fractionated proteins were electroblotted (Towbin, Staehelin & Gordon, 1979) onto PVDF-membranes (Millipore, Stockholm, Sweden). After blocking with 3 % BSA in PBS with 0.05 % Tween 20 (PBS-T), membranes were incubated with primary antibodies, washed in PBS-T and incubated with HRP-conjugated secondary antibodies. The ECL Western blot analysis system (Amersham Pharmacia Biotech) was used to detect the antibodies. To perform a second immunodetection, antibodies were removed from the membranes with a stripping buffer (62.5 mM Tris HC1, pH 6.8, 2 % SDS, and 0.7 % mercaptoethanol) for 30 min at 50°C, followed by three washes with large volumes of PBS-T.
Results
SUBCELLULAR DISTRIBUTION OF α-DYSTROBREVIN ISOFORMS
Immunoblotting of NP 40-soluble fractions (NP-sol) of MDCKI cells using α-dystrobrevin (α-DB) antibodies revealed protein bands of immunoreactivity corresponding to molecular weights of 35 kDa, 42 kDa, 54 kDa, and 79 kDa, and additional faint bands at 4d kDa, 59 kDa, 67 kDa, and 115 kDa (Fig. 1 a). The NP 40-insoluble fractions (NP-ins) showed protein bands at 42 kDa and 79 kDa, and faint bands at 115 kDa (Fig. 1b). In both fractions of HT 29 cells, the antibody detected protein bands at 76 kDa and in NP-sol a supplementary band at 54 kDa (Fig. 1 c,d). In addition, faint protein bands were found in NP-sol, corresponding to 42 kDa, 59 kDa, and 105 kDa, and in NP-ins corresponding to 54 kDa, 67 kDa, and 105 kDa (Fig. 1 c,d). Treatment with PMA did not change the subcellular distribution of α-dystrobrevin in either cell line.
Immunoblots of MDCK I and HT 29 cells before and after PMA treatment for 2 h or 18 h, respectively, showing α-DB isoforms in NP 40 soluble (NP-sol) and insoluble (NP-ins) fractions. (a, b) In NP-sol of MDCK I cells four bands of immunoreactivity, at 35 kDa, 42 kDa, 54 kDa, and 79 kDa, are detected and the NP-ins show two bands at 42 kDa and 79 kDa. (c, d) In HT 29 cells, both fractions show protein bands at 76 kDa and the NP-sol shows a supplementary band at 54 kDa. Additional faint protein bands were detected by the antibody, as depicted in the figure. PMA-treatment did not change the band pattern in neither cell line. C = control cells.
IMMUNOFLUORESCENCE LOCALIZATION OF α-DYSTROBREVIN
Expression of α-DB in both MDCK I and HT 29 cells was found using confocal microscopy. Vertical sections of untreated cells displayed a diffuse pattern, localized to the basolateral and the apical submembranous areas of the cells (Fig. 2 a, d). In MDCK I cells, PMA treatment caused concentration of α-DB at lateral cell contacts (Fig. 2 b, c), while in HT 29 cells, the distribution of α-DB seemed to be unaffected by PMA treatment (Fig. 2 e, f).
Confocal vertical images displaying the distribution of α-DB in MDCK I and HT 29 before and after PMA-treatment for 2 h or 1 8 h, respectively. Cells grown on glass cover slips were exposed to PMA, fixed in paraformaldehyde and permeabilized in Triton X-100. They were labelled with antibodies to a-DB, and secondary antibodies conjugated with Alexa 488. The cover slips were mounted in ProLong mounting medium and viewed in a confocal laser scanning microscope. Apical parts are facing downwards (a, d) Vertical sections show basolateral and apical submembranous staining of α-DB in control cells of MDCK I and HT 29. (b, c) In MDCK I cells treated with PMA for 2 h, α-DB is concentrated at the lateral contacts between cells while 18-h treated MDCK I cells show decreased labelling of a-dystrobrevin. (e, f) PMA-treatment of HT 29 cells did not seem to change the distribution of a-DB. Box = 10 × 10
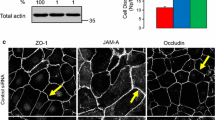
To more specifically determine whether there was also localization of α-DB at or near the tight junctions (TJs), untreated and PMA-treated cells were double-labelled with antibodies against α-DB and ZO-1. Untreated MDCK I cells displayed continuous ZO-1 staining at the TJs and a diffuse and spotty α-DB staining (Fig. 3 a). However, a 2 h treatment with PMA resulted in discontinuous ZO-1 labelling, where areas lacking ZO-1 labelling now showed a distinct labelling of α-DB near TJ areas (Fig. 3 b). In areas with ZO-1-labelled cell-cell contacts, α-DB and ZO-1 partially co-localized (Fig. 3 b). After an 18 h-treatment, the ZO-1-labelling at the TJs was strongly ruptured, and the cells also showed a decreased α-DB labelling (Fig, 3 c). Untreated HT 29 cells, known to have poorly developed TJs, displayed a mostly diffuse ZO-1 staining with few distinctly labelled cell-cell contacts and a diffuse α-DB staining (Fig. 3 d). PMA treatment for 2 h or 18 h of HT 29 cells increased the ZO-1 labelling of TJs, although not to the same extent as in untreated MDCK I cells. Cells with partly ZO-1-labelled cell borders displayed a distinct α-DB staining at the sites of cell-cell contacts adjacent to, and partially co-localized with, ZO-1 (Fig. 3 e, f).
Confocal horizontal images showing the distribution of α-DB and ZO-1 in MDCK I and HT 29 before and after PMA-treatment for 2 h and 18 h, respectively. Cells grown on glass cover slips were exposed to PMA, fixed in paraformaldehyde and permeabilized in Triton X-100. They were labelled with antibodies to α-DB and ZO-1, respectively, and secondary antibodies conjugated with Alexa 488 (green) for α-DB and Alexa 594 (red) for ZO-1. The cover slips were mounted in ProLong mounting medium and viewed in a confocal laser scanning microscope. (a) MDCK I control cells with continuous staining of ZO-1 display diffuse α-DB staining. (b) PMA-treated MDCK I cells display discontinuous staining of ZO-1 at the tight junctions and an accumulated α-dystrobrevin staining close to broken tight junction areas. (c) 18-h PMA treatment results in decreased labelling of both ZO-1 and α-DB in MDCK I cells. (d) HT 29 control cells have poorly developed tight junctions with few areas of ZO-1 staining and a diffuse α-DB staining. (e, f) In PMA-related HT 29 cells, there is increased staining of ZO-1 and a condensed α-DB staining at some cell-cell contacts. Bar = 10 μm.
The localization of α-DB was further examined, using occludin instead of ZO-1 as a marker for the TJ. The staining of occludin in MDCK I and HT 29 cells showed a similar pattern as the ZO-1 staining (Fig. 4). Thus, occludin in untreated MDCK I cells formed a honeycomb pattern at the apical part of the cells, which pattern was fragmented by PMA treatment. In HT 29 cells, PMA caused an increase in occludin localization at the TJ. α-DB showed a diffuse submembranous localization at the TJ area of untreated MDCK I cells, and PMA treatment resulted in accumulation of α-DB at the sites of fragmented occludin (Fig. 4 b). In PMA-treated HT 29 cells, α-DB was also concentrated to intercellular contacts close to occludin strands (Fig. 4 e, f). In both MDCK I and HT 29 cells, spots of co-localization between occludin and a-dystrobrevin were detected.
Confocal horizontal images displaying distribution of α-DB and occludin in MDCK I and HT 29 before and after PMA treatment for 2 h and 18 h, respectively. Cells grown on glass cover slips were exposed to PMA, fixed in paraformaldehyde and permeabilized in Triton X-100. They were labelled with antibodies to α-DB and occludin, respectively, and secondary antibodies conjugated with Alexa 488 (green) for α-DB and Alexa 594 (red) for ZO-1. The cover slips were mounted in ProLong mounting medium and viewed in a confocal laser scanning microscope. (a) MDCK I control cells show continuous staining of occludin and a diffuse α-DB staining (b) PMA-treated MDCK I cells display a scattered staining of occludin at the tight junctions and an accumulated α-dystrobrevin staining close to broken tight junction areas. (c) 18-h PMA treatment results in decreased labelling of both occludin and α-DB in MDCK I cells. (d) HT 29 control cells have poorly developed tight junctions with few areas of occludin staining and a diffuse α-DB staining. (e, f) PMA-treated HT 29 cells show increased staining of occludin and a condensed α-DB staining at apical cell-cell contacts. Bar = μm.
CO-PRECIPITATION OF α-DYSTROBREVIN WITH ZO-1 AND ACTIN
To investigate its association with TJ, α-DB immunoprecipitates were analyzed by immunoblotting with antibodies against ZO-1. The presence of α-DB and actin in the immunoprecipitates was also examined. Alpha-DB protein bands corresponding to molecular weights of 79 kDa and 76 kDa were enriched from MDCK I and HT 29 cells, respectively (Fig. 5 a, b). In addition, in HT 29 cells, α-DBs with the molecular weights of 67 kDa and 105 kDa were detected (Fig. 5 b). The intense protein bands at 54 kDa in all lanes probably represent both α-DB and the heavy chains of the immunoglobulin used for immunoprecipitation. ZO-1 co-precipitated with α-DB in NP-ins of untreated MDCK I cells (Fig. 5 c). Cells treated with PMA for 2 h had an increased amount of ZO-1 in the α-DB precipitate compared to untreated cells and cells treated for 18 h with PMA (Fig. 5 c). Co-precipitation of ZO-l with α-DB was also detected in the NP-ins of HT 29 cells, but PMA treatment did not seem to change the amount of precipitated ZO-1 (Fig. 5 d). In NP-sol of MDCK I and HT 29, no protein bands of ZO-1 were detected. Actin co-precipitated with α-DB in NP-sol and NP-ins of both HT 29 and MDCK I (Fig. 5 e, f).
Immunoblots showing co-precipitation of ZO-1 and actin with α-DB, and tyrosine phosphorylation of α-DB isoforms. For immunoprecipitation, cells were grown in tissue culture plates and treated with PMA for 2 h or 18 h, respectively. α-DB was immunoprecipitated from NP-40 detergent soluble (NP-sol) and insoluble (NP-ins) fractions and α-DB, ZO-1, actin and phosphotyrosine were detected by electrophoresis and immunoblotting. (a, b) Two isoforms of a-DB, with the molecular weights 59 kDa and 67 kDa are enriched in both fractions of MDCKI and HT 29. The intense protein bands at 54 kDa probably represent both α-DB and the heavy chains of IgG. Supplementary bands corresponding to molecular weights of 76 kDa and 105 kDa are detected in the NP-ins of HT 29. The NP-ins of MDCK I cells show faint bands at 79 kDa. (c, d) ZO-1 co-precipitates with α-DB in NP-ins of MDCK I and to a lesser degree in the NP-ins of HT 29. (e, f) Co-precipitation of actin with α-dystrobrevin. (g, h) In HT 29, weak bands of phosphotyrosine proteins were detected at 76 kDa in the NP-ins, and strong bands at 54 kDa and 67 kDa in both fractions. Phosphotyrosine bands were also detected at 54, 67, and 79 kDa in all lanes of MDCK 1. C = control cells; M = molecular mass standard; DB = α-dystrobrevin; ptyr = tyrosine-phosphorylated proteins; * marks the rabbit IgG heavy chain.
PHOSPHORYLATION STATE OF α-DYSTROBREVIN ISOFORMS
Alpha-DB immunoprecipitates from MDCK I and HT 29 were analyzed by immunoblotting with antibodies to phosphotyrosine. In both fractions of MDCK I and HT 29, protein bands were detected at 54 kDa and 67 kDa (Fig. 5 g, h) and in the NP-ins of HT 29, additional weak bands were seen at 76 kDa, representing tyrosine-phosphorylated α-DB 1 (Fig. 5 h). PMA treatment of MDCK I cells decreased the tyrosine phosphorylation of α-DB in the NP-ins (Fig. 5 g), but did not change the apparent amount of tyrosine-phosphorylated α-DB in the NP-sol and in the two fractions of HT 29 (Fig. 5 g, h).
COMPARISON WITH OTHER EPITHELIAL CELL LINES
TER measurements and immunofluorescence experiments were repeated with two additional cell lines, i.e. LLC-PK1 and Caco-2 (figures not shown). PKC activation by PMA decreased TER in LLC-PK1 and also in Caco-2, although more slowly. In LLC-PK1 cells, PKC activation for 2 h change neither the ZO-1 pattern nor the subcellular distribution of α-DB. On the contrary, in Caco-2 cells, PKC activation for 2 h caused fragmentation of the ZO-1 staining and clustering of α-DB close to, and in some cells co-localized with ZO-1.
Discussion
In this paper we have assessed the expression and distribution of α-dystrobrevins (α-DBs) in the epithelial cell lines MDCKI and HT 29, before and after activation of protein kinase C (PKC). One major difference between the two cell lines is their barrier characteristics; MDCK I cell monolayers form a very tight barrier (measured as high TER and low permeability to molecules), while HT 29 cell monolayers form a leaky barrier (low TER and high permeability). This suggests that the cells, being of kidney and intestinal origin, have different functions and are at different developmental stages. Accordingly, we have recently reported that PKC activation with the phorbolester PMA elicits distinct responses in the two cell lines, viz. disassembly or assembly of tight junctions (TJ), respectively (Sjo et al., 2003). The α-DBs are expressed as several isoforms by alternative splicing (Blake et al., 1996; Sadoulet-Puccio et al., 1996). They have been grouped into three classes (α-DB 1, α-DB 2, and α-DB 3), each consisting of multiple splice variants (Enigk & Maimone, 1999). In mouse skeletal muscle, the molecular weights of the isoform classes are 94 kDa, 62 kDa, and 42 kDa (Nawrotzki et al., 1998). In the present study, the molecular masses of α-DBs found in MDCK I cells and HT 29 cells were 79/76 kDa and 54 kDa (Fig. 1), probably corresponding to splice variants of α-DB 1 and α-DB 2, respectively. The discrepancies in the molecular sizes of α-DBs could also reflect varying degrees of phosphorylation since some variants of α-DB have multiple phosphorylation sites. The 79/76 kDa protein was represented in both the NP40-soluble and -insoluble fractions, while the 54 kDa protein was concentrated to the NP40-soluble fractions. This indicates that the 79/76 kDa isoform of α-DB has a stronger association to detergent-insoluble protein complexes, such as the TJ. Additional isoforms, with molecular masses of 35 kDa and 42 kDa, were detected by the antibody and might represent splice variants of α-DB 3. The faint bands probably represent additional splice variants or possibly modified proteins or degraded products of α-DB isoforms. PKC activation by PMA did not change the immunoblot band patterns or the detergent solubility of the α-DBs.
DB was originally discovered as a member of the dystrophin-associated complex in skeletal muscle cells (Blake et al., 2002). Later, DB-containing complexes have been discovered in epithelial cells where they were found to localize to the basal or basolateral region (Kachinsky et al., 1999; Loh et al., 2000). In the former studies, the DB isoform was proposed to belong to the β-DB family. In the present investigation, using α-DB antibodies (Kulyte et al., 2002), we demonstrate both basolateral and apical submembranous localization of α-DB in epithelial cells (Fig. 2). First, these results suggest that a-DB-containing complexes exist at the basolateral membrane area, possibly mediating contact with the extracellular matrix and adjacent cells via transmembrane proteins and the filamentous actin cytoskeleton (Koenig & Kunkel, 1990; Yoshida & Ozawa, 1990). Earlier, it has also been demonstrated that the DGC-protein dystroglycan binds to laminin, a component of the basement membrane that plays an important role in the differentiation of epithelia (Durbeej et al., 1995; Ekblom et al., 1998; Klein et al., 1988). Accordingly, in our study we detected co-precipitation of α-DB and actin (Fig. 5 e, f). Secondly, our results propose that a-DB-containing complexes exist at the apical membranes of the epithelial cells. These complexes might serve as signalling and/or stabilizing components in the membrane, e.g., scaffolds for receptors and ion channels, but their specific role remains to be elucidated.
Expression of α-DB isoforms has been described predominantly in muscle cells (Nawrotzki et al., 1998; Peters et al., 1998), while it is sparsely investigated in epithelia. However, α-DB 1 has been found in the renal cortex of mouse kidney where it forms a complex with the dystrophin homologue utrophin (Loh et al., 2000). In addition, the two α-DB isoforms, 65 and 87 kDa, were detected in ciliary epithelial cells (Ueda et al., 2000). Interestingly, Kachinsky et al. report that syntrophin preparations from MDCK II cells contained only one DB isoform, the 65 kDa protein, and this isoform was believed to be β-DB (Kachinsky et al., 1999). Thus, the α-DB isoforms detected in MDCK I in our study were not found in the syntrophin preparation by Kachinsky. Taken together, these results suggest that α-DBs in epithelia exist in multiple distinct DGC-like complexes, possibly binding to specific dystrophin- and syntrophin-like proteins, not yet identified.
By labelling the MDCK I cells with antibodies to α-DB and the TJ-associated proteins ZO-1 and occludin, respectively, we found continuously labelled bands at the TJs and a diffuse submembranous localization of a-DB, usually not co-localized with ZO-1 or occludin (Fig. 3a and 4a). However, disassembly of TJs in MDCK I cells, as revealed by a scattered staining of ZO-1 and occludin, caused a concentration of α-DB at the membrane that partially co-localized with ZO-1 and occludin (Fig. 2b , 3b and 4b). On the contrary, in HT 29 cells, it was Tj assembly that was accompanied with increased localization of α-DB to apical cell-cell contacts (Fig. 3e, f and 4e, f). Accordingly, the condensation of α-DB at cell-cell contacts and its co-localization with ZO-1 and occludin correlate with reorganizing TJs. To further examine the association of α-dystrobrevin with TJ, we repeated the immunofluorescence experiment with two additional cell lines, i.e., LLC-PK1, originating from pig kidney, and Caco 2, a commonly used human colon cancer cell line. In LLC-PK1 cells, PKC activation for 2 h did neither change the ZO-1 pattern nor the subcellular distribution of a-DB. On the contrary, in Caco-2 cells, PKC activation for 2 h caused fragmentation of the ZO-1 staining and clustering of α-DB close to, and in some cells co-localized with ZO-1. We conclude that our results with LLC-PK1 and Caco 2 support our proposition that α-dystrobrevin accumulates at or near reorganizing TJ.
The presence of tyrosine phosphorylated α-DBs in either cell line (Fig. 5g, h), and the PKC-induced change in tyrosine phosphorylation in MDCK I cells, suggest that α-DB has a signalling as well as a structural role in epithelial cells. Its putative role in the PKC-induced regulation of the epithelial barrier needs to be further investigated. It has recently been reported that PKC-induced modulation of cell-cell adhesion involves activation also of the epidermal growth factor receptor (Barbosa et al., 2003). The latter has actually been found in the sarcoglycans of the DGC (McNally et al., 1996), suggesting that they participate in growth regulation and signalling processes.
In muscle cells, α-DBs 1 and 2 bind to syntrophins, which have been shown to be associated with a variety of signalling molecules via their PDZ domain, forming homo- or heterodimers (Brenman et al., 1996; Gee et al., 1998; Schultz et al., 1998; Hasegawa et al., 1999). In epithelial cells, the only binding molecule that has been identified for syntrophin is β-DB (Kachinsky et al., 1999), but possibly α-DB associates with isoforms of syntrophin or syntrophin-like proteins, not yet identified, thereby recruiting signalling molecules via their PDZ domain. At the TJ of epithelial cells, numerous PDZ-containing proteins have been found (Gonzalez-Mariscal et al., 2003). ZO-1 is such a PDZ-protein, making it a possible candidate for association to syntrophin. In our study co-precipitation of α-DB and ZO-1 was seen in the NP 40-insoluble fractions of MDCK I and HT 29, indicating that these proteins co-exist in multiprotein complexes (Fig. 5 c, d). Taken together, our findings suggest that α-DB is associated with reorganizing TJ via ZO-1 and occludin. Whether the α-DB isoforms also associate with other TJ proteins during different stages of epithelial formation and maintenance remains to be elucidated.
This research was financially supported by the King Gustaf Vth 80-year Foundation, the Nanna Schwartz Foundation, the Swedish Research Council (VR:M and VR:NT), the Magn. Bergwall Foundation, and theLars Hiertas Minne Foundation. We are very grateful to Agné Kulyté, Center for Genomics and Bioinformatics, Karolinska Institute, Stockholm, Sweden, for providing antibodies to α-dystrobrevin.
References
M. Akaaboune R.M. Grady S. Turney J.R. Sanes J.W. Lichtman (2002) ArticleTitleNeurotransmitter receptor dynamics studied in vivo by reversible photo-unbinding of fluorescent ligands Neuron 34 865–876
S. Balasubramanian E.T. Fung R.L. Huganir (1998) ArticleTitleCharacterization of the tyrosine phosphorylation and distribution of dystrobrevin isoforms FEBS Lett. 432 133–140
M.S. Balda L. Gonzalez-Mariscal K. Matter M. Cereijido J.M. Anderson (1993) ArticleTitleAssembly of the tight junction: the role of diacylglycerol J. Cell Biol. 123 293–302
L.A. Barbosa L. Goto-Silva P.A. Redondo S. Oliveira G. Montesano W. Souza ParticleDe J.A. Morgado-Diaz (2003) ArticleTitleTPA-induced signal transduction; a link between PKC and EGFR signaling modulates the assembly of intercellular junctions in Caco-2 cells Cell Tissue 312 319–331
D.J. Blake R. Nawrotzki N.Y. Loh D.C. Gorecki K.E. Davies (1998) ArticleTitleBeta-dystrobrevin, a member of the dystrophin-related protein family Proc. Natl. Acad. Sci. USA 95 241–246
D.J. Blake R. Nawrotzki M.F. Peters S.C. Froehner K.E. Davies (1996) ArticleTitleIsoform diversity of dystrobrevin, the murine 87-kDa postsynaptic protein J. Biol. Chem. 271 7802–7810
D.J. Blake J.M. Tinsley K.E. Davies A.E. Knight S.J. Winder J. Kendrick-Jones (1995) ArticleTitleCoiled-coil regions in the carboxy-terminal domains of dystrophin and related proteins: potentials for protein-protein interactions Trends Biochem. Sci. 20 133–135
D.J. Blake A. Weir S.E. Newey K.E. Davies (2002) ArticleTitleFunction and genetics of dystrophin and dystrophin-related proteins in muscle Physiol. Rev. 82 291–329
J.E. Brenman D.S. Chao S.H. Gee A.W. McGee S.E. Craven D.R. Santillano Z. Wu F. Huang H. Xia M.F. Peters S.C. Froehner D.S. Bredt (1996) ArticleTitleInteraction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alphal-syntrophin mediated by PDZ domains Cell 84 757–767
C. Carr G.D. Fischbach J.B. Cohen (1989) ArticleTitleA novel 87,000-Mr protein associated with acetylcholine receptors in Torpedo electric organ and vertebrate skeletal muscle J. Cell Biol. 109 1753–1764
M. Cereijido L. Shoshani R.G. Contreras (2000) ArticleTitleMolecular physiology and pathophysiology of tight junctions. I. Biogenesis of tight junctions and epithelial polarity Am. J. Physiol. 279 G477–G482
H. Clarke C.W. Marano A. Peralta Soler J.M. Mullin (2000) ArticleTitleModification of tight junction function by protein kinase C isoforms Adv. Drug. Deliv. Rev. 41 283–301
M. Durbeej E. Larsson O. Ibraghimov-Beskrovnaya S.L. Roberds K.P. Campbell P. Ekblom (1995) ArticleTitleNon-muscle alpha-dystroglycan is involved in epithelial development J. Cell Biol. 130 79–91
M. Ekblom M. Falk K. Salmivirta M. Durbeej P. Ekblom (1998) ArticleTitleLaminin isoforms and epithelial development Ann. N.Y. Acad. Sci. 857 194–211
R.E. Enigk M.M. Maimone (1999) ArticleTitleDifferential expression and developmental regulation of a novel alpha-dystrobrevin isoform in muscle Gene 238 479–488
S.H. Gee R. Madhavan S.R. Levinson J.H. Caldwell R. Sealock S.C. Froehner (1998) ArticleTitleInteraction of muscle and brain sodium channels with multiple members of the syntrophin family of dystrophin-associated proteins J. Neurosci. 18 128–137
L. Gonzalez-Mariscal A. Betanzos A. Avila-Flores (2000) ArticleTitleMAGUK proteins: structure and role in the tight junction Semin. Cell Dev. Biol. 11 315–324
L. Gonzalez-Mariscal A. Betanzos P. Nava B.E. Jaramillo (2003) ArticleTitleTight junction proteins Prog. Biophys. Mol. Biol. 81 1–44
R.M. Grady M. Akaaboune A.L. Cohen M.M. Maimone J.W. Lichtman J.R. Sanes (2003) ArticleTitleTyrosine-phosphorylatcd and nonphosphorylated isoforms of alpha-dystrobrevin: roles in skeletal muscle and its neuromuscular and myotendinous junctions J. Cell Biol. 160 741–752
R.M. Grady R.W. Grange K.S. Lau M.M. Maimone M.C. Nichol J.T. Stull J.R. Sanes (1999) ArticleTitleRole for alpha-dystrobrevin in the pathogenesis of dystrophin-dependent muscular dystrophies Nat. Cell Biol. 1 215–220
R.M. Grady H. Zhou J.M. Cunningham M.D. Henry K.P. Campbell J.R. Sanes (2000) ArticleTitleMaturation and maintenance of the neuromuscular synapse: genetic evidence for roles of the dystrophin–glycoprotein complex Neuron 25 279–293
M. Hasegawa A. Cuenda M.G. Spillantini G.M. Thomas V. Buee-Scherrer P. Cohen M. Goedert (1999) ArticleTitleStress-activated protein kinase-3 interacts with the PDZ domain of alpha1-syntrophin. A mechanism for specific substrate recognition J. Biol. Chem. 274 12626–12631
P.J. Holzfeind H.J. Ambrose S.E. Newey R.A. Nawrotzki D.J. Blake K.E. Davies (1999) ArticleTitleTissue-selective expression of alpha-dystrobrevin is determined by multiple promoters J. Bio. Chem. 274 6250–6258
R.N. Hull W.R. Cherry G.W. Weaver (1976) ArticleTitleThe origin and characteristics of a pig kidney cell strain, LLC-PK In Vitro 12 670–677
O. Ibraghimov-Beskrovnaya J.M. Ervasti C.J. Leveille C.A. Slaughter S.W. Sernett K.P. Campbell (1992) ArticleTitlePrimary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix Nature 355 696–702
M. James A. Nuttall J.L. Ilsley K. Ottersbach J.M. Tinsley M. Sudol S.J. Winder (2000) ArticleTitleAdhesion-dependent tyrosine phosphorylation of (beta)-dystroglycan regulates its interaction with utrophin J. Cell Sci. 113 1717–1726
A.M. Kachinsky S.C. Froehner S.L. Milgram (1999) ArticleTitleA PDZ-containing scaffold related to the dystrophin complex at the basolateral membrane of epithelial cells J. Cell Biol. 145 391–402
G. Klein M. Langegger R. Timpl P. Ekblom (1988) ArticleTitleRole of laminin A chain in the development of epithelial cell polarity Cell 55 331–341
M. Koenig L M. Kunkel (1990) ArticleTitleDetailed analysis of the repeat domain of dystrophin reveals four potential hinge segments that may confer flexibility J. Biol. Chem. 265 4560–4566
A. Kulyte R. Navakauskiene G. Treigyte A. Gineitis T. Bergman K.E. Magnusson (2002) ArticleTitleCharacterization of human alpha-dystrobrevin isoforms in HL-60 human promyelocytic leukemia cells undergoing granulocytic differentiation Mol. Biol. Cell 13 4195–4205
U.K. Laemmli (1970) ArticleTitleCleavage of structural proteins during the assembly of the head of bacteriophage T4 Nature 227 680–685
N.Y. Loh S.E. Newey K.E. Davies D.J. Blake (2000) ArticleTitleAssembly of multiple dystrobrevin-containing complexes in the kidney J. Cell Sci. 113 2715–2724
K. Matter M.S. Balda (2003) ArticleTitleSignalling to and from tight junctions Nat. Rev. Mol. Cell Biol. 4 225–236
E.M. McNally D. Duggan J.R. Gorospe C.G. Bonnemann M. Fanin E. Pegoraro H.G. Lidov S. Noguchi E. Ozawa R.S. Finkel R.P. Cruse C. Angelini L.M. Kunkel E.P. Hoffman (1996) ArticleTitleMutations that disrupt the carboxyl-terminus of gamma-sarcoglycan cause muscular dystrophy Hum. Mol. Genes. 5 1841–1847
L. Metzinger D.J. Blake M.V. Squier L.V. Anderson A.E. Deconinck R. Nawrotzki D. Hilton- Jones K.E. Davies (1997) ArticleTitleDystrobrevin deficiency at the sarcolemma of patients with muscular dystrophy Hum. Mol. Genet. 6 1185–1191
R. Nawrotzki N.Y. Loh M.A. Ruegg K.E. Davies D.J Blake (1998) ArticleTitleCharacterisation of alpha-dystrobrevin in muscle J. Cell. Sci. 111 2595–2605
M. Neutra D. Louvard (1989) Differentiation of Intestinal Cells in Vitro Alan R. Liss, Inc. New York
M.F. Peters K.F. O’Brien H.M. Sadoulet-Puccio L.M. Kunkel M.E. Adams S.C. Froehner (1997) ArticleTitleBeta-dystrobrevin, a new member of the dystrophin family. Identification, cloning, and protein associations J. Biol. Chem. 212 31561–31569
M.F. Peters H.M. Sadoulet-Puccio MR. Grady N.R. Kramarcy L.M. Kunkel J.R. Sanes R. Sealock S.C. Froehner (1998) ArticleTitleDifferential membrane localization and intermolecular associations of alpha-dystrobrevin isoforms in skeletal muscle J. Cell Biol. 142 1269–1278
M. Pinto S Robine-Leon et al. (1983) ArticleTitleEnterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture Biol. Cell 47 323–330
Roberts R.G. 2001. Dystrophins and dystrobrevins.Genome Biol. 2REVIEWS3006
H.M. Sadoulet-Puccio T.S. Khurana J.B. Cohen L.M. Kunkel (1996) ArticleTitleCloning and characterization of the human homologue of a dystrophin related phosphoprotein found at the Torpedo electric organ post-synaptic membrane Hum. Mol. Genet. 589 489–496
J. Schultz U. Hoffmuller G. Krause J. Ashurst M.J. Macias P. Schmieder J. Schneider-Mergener H. Oschkinat (1998) ArticleTitleSpecific interactions between the syntrophin PDZ domain and voltage-gated sodium channels Nat. Struct. Biol. 5 19–24
A. Sjo K.E. Magnusson K.H. Peterson (2003) ArticleTitleDistinct effects of protein kinase C on the barrier function at different developmental stages Biosci. Rep. 23 87–102
B.R. Stevenson J.D. Siliciano M.S. Mooseker D.A. Goodenough (1986) ArticleTitleIdentification of ZO-1 : a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia J. Cell Biol. 103 755–766
H. Towbin T. Staehelin J. Gordon (1979) ArticleTitleElectrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications Proc. Natl. Acad. Sci. USA 76 4350–4354
H. Ueda T. Baba K. Kashiwagi H. lijima S. Ohno (2000) ArticleTitleDystrobrevin localization in photoreceptor axon terminals and at blood-ocular barrier sites Invest. Ophthalmol. Vis. Sci. 41 3908–3914
K.R. Wagner J.B. Cohen R.L. Huganir (1993) ArticleTitleThe 87K postsynaptic membrane protein from Torpedo is a protein-tyrosine kinase substrate homologous to dystrophin Neuron 10 511–522
M. Yoshida H. Hama M. Ishikawa-Sakurai M. Imamura Y. Mizuno K. Araishi E. Wakabayashi-Takai S. Noguchi T. Sasaoka E. Ozawa (2000) ArticleTitleBiochemical evidence for association of dystrobrevin with the sarcoglycan-sarcospan complex as a basis for understanding sarcoglycanopathy Hum. Mol. Genet. 9 1033–1040
M. Yoshida E. Ozawa (1990) ArticleTitleGlycoprotein complex anchoring dystrophin to sarcolemma J. Biochem. (Tokyo) 108 748–752
M. Yoshida A. Suzuki H. Yamamoto S. Noguchi Y. Mizuno E. Ozawa (1994) ArticleTitleDissociation of the complex of dystrophin and its associated proteins into several unique groups by n-octyl beta-D-glucoside Eur. J. Biochem. 222 1055–1061
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sjö, A., Magnusson, K. & Peterson, K. Association of α-Dystrobrevin with Reorganizing Tight Junctions. J Membrane Biol 203, 21–30 (2005). https://doi.org/10.1007/s00232-004-0728-1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00232-004-0728-1