Abstract
Purpose
The aim of the present study was to evaluate the safety, pharmacokinetic (PK) and pharmacodynamic (PD) properties of remimazolam besylate following single ascending dose (SAD) and continuous infusion in healthy Chinese volunteers.
Methods
This was a randomized phase I study conducted in two parts. Part I was a double-blind, placebo- and midazolam-controlled, SAD study among healthy Chinese participants with a remimazolam dose of 0.025–0.4 mg/kg. Part II was an open-label, midazolam-controlled, continuous infusion study. Bispectral index (BIS) monitoring and Modified Observers Assessment of Alertness and Sedation (MOAA/S) score assessment were used to assess the PD properties.
Results
The half-life range of remimazolam was from 34.1 ± 8.1 to 59.8 ± 20.5 min in the SAD study. The sedation function was initially observed at the dose of 0.05 mg/kg remimazolam. Doses of ≥ 0.075 mg/kg exerted a peak sedation effect within 1–2 min after injection, resulting in a deeper and more rapid sedation. In the 2 h continuous infusion, remimazolam showed a deeper sedation and more rapid recovery than midazolam. For general anesthesia, an induction dosage of 0.2 mg/kg/min and a maintenance dosage of 1 mg/kg/h can achieve a satisfactory efficacy effect.
Conclusions
Remimazolam was safe and well tolerated in healthy Chinese participants. Based on the phase I clinical study, we suggest that remimazolam besylate demonstrates greater sedation and quicker recovery from sedation than midazolam.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Benzodiazepines have been used for sedation and as adjuvants to general anesthetics for decades [1, 2]. Midazolam is the representative drug of benzodiazepine anesthetics [3]. With advantages such as relatively rapid onset of action, multiple acceptable administration, and nonpainful induction [4], midazolam is considered the cornerstone of moderate sedation [5]. However, with an elimination half-life of 1.8–6.4 h, midazolam has a long-acting metabolite, thereby exerting greater cumulative effects and slower recovery of neuropsychiatric function [6, 7]. Remimazolam is an ultra-short-acting benzodiazepine in development for the induction and maintenance of anesthesia and procedural sedation [8]. Similar to midazolam, remimazolam works as γ-aminobutyric acid A (GABAA) receptor [9]. Because of the carboxylic ester linkage, remimazolam can be rapidly hydrolyzed to its pharmacologically inactive metabolite, carboxylic acid (RF7054), by nonspecific tissue esterases [10]. With an organ-independent metabolism and a first-order pharmacokinetics (PK), the extent and duration of the sedation of remimazolam are dose dependent. Prolonged infusions or higher doses are unlikely to result in accumulation and extended effect [11]. Owing to the stability issue associated with the free base of remimazolam, salts of the compound have been studied. A study on remimazolam tosylate in healthy Chinese participants has been published [12]. However, owing to its good thermal stability, low hygroscopicity, and high water solubility, remimazolam besylate is considered the most preferred compound. Remimazolam besylate was developed by PAION and its collaborators, and some related clinical trials have been completed. The first-in-human single ascending-dose (SAD) study was conducted in the USA, in which remimazolam (0.01–0.30 mg/kg) provided rapid onset and offset sedation and consistent PK and was well tolerated following a single bolus administration [13, 14]. Phase Ib and phase II study have stated that remimazolam provides adequate sedation for the participants undergoing upper gastrointestinal endoscopy and colonoscopy, and its sedative effects were easily reversed with flumazenil [15,16,17]. Phase III studies of remimazolam are being conducted in different countries, in which remimazolam has shown a superior sedation profile in the completed studies [5, 18]. However, the efficiency and safety data of remimazolam besylate among the Chinese population are limited. Yichang Humanwell Pharmaceutical Co., Ltd. (Hubei, China) became the only collaborator of PAION to develop and promote the marketing of remimazolam besylate in China in 2012. To estimate the safety PK and pharmacodynamic (PD) properties of remimazolam besylate in the Asian population, SAD or continuous infusion studies were conducted, which support the further clinical development of remimazolam besylate.
Most PK–PD studies on anesthetics are currently based on arterial blood samples. However, placing arterial catheters in healthy volunteers is difficult to be accepted. In the phase I study, placing an arterial catheter can be avoided if venous sampling can provide enough information to support a further study. The PK parameters of arterial and venous samples of remimazolam were first compared in this study.
Methods
Study population
Healthy Chinese males or females who met the following criteria were enrolled in the study: ages 18 to 45 years, body mass index (BMI) 19 to 24 kg/m2, systolic blood pressure 90 to 140 mmHg, diastolic blood pressure 50 to 90 mmHg, resting heart rate 50 to 100 bpm, and blood oxygen saturation ≥ 95%. Exclusion criteria included a history of organ diseases or medical conditions that could impair the participant’s ability to participate in or complete the study. Participants with a Mallampati score of 3 or 4 were also excluded. The detailed criteria of the study have been reported in the website of the Chinese Critical Trial Registry (http://www.chictr.org.cn; ChiCTR1800015185 and ChiCTR1800015186).
Study design
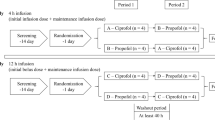
The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization of Good Clinical Practice at one center (Peking University First Hospital, Beijing) in China. The study protocol and informed consent were reviewed and approved by the Independent Ethics Committee of the Peking University First Hospital before the study started. The study was conducted in two parts: a placebo- and midazolam-controlled, randomized, double-blind, SAD study and a randomized, open-label, continuous infusion study.
In the SAD study, 60 healthy volunteers were planned for enrollment in seven groups. The doses of remimazolam were 0.025, 0.05, 0.075, 0.1, 0.2, 0.3, and 0.4 mg/kg in groups 1 to 7, respectively. The dose of midazolam was 0.075 mg/kg in every group. In groups 1 and 2, 5 male volunteers were randomized to receive a single dose of remimazolam besylate, placebo, or midazolam in 1 min at a 3:1:1 ratio. In groups 3 to 7, 10 male or female volunteers were randomized to receive a single dose of remimazolam besylate, placebo, or midazolam in 1 min at a 8:1:1 ratio. After completion of the first 2 participants in group 6, another 10 participants were enrolled, and arterial blood samples were added according to the expert seminar. Therefore, there were 12 patients in group 6.
In the continuous infusion study, 24 healthy males or females were planned for enrollment in two groups. In each group, 12 volunteers were randomized to receive either remimazolam besylate or midazolam at a 9:3 ratio. Participants were first induced with 0.2 mg/kg/min remimazolam in 1-min intravenous injection and then maintained at 1 mg/kg/h (group 1) and 2 mg/kg/h (group 2) by an infusion pump over a 2-h period. Midazolam was given at 0.15 mg/kg/min in 1 min as an induction dosage and then maintained at 0.05 mg/kg/h using an infusion pump over a 2-h period. After group 1, the stop criterion was met (the bispectral index (BIS) of > 50% of the participants decreased to 50) and the satisfactory efficacy effect was achieved. Group 2 was not studied (Supplementary Material 1).
Safety and tolerability evaluation
Safety and tolerability were evaluated using physical examination, vital signs (blood pressure, heart rate, and respiration rate), 12-lead ECGs, clinical laboratory parameters, airway assessment, and participants’ chief complaint. To ensure the safety of the participants, bedside ECG monitoring was used during drug administration.
In the SAD study, the participants were monitored by a bedside 5-lead ECG monitor at 10 min predose to 60 min postdose. SpO2 and noninvasive hemodynamic monitoring (supine blood pressure, heart rate, and respiration rate) were recorded predose and at 0, 1, 2, 5, 10, 20, 30, 40, 50, and 60 min postdose. Standard 12-lead ECGs also were performed at these time points.
In the continuous infusion study, the participants were monitored by a bedside 5-lead ECG monitor at 10 min predose to 3 h postdose. SpO2 and noninvasive hemodynamic monitoring were recorded before dosing and at 0, 1, 2, 5, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 121, 122, 125, 130, 140, 150, 160, and 170 min, and 3, 4, 6, 8, and 12 h after injection. Standard 12-lead ECGs also were performed at these time points. All ECGs were evaluated by a cardiologist.
PK evaluation
In the SAD study, blood samples were drawn into EDTA tubes before dosing and at 0, 1, 2, 3, 4, 5, 6, 8, 10, 12, 15, 20, 30, and 45 min, and 1, 1.5, 2, 3, 4, 8, and 12 h after injection for PK analysis. Venous blood samples were collected from the participants from groups 1 to 5 and 7 and the first two participants from group 6. Both venous and arterial blood samples were taken from another 10 participants from group 6. Voided urine was collected over the following sampling periods: 0–4, 4–8, 8–12, and 12–24 h after dosing.
In the continuous infusion study, venous and arterial blood samples (3 mL) were drawn into EDTA tubes predose and at 0 (immediately after the induction dose), 1, 5, 15, 30, 45, 60, 90, 120, 121, 122, 123, 124, 125, 126, 128, 130, 132, 135, 140, 150, 165, 180, and 210 min, and 4, 5, 6, 8, and 12 h after the start of infusion for PK analysis. Voided urine was collected over the following sampling periods: 0 to 4, 4 to 8, 8 to 12, and 12 to 24 h after dosing.
Blood samples were centrifuged for 10 min at 3000 rpm at 4 °C within 30 min of collection and then stored at − 70 °C until analysis. Two aliquots of 5 mL urine at each sampling period were stored at − 70 °C until PK analysis.
PK parameters were calculated using the noncompartmental model. The calculations were performed and a descriptive statistical summary of the PK parameters was obtained using WinNonLin™ software (version 6.3; Pharsight Corporation, Mountain View, CA, USA). Description of the PK parameters analysis was shown in the Supplementary Material 1. The fraction of remimazolam and RF7054 excreted in urine was determined by the quotient of the sum of remimazolam and RF7054 excreted over all dosing intervals and the dose administered.
Bioanalytical assay
The concentrations of remimazolam and RF7054 in plasma and urine were analyzed using a validated high-performance liquid chromatography-tandem mass spectrometry assay at the central laboratory of WuXi AppTec Co., Ltd. (Shanghai, China). Samples were prepurified by the protein precipitation procedure and analyzed using metolazone as internal standard. The calibration ranges were from 1 to 1000 ng/mL for remimazolam and RF7054 (plasma and urine). For remimazolam and RF7054 in plasma and urine, all the observed data for the intrarun and interbatch precision were less than 8.4% and accuracy were − 15.4 to 6.0% (Supplementary Material 1).
PD evaluation
Sedation levels were assessed by BIS monitoring [19] and Modified Observers Assessment of Alertness and Sedation (MOAA/S) score assessments [20]. The MOAA/S score, which was widely used in clinical research regarding sedation, was described in Supplementary Material 1. The BIS value was displayed on a BIS monitor (BIS EEG VISTA, USA). At each time point, an anesthetist recorded the BIS value immediately before MOAA/S assessment.
In the SAD study, for groups 1 to 5 and the first two participants in group 6, BIS and MOAA/S scores were recorded at the same time with SpO2 and noninvasive hemodynamic monitoring reporting. For the last 10 participants in group 6 and 7, BIS and MOAA/S scores were recorded predose and at 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 15, 20, 25, 30, 35, 40, 45, 50, 55, and 60 min postdose.
In the continuous infusion study, BIS and MOAA/S scores were recorded predose and at 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 70, 80, 90, 100, 110, 120, 121, 122, 125, 130, 135, 140, 145, 150, 155, 160, 165, 170, 175, and 180 min postdose.
Statistical analyses
Statistical comparisons of the groups were conducted using IBM SPSS Statistics version 19. The values of AUC0–∞, AUC0–t, and Cmax were logarithmically transformed by assuming a log-normal distribution. To compare the arterial and venous sample PK profile of remimazolam and RF7054, paired t test was used. The dose proportionality of exposure was analyzed using the power model: Y = a + Dβ, where Y represents the PK parameters (AUC and Cmax), D represents the dose, a represents the intercept, and β represents the slope. If the 95% confidence interval of β was within 80 to 125%, the exposure was judged to be proportional to the dose. Safety statistical analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Results
Demographics
In the SAD study, 62 healthy Chinese participants (mean (range) age, 28.26 (19–41) years) completed the study, of whom 48 received remimazolam besylate, 7 received midazolam, and 7 received placebo. Forty-seven participants were males (75.81%) and 15 participants were females (24.19%). In the overall population, the mean BMI was 22.32 (19.3–24) kg/m2 (Supplementary Table 1).
In the continuous infusion study, 12 healthy Chinese participants (8 males and 4 females) completed the study. Among them, 9 participants (7 males and 2 females) received remimazolam besylate, with an average (range) age of 29.1 (24–44) years and BMI of 22.4 ± 1.4 kg/m2. Another three participants (1 male and 2 females) received midazolam, with an average (range) age of 24.0 (23–26) years and BMI of 20.8 ± 0.6 kg/m2.
Safety and tolerability
SAD study
No serious or significant adverse events (AE) occurred during the SAD study. Fifty-four AEs were reported by 41 participants, and 45 of them were considered to be related to the trial medication (Supplementary Table 2). All treatment-emergent AEs had disappeared without medication by the end of the study. No events that met the stop rule for terminating dose escalation occurred during the study. Thus, we concluded that remimazolam would be safe and well tolerated at doses of 0.025 to 0.4 mg/kg.
Continuous infusion study
In the continuous infusion study, 8 remimazolam participants reported 13 AEs (88.89%) and 3 midazolam participants reported 6 AEs (100%). Among them, 18 AEs were considered to be related to the trial medication. In detail, two of six lower SpO2 were considered to be moderate AEs. One subject was given mask oxygen inhalation and oropharyngeal airway and the other was given oxygen inhalation through a nasal catheter. Hypotension was observed in five participants: three in the midazolam group and two in the remimazolam group. Hiccup, bradycardia, faster respiratory frequency, lower hemoglobin, positive urine protein, and abnormal ECGs were also reported once or twice. No serious AEs occurred and remimazolam was safe and well tolerated during the study. After completion of the first dose group, the termination criteria were met, and no further exploration was carried out.
PK properties
SAD study
One subject at 0.075 mg/kg dose and one subject at 0.1 mg/kg dose were excluded for skipping more than three samples in the first 5 min in the data analysis. Sixty participants were included in the PK analysis. The key PK parameters for remimazolam and RF7054 are shown in Tables 1 and 2, and the venous plasma PK profiles for remimazolam and RF7054 are shown in Fig. 1. At 0.3 mg/kg, the Cmax for arterial and venous sampling was 3950 ± 1620 and 1510 ± 987 ng/mL, respectively (P < 0.01). The MRT for venous blood was longer than that for arterial blood (P < 0.05). Except for Cmax and MRT, no significance difference was found between venous and arterial remimazolam PK parameters. There was no significance for the venous and arterial plasma PK parameters of RF7054. Based on the power model assessment, among the seven doses, the slope values (95% confidence interval (95% CI)) for Cmax and AUC0–t were 1.27 (1.036, 1.495) and 0.96 (0.879, 1.031) for remimazolam and 0.96 (0.907, 1.014) and 1.01 (0.932, 1.094) for RF7054, respectively. According to the exposure proportional judgment of the 95% CI of β, which was within 80 to 125%, the AUC0-t for remimazolam and both AUC0–t and Cmax for RF7054 were dose proportional across all the studied doses. The Cmax for remimazolam had a slight deviation from dose proportionality.
After the single intravenous injection of remimazolam, there was almost no remimazolam recovered in urine, whereas the urine percent recovered (%) from the metabolite RF7054 was about 70.8 to 89.1%.
Continuous infusion study
The venous and arterial key PK parameters from nine participants of remimazolam and RF7054 are shown in Table 3, and the PK profiles for remimazolam and RF7054 are shown in Fig. 2. Remimazolam reached significantly higher Cmax (p < 0.01) immediately after dosing in the arterial blood, much quicker than in the venous Tmax (p < 0.01) in the continuous infusion. The MRT for venous blood was longer than that for arterial blood (p < 0.05). For RF7054, there was no significance for the venous and arterial plasma PK parameters.
PD properties
SAD study
Dose escalation proceeded as planned throughout the groups, and good tolerance was observed even in the highest dose (0.4 mg/kg) in the SAD study. The sedation function was first observed in the dose of 0.05 mg/kg remimazolam. Doses of ≥ 0.075 mg/kg had a peak sedation effect in 1 to 2 min after injection, resulting in a deeper and more rapid sedation. The degree and duration of sedation for remimazolam were dose dependent. Compared with midazolam, remimazolam at ≥ 0.1 mg/kg appeared to produce a deeper sedation and maintained a quicker recovery from sedation at ≤ 0.3 mg/kg (Supplementary Fig. S1). The mean times from first MOAA/S of < 5 to fully alert of 0.1 mg/kg remimazolam was 12.63 min, similar to that of midazolam (Supplementary Table 3). As suggested by the PD results, and short-term operations such as endoscopy, a single dose of 0.1 mg/kg remimazolam could achieve a similar effect as that of midazolam.
Continuous infusion study
Both remimazolam and midazolam observed a rapid onset of sedation after the induction dose, but remimazolam showed a deeper sedation than midazolam in the 2 h continuous infusion. The recovery from sedation after dosing of remimazolam was more rapid compared with midazolam (Supplementary Fig. S2).
PK–PD correlation
In Fig. 3, the MOAA/S score decreased to 2 to 3 when the venous plasma concentration of remimazolam was 400 to 500 ng/mL. The BIS score was 40 to 60 and 60 to 80 when the concentration was 800 to 1200 and 200 to 800 ng/mL, respectively. When the concentration was > 1200 ng/mL, the BIS score was < 40 and the MOAA/S score was 0. Based on the above results, we suggested that good sedation can be reached when the plasma concentration was between 400 and 1200 ng/mL.
Discussion
Remimazolam besylate is a novel sedative agent developed for procedural sedation, general anesthesia, and sedation in the intensive care unit. It was produced using the same method as that of remifentanil, so-called soft drug development, which is defined as a strategy in which novel active compounds are specifically designed to be vulnerable to rapid biotransformation into inactive metabolites [11, 21]. It allows for the rapid onset of sedation and a rapid offset with a predictable short duration of action [7]. Preclinical trials showed that the main route of metabolism of remimazolam is via the inactive metabolite RF7054 by tissue carboxylesterases without the involvement of cytochrome P450 isoenzymes [10]. Based on its PK properties, remimazolam has limited potential to be influenced by ethnicity. In the first-in-human study of remimazolam besylate conducted in the USA, healthy participants received a single 1-min intravenous infusion of remimazolam (0.01–0.3 mg/kg) and radial arterial PK samples were used for the PK analysis [13]. The initial maximum dose was 0.35 mg/kg in that study, but the stop criterion (> 50% of the participants with loss of consciousness for a minimum of 5 min) was met at a dose of 0.3 mg/kg; hence, 0.35 mg/kg was not studied. However, the maximum tolerated single intravenous dose of remimazolam in our study was 0.4 mg/kg. The PK properties could explain the causes of the difference. In the SAD study, arterial sample PK parameters were only analyzed at the dose of 0.3 mg/kg remimazolam. We compared the PK parameters of the two studies in the same dose (0.3 mg/kg). The exposure-related parameters AUC0–∞ and Cmax were lower in Chinese participants (14.6 μg min/mL = 243.3 ng h/mL vs. 339.3 ng h/mL and 3950 vs. 6095 ng/mL), but the CL was consistent with that observed in previous studies (22.2 mL/min/kg = 81.2 L/kg vs. 73.71 L/kg). As for PD, the duration of loss of consciousness and the median time to fully alert were shorter in our study (8 vs. 12 min and 25 vs. 31.5 min, respectively). This might be related to remimazolam in both studies as administered based on weight (mg/kg), and the weight of Chinese participants was much lighter (61.0 vs. 76.8 kg). Therefore, the total dose in Chinese participants was lower at the same group (0.3 mg/kg).
As a GABAA receptor, midazolam is the shortest-acting benzodiazepine available in China. In this study, midazolam was added as a positive control drug to compare the PD and safety properties. Remimazolam differs from midazolam by its carboxylic ester linkage, enabling its rapid breakdown to inactive metabolites only [18]. The half-life of remimazolam after a single dose in this study was 34.1 ± 8.1 to 59.8 ± 20.5 min, which was much shorter than that of midazolam. In the dose range of 0.075 to 0.4 mg/kg, the onset time for remimazolam was as rapid as midazolam. The average time to fully alert for remimazolam was 12.3 to 25 min (0.075–0.3 mg/kg), which was comparable with midazolam of 16.3 min. Recovery took up to 50 min for 0.4 mg/kg remimazolam, of which doses were unlikely to be useful in the short-term procedure or diagnostic sedation. When the concentration of remimazolam was between 400 and 1200 ng/mL, remimazolam was proven to achieve better sedation and shorter recovery time compared with midazolam. When the concentration was > 1200 ng/mL, the BIS score was < 40 and the MOAA/S score was 0. The Cmax was 317 ± 246 to 1510 ± 987 ng/mL for 0.075–0.3 mg/kg and 2170 ± 1950 ng/mL for 0.4 mg/kg remimazolam. Remimazolam is currently in advanced development in numerous indications, and major clinical trials are ongoing. Considering the safety and efficacy properties, the single dose of remimazolam was recommended at 0.075 to 0.3 mg/kg in the short-term procedure or diagnostic sedation.
To investigate the use of remimazolam as a sedative in general anesthesia, we employed the continuous infusion study. To our knowledge, this is the first report of the PK–PD properties of remimazolam besylate in general anesthesia in healthy participants. For the induction of general anesthesia, 0.2 mg/kg/min remimazolam in 1-min intravenous injection was given, and the maintenance dosage was planned at 1 mg/kg/h in groups 1 and 2 mg/kg/h in group 2 by an infusion pump over a 2-h period. After group 1, the stop criterion was met and the satisfactory efficacy effect was achieved. Group 2 was not studied. Based on these results, the recommended dosage for phase II study of remimazolam besylate in general anesthesia in Chinese participants is at an induction dosage of 0.2 mg/kg/min and a maintenance dosage of 1 mg/kg/h.
Venous blood samples are used in most PK studies. The existence of drug concentration differences between arterial and venous blood has been documented for various drugs [22, 23]. For drugs rapidly eliminated in peripheral tissues, the delay from the arterial circulation to the venous sampling site should be taken into account [23]. Most contemporary PK–PD studies for anesthetics are based on arterial blood samples. Tuk et al. [24] quantified the extent of arteriovenous concentration differences of midazolam in rats and determined the consequences of these differences on the PD estimates. Although arterial blood carries the drug or active metabolite to various parts of the body to produce pharmacological effects, the observed arteriovenous concentration differences did not result in biased PD estimates for midazolam in rats using an effect-linked PD model [23, 24]. During the course of the clinical trial, arterial blood was more difficult to collect compared with venous samples. Furthermore, placing arterial catheters in healthy volunteers is difficult to be accepted. Additionally, complications such as radial artery occlusion, nerve damage, or pseudo-aneurysm of the radial artery are serious and need to be carefully balanced against the gain of obtaining arterial blood samples [25]. Thus, placing an arterial catheter for healthy volunteers is unnecessary if similar results can be obtained using venous blood samples. To compare the difference between arterial and venous samples of remimazolam, both arterial and venous blood samples were collected in group 6 of the SAD and continuous infusion studies. Apart from Cmax, Tmax, and MRT, no significant difference was found between the other PK parameters of remimazolam and the parameters of RF7054. The differences in concentration could not result in an influence on PD and safety estimates and dose recommendation for remimazolam. Venous samples can be used in the phase I PK/PD study of remimazolam to obtain appropriate doses suggested for the planning of phase II development of this drug.
Most AEs in this study were caused by the pharmacological effects of remimazolam. Although these AEs are considered drug related, most were mild and temporary and did not cause clinical changes in patients. There was no apparent remimazolam dose response in the number and frequency of AEs. Two moderate AEs were reported in the continuous infusion study, the lowest SpO2 of 76% was observed in one subject, the duration time for SpO2 of < 90% was 8 min, and the mask oxygen inhalation and oropharyngeal airway was given to the subject. A lowest SpO2 of 81% was observed in another subject, the duration time for SpO2 of < 90% was 16 min, and oxygen inhalation through a nasal catheter was given to the subject. Both participants returned to normal SpO2 under normal breathing conditions after 8 and 16 min. In conclusion, we suggested that remimazolam showed good safety and tolerance in this study.
Conclusion
As the first study that assessed the safety, pharmacokinetic and pharmacodynamic of remimazolam besylate in healthy Chinese volunteers after single-dose and continuous infusion injection, we found that remimazolam was safe and well tolerated with predictable PK properties and dose-dependent PD properties. Based on the phase I clinical study, we suggested that remimazolam might have greater sedation and a quicker recovery from sedation compared with midazolam.
References
Cornett EM, Novitch MB, Brunk AJ, Davidson KS, Menard BL, Urman RD, Kaye AD (2018) New benzodiazepines for sedation. Best Pract Res Clin Anaesthesiol 32(2):149–164. https://doi.org/10.1016/j.bpa.2018.06.007
Saari TI, Uusi-Oukari M, Ahonen J, Olkkola KT (2011) Enhancement of GABAergic activity: neuropharmacological effects of benzodiazepines and therapeutic use in anesthesiology. Pharmacol Rev 63(1):243–267. https://doi.org/10.1124/pr.110.002717
Olkkola KT, Ahonen J (2008) Midazolam and other benzodiazepines. Handb Exp Pharmacol 182:335–360. https://doi.org/10.1007/978-3-540-74806-9_16
Reves JG, Fragen RJ, Vinik HR, Greenblatt DJ (1985) Midazolam: pharmacology and uses. Anesthesiology 62(3):310–324
Pastis NJ, Yarmus LB, Schippers F, Ostroff R, Chen A, Akulian J, Wahidi M, Shojaee S, Tanner NT, Callahan SP, Feldman G, Lorch DG Jr, Ndukwu I, Pritchett MA, Silvestri GA, Investigators PAION (2019) Safety and efficacy of remimazolam compared with placebo and midazolam for moderate sedation during bronchoscopy. Chest 155(1):137–146. https://doi.org/10.1016/j.chest.2018.09.015
Ulmer BJ, Hansen JJ, Overley CA, Symms MR, Chadalawada V, Liangpunsakul S, Strahl E, Mendel AM, Rex DK (2003) Propofol versus midazolam/fentanyl for outpatient colonoscopy: administration by nurses supervised by endoscopists. Clin Gastroenterol Hepatol 1(6):425–432
Pambianco DJ, Cash BD (2016) New horizons for sedation: the ultrashort acting benzodiazepine remimazolam. Tech Gastrointest Endosc 18(1):22–28. https://doi.org/10.1016/j.tgie.2016.02.004
Rogers WK, McDowell TS (2010) Remimazolam, a short-acting GABA(A) receptor agonist for intravenous sedation and/or anesthesia in day-case surgical and non-surgical procedures. IDrugs 13(12):929–937
Goudra BG, Singh PM (2014) Remimazolam: the future of its sedative potential. Saudi J Anaesth 8(3):388–391. https://doi.org/10.4103/1658-354X.136627
Kilpatrick GJ, McIntyre MS, Cox RF, Stafford JA, Pacofsky GJ, Lovell GG, Wiard RP, Feldman PL, Collins H, Waszczak BL, Tilbrook GS (2007) CNS 7056: a novel ultra-short-acting benzodiazepine. Anesthesiology 107(1):60–66
Wesolowski AM, Zaccagnino MP, Malapero RJ, Kaye AD, Urman RD (2016) Remimazolam: pharmacologic considerations and clinical role in anesthesiology. Pharmacotherapy 36(9):1021–1027. https://doi.org/10.1002/phar.1806
Zhou Y, Hu P, Huang Y, Nuoer S, Song K, Wang H, Wen J, Jiang J, Chen X (2018) Population pharmacokinetic/pharmacodynamic model-guided dosing optimization of a novel sedative HR7056 in Chinese healthy subjects. Front Pharmacol 9:1316. https://doi.org/10.3389/fphar.2018.01316
Antonik LJ, Goldwater DR, Kilpatrick GJ, Tilbrook GS, Borkett KM (2012) A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part I. safety, efficacy, and basic pharmacokinetics. Anesth Analg 115(2):274–283. https://doi.org/10.1213/ANE.0b013e31823f0c28
Wiltshire HR, Kilpatrick GJ, Tilbrook GS, Borkett KM (2012) A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part II. Population pharmacokinetic and pharmacodynamic modeling and simulation. Anesth Analg 115(2):284–296. https://doi.org/10.1213/ANE.0b013e318241f68a
Worthington MT, Antonik LJ, Goldwater DR, Lees JP, Wilhelm-Ogunbiyi K, Borkett KM, Mitchell MC (2013) A phase Ib, dose-finding study of multiple doses of remimazolam (CNS 7056) in volunteers undergoing colonoscopy. Anesth Analg 117(5):1093–1100. https://doi.org/10.1213/ANE.0b013e3182a705ae
Borett KM, Riff DS, Schwartz HI, Winkle PJ, Pambianco DJ, Lees JP, Wilhelm-Ogunbiyi K (2015) A phase IIa, randomized, double-blind study of remimazolam (CNS 7056) versus midazolam for sedation in upper gastrointestinal endoscopy. Anesth Analg 120(4):771–780. https://doi.org/10.1213/ANE.0000000000000548
Pambianco DJ, Borkett KM, Riff DS, Winkle PJ, Schwartz HI, Melson TI, Wilhelm-Ogunbiyi K (2016) A phase IIb study comparing the safety and efficacy of remimazolam and midazolam in patients undergoing colonoscopy. Gastrointest Endosc 83(5):984–992. https://doi.org/10.1016/j.gie.2015.08.062
Rex DK, Bhandari R, Desta T, DeMicco MP, Schaeffer C, Etzkorn K, Barish CF, Pruitt R, Cash BD, Quirk D, Tiongco F, Sullivan S, Bernstein D (2018) A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc 88(3):427–437. https://doi.org/10.1016/j.gie.2018.04.2351
Bower AL, Ripepi A, Dilger J, Boparai N, Brody FJ, Ponsky JL (2000) Bispectral index monitoring of sedation during endoscopy. Gastrointest Endosc 52(2):192–196
Chernik DA, Gillings D, Laine H, Hendler J, Silver JM, Davidson AB, Schwam EM, Siegel JL (1990) Validity and reliability of the observer’s assessment of alertness/sedation scale: study with intravenous midazolam. J Clin Psychopharmacol 10(4):244–251
Egan TD (2009) Is anesthesiology going soft? Trends in fragile pharmacology. Anesthesiology 111(2):229–230. https://doi.org/10.1097/ALN.0b013e3181ae8460
Ericsson H, Bredberg U, Eriksson U, Jolin-Mellgård A, Nordlander M, Regårdh CG (2000) Pharmacokinetics and arteriovenous differences in clevidipine concentration following a short- and a long-term intravenous infusion in healthy volunteers. Anesthesiology 92(4):993–1001. https://doi.org/10.1097/00000542-200004000-00016
Sun L, Lau CE (2001) Arteriovenous serum cocaine concentration difference after intravenous bolus injection and constant-rate infusions: relation to pharmacodynamic estimates in rats. Eur J Pharm Sci 14(4):261–269
Tuk B, Herben VM, Mandema JW, Danhof M (1998) Relevance of arteriovenous concentration differences in pharmacokinetic-pharmacodynamic modeling of midazolam. J Pharmacol Exp Ther 284(1):202–207
Olofsen E, Mooren R, van Dorp E, Aarts L, Smith T, den Hartigh J, Dahan A, Sarton E (2010) Arterial and venous pharmacokinetics of morphine-6-glucuronide and impact of sample site on pharmacodynamic parameter estimates. Anesth Analg 111(3):626–632. https://doi.org/10.1213/ANE.0b013e3181e5e8af
Acknowledgments
This study was sponsored by Yichang Humanwell Pharmaceutical Co., Ltd. We thank the contributions of WuXi AppTec Co., Ltd. for performing the concentration assays. We also thank the Department of Health Statistics, Second Military Medical University, for performing the statistical analysis.
Author information
Authors and Affiliations
Contributions
Li-e Li, Xia Ye, Xue-yuan Yang, Xia Zhao, and Yi-min Cui designed the study. Xiao-yan Sheng, Yan Liang, Xue-yuan Yang, Xia Zhao, and Yi-min Cui conducted the clinical trial. Xue-yuan Yang is the anesthesiologist of the study, who assessed the pharmacodynamic parameters and safety of the subjects. Xiao-yan Sheng and Yan Liang analyzed the results and drafted the manuscript. Li-e Li, Xia Ye, Xia Zhao, and Yi-min Cui participated in the interpretation of results and revised the manuscript. All authors read and approved the final manuscript. The authors confirm that the PI for this paper is Yi-min Cui.
Corresponding author
Ethics declarations
Conflict of interest
Li-e Li and Xia Ye are employees of Yichang Humanwell Pharmaceutical Co., Ltd., which sponsored the clinical trial. The other authors have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sheng, Xy., Liang, Y., Yang, Xy. et al. Safety, pharmacokinetic and pharmacodynamic properties of single ascending dose and continuous infusion of remimazolam besylate in healthy Chinese volunteers. Eur J Clin Pharmacol 76, 383–391 (2020). https://doi.org/10.1007/s00228-019-02800-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-019-02800-3