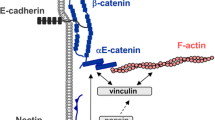
Abstract
Adhesion, segregation, and cellular plasticity are regulated by actin filaments anchored at the plaques of adherens junctions, sites of mechanical stabilization, and interfaces of multiple signaling networks. Drebrins were originally identified in neuronal cells, but the isoform drebrin E was also detected at adherens junctions of a wide range of non-neuronal cells, including polarized epithelia, endothelia, and fibroblasts. Here the protein is enriched at actin filament bundles associated with junctional plaques. Polarized epithelial cells contain two types of actin-associated complexes, one comprising drebrin but not vinculin and the other involving vinculin, but not drebrin. At gap junctions drebrin interacts with connexin 43, stabilizes this protein at membranes, and links it to the actin cytoskeleton. In vivo drebrin is widespread in diverse non-neuronal tissues of epithelial, endothelial, and smooth muscle origin, but not ubiquitous. In intestinal cells it is involved in cell compaction, linking of actin filaments to microtubules and formation and stabilization of the terminal web. Upregulation of drebrin was noted in several types of cancers, e.g., basal cell carcinomas for which it may serve as marker, liver metastases of colon carcinomas, and bladder cancer, suggesting that it is involved in regulating actin dynamics during tumor development, progression, and metastasis.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The ability of cells to communicate and adhere to each other is indispensable for the formation and maintenance of multicellular organisms. Adhesion, segregation, and cellular plasticity are regulated by actin filaments associated with specific domains of the plasma membrane. Among these, adherens junctions (zonulae adhaerentes) are the most prominent structures anchoring actin filaments. In adherens junctions intercellular contacts are mediated by classical cadherins, calcium-dependent transmembrane proteins that may form homophilic interactions with identical cadherins on neighboring cells, or heterophilic interactions with other classical cadherins on different types of cells. At their cytoplasmic tail, cadherins are anchored in a plaque in which they bind to β-catenin , protein p120, and plakoglobin. In turn, β-catenin is linked to α-catenin, which connects adherens junctions to the actin cytoskeleton. In addition to their mechanically stabilizing function, adherens junctions have emerged as important regulators of tissue morphogenesis and plasticity and as the interface of multiple signaling networks (Takeichi 2014). These networks are required for coupling and detachment of cadherin-catenin complexes from actin filaments, allowing cell sorting, migration, and invasion. Remodeling of adherens junctions induces reshaping of cells in various ways, including, e.g., apical constriction of polarized cells driven by actomyosin contraction. The plasticity of actin filaments is regulated by Rho GTPases and their effectors as well by a large number of actin-binding proteins accumulated at adherens junctions and other sites of the actin cytoskeleton, including drebrin.
2 Detection of Drebrin in Diverse Non-Neuronal Cells
Drebrins were initially identified in the brain and considered to be, by and large, brain-specific. However, brain specificity has only been documented for the adult isoform drebin A. Signals of mRNA corresponding to embryonic drebrin isoforms have been reported in several non-neuronal tissues and cell cultures (Fisher et al. 1994). Furthermore, drebrin has been identified in F-actin overlay assays among the actin-binding proteins of fibroblasts and carcinoma cells (Luna et al. 1997).
The first studies investigating the occurrence and distribution of drebrin outside the brain in greater detail were performed by our group (Peitsch et al. 1999) and by Keon et al. (2000). In immunoblot experiments, drebrin was detected in a wide range of non-neuronal cell lines, including cultures from simple epithelia such as human primary liver carcinoma (PLC), human cervix adenocarcinoma (HeLa), human mammary carcinoma (MCF-7) and human colonic adenocarcinoma (CaCo-2), primary human keratinocytes, human SV80 and bovine B1 fibroblasts and endothelial cell cultures from human umbilical vein (HUVEC), human umbilical artery (HUAEC), and human dermal microvasculature (HDMEC) (Peitsch et al. 1999, 2005; Keon et al. 2000). However, according to the observations of both groups, the human squamous carcinoma line A-431 did not contain any drebrin at the level of sensitivity applied (Peitsch et al. 1999; Keon et al. 2000).
Immunoblotting of murine and bovine tissues revealed drebrin-positive reactions in bovine muzzle epithelium, lingual mucosa, kidney, and aortic endothelium (Peitsch et al. 1999) as well as in the stomach and kidney of adult mice (Keon et al. 2000). Furthermore, lower amounts of drebrin were found in murine colon and bladder and trace amounts in the heart, lung, liver, and epididymis (Keon et al. 2000). By contrast, the protein was not detectable in murine duodenum and small intestine and in bovine skeletal muscle (Peitsch et al. 1999; Keon et al. 2000). Taken together, these results indicate that drebrin is widespread but not ubiquitous outside the nervous system.
The protein detected by immunoblot was unambiguously identified as drebrin in coimmunoprecipitation experiments followed by mass spectrometry (Peitsch et al. 1999; Keon et al. 2000). The isoform expressed in non-neuronal cells was determined to be drebrin E (Keon et al. 2000).
3 Drebrin Enrichment at Plaques of Adherens Junctions
Immunostaining of different epithelial and endothelial monolayer cell cultures showed drebrin reactions along cell–cell junctions and, occasionally, small drebrin-positive cytoplasmic dots in the cytoplasm (Peitsch et al. 1999, 2005; Keon et al. 2000). When the cells were double-labeled with drebrin antibodies and phalloidin to visualize actin filaments, colocalization of drebrin and actin was observed at adherens junctions (zonulae adhaerentes, Fig. 18.1a, a′), whereas cytoplasmic actin filament bundles (“stress fibers”) were free of drebrin (Fig. 18.1b, b′; Peitsch et al. 1999; Keon et al. 2000).
Double-label fluorescence confocal laser microscopy of cultured MDBK cells, labeled with antibodies against drebrin (a, b, c, red) in combination with phalloidin (a′, b′, green) or with desmoplakin antibodies (c, green). Different focal plains of the same cell are shown in (a) and (b). In the subapical region (a), drebrin is enriched along the plasma membrane, colocalizing with actin, whereas the stress fibers of actin seen in a more central plain (b) are devoid of drebrin. Staining patterns observed with drebrin and desmoplakin antibodies are mutually exclusive (c). Bars: 10 μm. Source: Peitsch et al. (1999)
The localization of drebrin in comparison to other actin-binding proteins enriched at cell–cell contacts was analyzed by double-label confocal fluorescence laser scanning microscopy . Double immunostaining of epithelial cells for drebrin and the desmosomal marker protein desmoplakin revealed differential distribution patterns, with desmoplakin-positive punctate staining at desmosomes and drebrin reactions in distinct streaks and dot- or whisker-like structures along cell boundaries (Fig. 18.1c). Plaque proteins of adherens junctions , e.g., α- and β-catenin, protein p120 and α-actinin, colocalized with drebrin to some extent (Peitsch et al. 1999). The same observation was made when the drebrin-negative cell line A-431 was stably transfected with drebrin-EGFP constructs. In transfectants containing moderate or low amounts of drebrin-EGFP, the fusion protein was recruited to adherens junctions where it was partially codistributed with the plaque proteins (Fig. 18.2a–a″, b–b″) and showed some overlap with E-cadherin (Fig. 18.2c–c″; Peitsch et al. 2005). By contrast, transfectants overexpressing drebin-EGFP formed numerous actin- and drebrin-rich cell processes (Peitsch et al. 2005, 2006). Similar observations were made upon stable transfection of Madin–Darby canine kidney (MDCK) cells (Keon et al. 2000), indicating that drebrin accumulates at junctional plaques of non-neuronal cells when expressed in moderate or low levels, whereas overexpression of the protein induces extensive morphological changes characterized by formation of cell processes, as noted in neuronal cells and fibroblasts (Shirao et al. 1992; Hayashi and Shirao 1999).
Accumulation of drebrin-EGFP fusion proteins close to adherens junctions. A-431 cells that do not contain endogenous drebrin were stably transfected with drebin-EGFP-C1 constructs (green in a–d), immunostained with antibodies against components of adherens junctions (red in a′–d′) and analyzed by confocal laser scanning microscopy. The drebrin constructs are concentrated along cell–cell contacts, but some drebrin-transfected cells also exhibit numerous small drebrin-rich cell processes (asterisks in a, a″, and d″). Plakoglobin (a′, a″), β-catenin (b′, b″) and E-cadherin (c′, c″) partially colocalize with drebrin-EGFP along intercellular contacts. Vinculin antibodies label focal contacts (d′), which are free of drebrin (d″, merge). Bars: 20 μm. Source: Peitsch et al. (2005)
Remarkably, antibodies against drebrin and vinculin , an actin-binding protein occurring both at adherens junctions and at cell-matrix adhesions, reacted in mutually exclusive pattern (Figs. 18.2d–d″ and 18.3). When cells derived from simple epithelia, e.g., Madin–Darby bovine kidney (MDBK) or PLC cells, were double labeled with drebrin and vinculin antibodies and investigated by confocal laser scanning microscopy, vinculin was enriched at focal adhesions in the near-bottom plane (Fig. 18.3a, b). More apically, it appeared in linear cortical structures at adherens junctions (Fig. 18.3c–f). Drebrin immunoreactions were also seen along adherens junctions, but the vinculin-positive domains were consistently drebrin-negative (Fig. 18.3a–f). Focal adhesions were free of drebrin, even if it has been reported that expression of drebrin in cDNA-transformed fibroblasts results in increased assembly of vinculin in adhesion plaques (Ikeda et al. 1996). Thus, polarized epithelial cells appear to contain two actin-associated complexes, one comprising drebrin but no—or very little—vinculin and the other involving vinculin, but not drebrin. Whether this differential localization of drebrin and vinculin correlates with the specific patterns of other plaque components and is of functional relevance remains to be investigated.
Confocal laser scanning microscopy of MDBK cells double-stained with antibodies to drebrin (red) and vinculin (green), focussing from the bottom (a) through central regions (b) to more apical plains containing the adherens junction (c–f). Vinculin is accumulated in focal adhesions at the bottom, in puncta adhaerentia in central regions (b) and at adherens junctions (c–f) in the apical region. Drebrin reactions appear in streaks or whisker-like structures along cell–cell contacts in all optical plains and sometimes additionally in small cytoplasmic dots (a–f). The distribution of drebrin and vinculin is strikingly different, suggesting existence of two actin filament systems, one associated with drebrin and the other with vinculin. A deconvolution procedure was used to increase the resolution on the Z-axis. Bars: 10 μm. Source: Peitsch et al. (1999)
As a component of endothelial adherens junctions, drebrin plays a crucial role in maintaining endothelial integrity under vascular flow conditions (Rehm et al. 2013; see Chap. 23). Drebrin knockdown was demonstrated to cause weakening of adherens junctions and loss of nectin from these sites. Stabilization of nectin at adherens junctions is achieved through a chain of interactions requiring binding of drebrin to actin, interactions between drebrin and afadin through their polyproline and PR1-2 domains, and interactions between afadin and nectin (Rehm et al. 2013). These processes are described in greater detail in Chap. 23.
4 Functions of Drebrin in Polarized Cells of the Gastrointestinal Tract
When immunoblot analysis was performed to examine the expression pattern of drebrin in murine tissues, particularly high amounts of the protein were detected in stomach (Keon et al. 2000). Immunofluorescence microscopy revealed specific enrichment of drebrin in acid-secreting parietal cells (Keon et al. 2000; Chew et al. 2005). Drebrin-positive reactions were particularly pronounced at the apical membrane of the intracellular canaliculi and in microvilli (Keon et al. 2000; Chew et al. 2005). The protein was differentially distributed in parietal cells along the gland axis, indicating that it may serve as a marker of parietal cell differentiation and supporting the notion that different populations of parietal cells are functionally heterogeneous (Chew et al. 2005). In parietal cells, activation of HCl secretion by cAMP-dependent agonists such as histamine involves distinct morphological changes, in particular, formation and elongation of actin-rich microvilli within intracellular canaliculi, which are the sites of acid secretion. Overexpression of epitope-tagged drebrin E induced formation of microspikes and F-actin-rich ringlike structures in cultured parietal cells and suppressed cAMP-dependent acid secretory responses (Chew et al. 2005). In MDCK cells, coexpression of epitope-tagged drebrin and the Rho family GTPase Cdc42, known to induce filopodial extension , resulted in additional elongation of the microspikes. However, coexpression of drebrin and dominant negative Cdc42 did not preclude drebrin-induced microspike formation, indicating that drebrin can induce the formation of actin-rich membrane projections both by Cdc42-dependent and by Cdc42-independent mechanisms (Chew et al. 2005).
Intestinal epithelial cells differentiate in the course of their migration along the crypt-villus axis, become elongated, and form an apical brush border of microvilli which are anchored in a dense actin-based network, the so-called terminal web . In highly polarized columnar human intestinal cells of line CaCo2/TC7 and in human enterocytes drebrin was found to be accumulated in puncta along the lateral membrane and in a dense network in the subapical region (Bazellières et al. 2012). Depletion of drebrin E affected neither cell polarity nor the composition and function of tight junctions. However, cell compaction and elongation processes were significantly impaired, i.e., drebrin-depleted cells were flatter and larger than controls. Drebrin knockdown blocked accumulation of actin in the subapical network, indicating that drebrin is crucial for formation and stabilization of the terminal web (Bazellières et al. 2012). Furthermore, myosin-IIB and βII-spectrin were downregulated, and apical accumulation of these two proteins was blocked upon drebrin depletion. The microvilli of drebrin-depleted cells were considerably less dense and disorganized, indicating that drebrin is required for brush border formation.
In polarized epithelial cells, a dense microtubular network runs parallel to the terminal web. Drebrin binds to the microtubule-plus-end-binding protein EB3 and tethers actin filaments and microtubules together by this way in growth cones (Geraldo et al. 2008). In CaCo2 cells transfected with an EB3-GFP construct drebrin, myosin-IIB and βII-spectrin coimmunoprecipitated with EB3 (Bazellières et al. 2012). However, in drebrin-depleted CaCo2, neither myosin-IIB nor βII-spectrin could be coprecipitated with this protein, proving that drebrin is indispensable for formation of the complex. In addition, accumulation of EB3 in the subapical region was disturbed upon drebrin depletion. Taken together, these observations suggest that the complex of drebrin, EB3, myosin-IIB , and βII-spectrin links F-actin and microtubule networks in the subapical region of polarized intestinal cells. Drebrin contributes to stabilizing the actin-based terminal web and promotes cell compaction dependent on myosin-IIB but not on EB3 (Bazellières et al. 2012).
Drebrin depletions also modify the distribution of several other apical marker proteins in CaCo2 cells, including the digestive enzyme dipeptidyl peptidase IV (DPPIV) which is specific for the brush border (Vacca et al. 2014). Instead of accumulating at the apical surface, DPPIV was redistributed to an enlarged subapical compartment related to lysosomes upon drebrin knockdown.
Mutations in the MYO VB gene encoding the motor protein MyoVb cause microvillar inclusion disease, a rare genetic disease characterized by intestinal malabsorption. In the apical recycling pathway, MyoVb interacts with Rab8a, which serves as a linker to transport vesicles. Conditional knockout of Rab8a leads to redistribution of apical membrane proteins into a lysosomal compartment and decrease in their protein expression levels. Interestingly, CaCo2 cells depleted of drebrin E shared several features with Rab8a-depleted cells and with the phenotype observed in microvillar inclusion disease (Vacca et al. 2014). In summary, these findings suggest that drebrin redirects the apical recycling pathway of intestinal epithelial cells to the lysosomes and that this protein is an important regulator of apical trafficking.
5 Drebrin as Binding Partner of Connexin 43 at Gap Junctions
Gap junctions are transmembrane channels that mediate intercellular communication by allowing the passage of ions, metabolites, and second messengers. They are formed by hexameric assemblies of six connexins, which align head to head between neighboring cells. Connexins are composed of four transmembrane helices, two extracellular loops kept together by disulfide linkages, and three variable cytoplasmic domains. Their functional importance is underscored by the fact that connexin defects can cause a wide range of human diseases. Connexin 43 is the main connexin of gap junctions in heart and in several other tissues; its null mutations in mice lead to lethal cardiac malformations.
In a screen for interaction partners of connexin 43, drebrin was identified as connexin 43 binding protein in murine brain membrane fractions (Butkevich et al. 2004). Drebrin was recovered in a pull-down assay with a GST-fusion protein encompassing the COOH-terminal domain of connexin 43. Double-label immunofluorescence microscopy revealed colocalization of drebin and connexin 43 in astrocytes and Vero cells, a kidney epithelial cell line from African green monkey. Drebrin was found to be accumulated in the plasma membrane fraction of Vero cells and to coimmunoprecipitate with connexin 43 from this fraction (Butkevich et al. 2004). Moreover, interactions between both proteins were confirmed by live-cell fluorescence resonance energy transfer (FRET) analyses of COS cells transfected with CFP-drebrin and connexin 43-YFP. Silencing of drebrin by siRNA resulted in internalization and degradation of connexin 43, and whole-cell voltage clamp recordings showed a marked decrease of cell–cell permeability upon drebrin knockdown. Conversely, overexpression of drebrin led to stabilization of gap junctions. Taken together, these findings indicate that drebrin stabilizes connexin 43 at the plasma membrane and thereby facilitates cell–cell coupling (Butkevich et al. 2004).
Based on these findings, it can be speculated that drebrin serves as a linker between connexin 43 and F-actin. In order to examine the relationship between the E-cadherin/α- and β-catenin/actin system and the connexin 43/drebrin/actin system, Vero cells were cotransfected with GFP-E-cadherin, connexin 43-CFP, and YFP-drebrin, and the fluorescent signals were linearly separated. The initial contact between two cells was found to be mediated by E-cadherin . Connexin 43 was recruited to cell–cell contacts only after a stable interface had been established. At this later stage, drebrin colocalized more with connexin 43 than with E-cadherin . This colocalization was maintained even after treatment with Latrunculin B , suggesting that actin filaments are not crucial for the interaction between drebrin and connexin 43 once stable gap junctions have been formed (Butkevich et al. 2004).
Drebrin may interact simultaneously with connexin 43 and actin via its N-terminal ADF domain and its proline-rich binding domains. Therefore, the connexin 43/drebrin complex may serve as an interface between extracellular signals and cellular responses mediated by rearrangement of the submembranous actin cytoskeleton (Majoul et al. 2007).
The composition and function of gap junctions may be modified by components of the extracellular matrix, in particular, by laminin-integrin interactions . When the impact of laminin-111 on connexin 43 was studied in mouse embryonic stem cells, laminin-111 was found to reduce intercellular communication through gap junctions and to enhance cell proliferation (Suh et al. 2012). Laminin-111 stimulated the dissociation of the connexin 43 /ZO-1 complex, followed by disruption of the connexin 43/drebrin and the connexin 43/actin complexes. This process was reversed by inhibition of RhoA. In summary, laminin-111 appears to decrease gap junction intercellular communication via increased RhoA-dependent phosphorylation of connexin 43, resulting in instability of the connexin 43/drebrin complex and increased degradation of connexin 43 (Suh et al. 2012).
6 Drebrin Accumulations at Junctional Plaques of Diverse Cell Types and Tissues In Vivo
To examine the distribution of drebrin in a broad range of non-neuronal cell types and organs, sections of bovine and murine tissues, including lactating udder, testis, kidney, aorta, vena cava, tongue, snout, lung, retina, heart, liver, stomach and intestine, and human tissues, including epidermis, scalp, umbilical cord, testis, and colon carcinoma, were immunostained with drebrin antibodies. In bovine udder, myoepithelial cells were markedly immunoreactive, but drebrin staining was also noted along cell–cell contacts of secretory cells. Bovine intestinal epithelium and human colon adenocarcinoma were similarly immunostained. In bovine and human testis, cell–cell contacts of Sertoli cells were particularly rich in drebrin (Peitsch et al. 1999).
In human, bovine and murine kidneys drebrin antibodies stained both glomeruli and certain tubular epithelial cells, in particular, acid-secreting type A intercalated cells and distal tubule epithelia (Peitsch et al. 1999; Keon et al. 2000, see also Chap. 20). In acid-secreting intercalated cells , drebrin was specifically accumulated at the apical plasma membrane. In distal tubule epithelia, the protein was concentrated at the luminal surface and at interdigitations of the basolateral membranes.
Stratified epithelia , e.g., squamous epithelium of bovine snout and lingual mucosa as well as the pseudostratified bronchial epithelium, showed variable drebrin reactions. The protein was sometimes enriched at cell–cell contacts, however, with variable intensity, and occasionally predominant or exclusive staining of the basal layer was noted (Peitsch et al. 1999).
Normal human epidermis was essentially drebrin-negative (Fig. 18.4a). Drebrin was, however, concentrated at the ducts of eccrine sweat glands, particularly along their cell–cell junctions (Fig. 18.4b). In addition, intercellular junctions of hair follicles were intensely drebrin-positive both in the bulbs (Fig. 18.4c) and, in upper parts, in the companion layer and in the outer root sheath (Fig. 18.4d), whereas the inner root sheath did not contain significant amounts of drebrin (Peitsch et al. 2005).
Distribution of drebrin in normal human skin. Normal human epidermis (bracket in a) is virtually devoid of drebrin, but drebrin-positive reactions are noted in the endothelial cells of small blood vessels (asterisks in a and b). Prominent staining is observed in the ducts of eccrine sweat glands (b), particularly along the cell–cell contacts. Drebrin is also concentrated at the intercellular junctions of hair follicles, both in the bulb (c) and in the outer root sheath (d). (a′–d′): phase contrast micrographs. Bars: 25 μm. Source: Peitsch et al. (2005)
Furthermore, drebrin was sometimes detected in the endothelium of large blood vessels, including bovine aorta and vena cava and human umbilical vein and artery. Smooth muscle cells of the vascular walls as well as vasa vasorum were also drebrin-positive, but the intensity of the reaction was rather variable. In bovine retina the most intense drebrin immunoreaction was observed in the inner plexiform layer, while the zonula limitans externa, comprising densely aligned junctions between photoreceptor and Müller glia cells, was drebrin-negative (Peitsch et al. 1999).
Bovine and human skeletal and cardiac muscles were consistently negative for drebrin, in accordance with results from immunoblot analyses. Moreover, bovine and human hepatocytes did not display any drebrin immunoreactivity at the level of sensitivity applied (Peitsch et al. 1999).
Corresponding to the findings obtained in cultured cells, drebrin is a common but not ubiquitous component of cell–cell junctions in diverse human, bovine, and murine cell types and tissues in vivo. The principle underlying the distribution of drebrin in mammalian cells and tissues is still unclear, and it is so far unexplained why certain polarized cells are particularly rich in drebrin whereas others are devoid of the protein.
7 Role of Drebrin During Carcinogenesis
Since expression of several junction-associated proteins is altered during development and progression of tumors, the distribution of drebrin was examined in skin cancers and their precursors (Peitsch et al. 2005). With an annual incidence around 100 per 100,000 inhabitants, basal cell carcinomas are the most common skin carcinomas of the Caucasian population. They are considered as semimalignant tumors because they locally invade and destroy tissues but metastasize only extremely rarely. Basal cell carcinomas consistently showed intense drebrin-positive immunoreactions, which were sometimes enhanced in the periphery of the tumors (Fig. 18.5a). Immunoelectron microscopy revealed enrichment of drebrin in the actin filament network underneath the plaques of adherens junctions, even if β-catenin examined for control was located even closer to the membrane.
Immunolocalization of drebrin in epithelial skin tumors. In basal cell carcinoma (BCC, a), intense drebrin staining is seen throughout the tumor, whereas the overlying epidermis appears drebrin-negative (brackets). In squamous cell carcinoma (SCC, b) and keratoacanthomas (c, d) drebrin antibodies react in heterogeneous patterns, showing both strongly drebrin-positive (b, c) and drebrin-negative compartments (b, d). Two different parts of the same keratoacanthoma, both containing horn pearls (HP), are displayed in (c) and (d). Asterisks: drebrin-positive blood vessels. (a′–d′): phase contrast images. Bars: 25 μm. Source: Peitsch et al. (2005)
Both basal cell carcinomas and two types of benign neoplasms, trichoblastoma and trichoepithelioma, are thought to be derived from follicular germinative cells. These tumors are sometimes difficult to distinguish by routine histology. When drebrin immunostaining was compared in the three kinds of tumors, basal cell carcinomas showed intense and homogeneous immunoreactions mainly at cell–cell boundaries. By contrast, trichoblastomas and trichoepitheliomas were only weakly and heterogeneously stained (Mizutani et al. 2014). Therefore, drebrin may be a useful marker for differentiating basal cell carcinomas from the two types of benign neoplasms.
In contrast to the strong and mostly homogeneous drebrin immunostaining observed in basal cell carcinomas, squamous carcinoma cells exhibited heterogeneous drebrin staining (Peitsch et al. 2005). Certain parts of the tumors showed rather intense reactions, whereas others appeared to be drebrin-negative (Fig. 18.5b). Drebrin-positive areas were often seen at the invasive tumor front or near horn pearls of differentiated squamous carcinomas, but there was no correlation between expression of drebrin and proliferation or differentiation. The same phenomenon as in squamous cell carcinomas , i.e., mosaicism of drebrin-rich and drebrin-free tumor regions, was noted in keratoacanthomas (Fig. 18.5c, d) and in precancerous lesions such as actinic keratoses. According to immunoblot analyses, normal human skin and scalp contained comparatively low amounts of drebrin, whereas the protein was markedly upregulated in basal and squamous cell carcinomas as well as in other tumors such as melanoma metastases and leiomyosarcomas (Peitsch et al. 2005).
Moreover, there is evidence that drebrin is involved in dissemination of colorectal carcinoma . In proteome profiles comparing the human colon adenocarcinoma cell line HCT-116 to its metastatic derivative E1 with iTRAQ labeling technology, drebrin was noted to be markedly overexpressed in the metastatic derivative (Lin et al. 2014). This finding was confirmed by immunoblot as well as by immunohistochemistry of colorectal cancer tissues with matched lymph node and liver metastasis tissues. Primary colorectal carcinomas were drebrin-negative in the majority of cases. By contrast, all lymph node and liver metastases examined stained positive for drebrin (Lin et al. 2014).
Drebrin was also demonstrated to promote the development and progression of bladder carcinoma by interacting with progranulin . The autocrine growth factor progranulin is known to induce proliferation, transformation, and invasion of several types of cancer, including urothelial cancer. In a pull-down assay with recombinant progranulin and extracts from bladder cancer cells, drebrin was identified as a novel progranulin-interacting protein, a finding confirmed by coimmunoprecipitation (Xu et al. 2015). Drebrin was shown to play a critical role for progranulin-dependent activation of the Akt and MAPK pathways which induce migration and invasion. Silencing of the protein resulted in impaired migration, decreased invasion, reduced colony forming in a soft agar assay, and impaired tumor formation in mouse xenograft models (Xu et al. 2015). In addition, expression of drebrin was found to be upregulated in bladder cancers compared to normal bladder tissue. In summary, these data indicate that drebrin exerts an important function in regulating progranulin activity in bladder carcinomas (Xu et al. 2015).
Development and progression of tumors is associated with rearrangements of the actin cytoskeleton during tumor cell migration, invasion, and metastasis. The finding that drebrin is upregulated in several kinds of tumors or their metastases implies that it plays an important role in regulating actin dynamics during these processes.
8 Conclusion
Drebrin E is a widespread component of the actin filament system in non-neuronal cells in vitro and in vivo. In polarized epithelial and endothelial cells, it has to be added to the list of actin-binding proteins regulating actin dynamics at adherens junction. At gap junctions drebrin interacts with connexin 43. Upregulation of drebrin in several kinds of carcinomas implies a role in regulating actin dynamics during tumor development, progression, and metastasis. Since it is markedly enriched and homogeneously distributed in particular tumors such as basal cell carcinomas, it may serve as marker for their detection and differential diagnosis.
References
Bazellières E, Massey-Harroche D, Barthélémy-Requin M, Richard F, Arsanto JP, Le Bivic A (2012) Apico-basal elongation requires a drebrin-E-EB3 complex in columnar human epithelial cells. J Cell Sci 125:919–931
Butkevich E, Hülsmann S, Wenzel D, Shirao T, Duden R, Majoul I (2004) Drebrin is a novel connexin-43 binding partner that links gap junctions to the submembrane cytoskeleton. Curr Biol 14:650–658
Chew CS, Okamoto CT, Chen X, Thomas R (2005) Drebrin E2 is differentially expressed and phosphorylated in parietal cells in the gastric mucosa. Am J Physiol Gastrointest Liver Physiol 289:G320–G331
Fisher LW, McBride OW, Filpula D, Ibaraki K, Young MR (1994) Human drebrin (DBNl): cDNA sequence, mRNA tissue distribution and chromosomal localization. Neurosci Res Commun 14:35–42
Geraldo S, Khanzada UK, Parsons M, Chilton JK, Gordon-Weeks PR (2008) Targeting of the F-actin-binding protein drebrin by the microtubule plus-tip protein EB3 is required for neuritogenesis. Nat Cell Biol 10:1181–1189
Hayashi K, Shirao T (1999) Change in the shape of dendritic spines caused by overexpression of drebrin in cultured cortical neurons. J Neurosci 19:3918–3925
Ikeda K, Kaub PA, Asada H, Uyemura K, Toya S, Shirao T (1996) Stabilization of adhesion plaques by the expression of drebrin A in fibroblasts. Brain Res Dev Brain Res 91:227–236
Keon BH, Jedrzejewski PT, Paul DL, Goodenough DA (2000) Isoform specific expression of the neuronal F-actin binding protein, drebrin, in specialized cells of stomach and kidney epithelia. J Cell Sci 113:325–336
Lin Q, Tan HT, Lim TK, Khoo A, Lim KH, Chung MC (2014) iTRAQ analysis of colorectal cancer cell lines suggests Drebrin (DBN1) is overexpressed during liver metastasis. Proteomics 14:1434–1443
Luna EJ, Pestonjamasp KN, Cheney RE, Strassel CP, TH L, Chia CP, Hitt AL, Fechheimer M, Furthmayr H, Mooseker MS (1997) Actin-binding membrane proteins identified by F-actin blot overlays. Soc Gen Physiol Ser 52:3–18
Majoul I, Shirao T, Sekino Y, Duden R (2007) Many faces of drebrin: from building dendritic spines and stabilizing gap junctions to shaping neurite-like cell processes. Histochem Cell Biol 127:355–361
Mizutani Y, Iwamoto I, Kanoh H, Seishima M, Nagata K (2014) Expression of drebrin, an actin binding protein, in basal cell carcinoma, trichoblastoma and trichoepithelioma. Histol Histopathol 29:757–766
Peitsch WK, Grund C, Kuhn C, Schnölzer M, Spring H, Schmelz M, Franke WW (1999) Drebrin is a widespread actin-associating protein enriched at junctional plaques, defining a specific microfilament anchorage system in polar epithelial cells. Eur J Cell Biol 78:767–778
Peitsch WK, Hofmann I, Bulkescher J, Hergt M, Spring H, Bleyl U, Goerdt S, Franke WW (2005) Drebrin, an actin-binding, cell-type characteristic protein: induction and localization in epithelial skin tumors and cultured keratinocytes. J Investig Dermatol 125:761–774
Peitsch WK, Bulkescher J, Spring H, Hofmann I, Goerdt S, Franke WW (2006) Dynamics of the actin-binding protein drebrin in motile cells and definition of a juxtanuclear drebrin-enriched zone. Exp Cell Res 312:2605–2618
Rehm K, Panzer L, van Vliet V, Genot E, Linder S (2013) Drebrin preserves endothelial integrity by stabilizing nectin at adherens junctions. J Cell Sci 126:3756–3769
Shirao T, Kojima N, Obata K (1992) Cloning of drebrin A and induction of neurite-like processes in drebrin-transfected cells. Neuroreport 3:109–112
Suh HN, Kim MO, Han HJ (2012) Laminin-111 stimulates proliferation of mouse embryonic stem cells through a reduction of gap junctional intercellular communication via RhoA-mediated Cx43 phosphorylation and dissociation of Cx43/ZO-1/drebrin complex. Stem Cells Dev 21:2058–2070
Takeichi M (2014) Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. Nat Rev Mol Cell Biol 15:397–410
Vacca B, Bazellières E, Nouar R, Harada A, Massey-Harroche D, Le Bivic A (2014) Drebrin E depletion in human intestinal epithelial cells mimics Rab8a loss of function. Hum Mol Genet 23:2834–2346
Xu SQ, Buraschi S, Morcavallo A, Genua M, Shirao T, Peiper SC, Gomella LG, Birbe R, Belfiore A, Iozzo RV, Morrione A (2015) A novel role for drebrin in regulating progranulin bioactivity in bladder cancer. Oncotarget 6:10825–10839
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Japan KK
About this chapter
Cite this chapter
Ludwig-Peitsch, W.K. (2017). Drebrin at Junctional Plaques. In: Shirao, T., Sekino, Y. (eds) Drebrin. Advances in Experimental Medicine and Biology, vol 1006. Springer, Tokyo. https://doi.org/10.1007/978-4-431-56550-5_18
Download citation
DOI: https://doi.org/10.1007/978-4-431-56550-5_18
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-56548-2
Online ISBN: 978-4-431-56550-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)