Abstract
Purpose
The sedative effects of pregabalin during perioperative period have not been sufficiently characterized. The aim of this study was to verify the sedative effects of premedication with pregabalin on intravenous sedation (IVS) using propofol and also to assess the influences of this agent on circulation, respiration, and postanesthetic complications.
Methods
Ten healthy young volunteers underwent 1 h of IVS using propofol, three times per subject, on separate days (first time, no pregabalin; second time, pregabalin 100 mg; third time, pregabalin 200 mg). The target blood concentration (C T) of propofol was increased in a stepwise fashion based on the bispectral index (BIS) value. Ramsay’s sedation score (RSS) was determined at each propofol C T. Propofol C T was analyzed at each sedation level. Circulation and respiration during IVS and complications were also verified.
Results
Propofol C T was reduced at BIS values of 60 and 70 in both premedicated groups (100 mg: p = 0.043 and 0.041; 200 mg: p = 0.004 and 0.016, respectively) and at a BIS value of 80 in the pregabalin 200 mg group (p < 0.001). Propofol C T was decreased at RSS 4–6 in the pregabalin 100 mg group (RSS 4: p = 0.047; RSS 5: p = 0.007; RSS 6: p = 0.014), and at RSS 3–6 in the pregabalin 200 mg group (RSS 3–5: p < 0.001; RSS 6: p = 0.002).
Conclusion
We conclude that oral premedication with pregabalin reduces the amount of propofol required to obtain an acceptable and adequate sedation level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pregabalin has been used for the treatment of neuropathic pain and other chronic neuralgia [1, 2]. Recent studies have shown that pregabalin is effective for managing postoperative pain resulting from various surgical procedures [3,4,5,6,7,8]. Also, pregabalin increases the perioperative sedation level [9, 10]. In some studies, it has been reported that the sedative effect of pregabalin is also observed as its side effect [3, 11], although it has been suggested that preoperative administration of pregabalin does not reduce the propofol requirement for induction of general anesthesia [12]. Thus, the sedative effect of pregabalin has not been sufficiently characterized.
Pregabalin reduces depolarization-induced Ca2+ entry via presynaptic Ca2+ channels in the central nervous system (CNS) [13,14,15,16,17,18]; consequently, this agent suppresses the release of glutamate and other excitatory neurotransmitters [18]. The influence of inhibiting excitatory neurotransmitter release from presynaptic terminals on anesthetic depth remains uncertain. It is not likely that oral administration of pregabalin alone obtains a sufficient sedative effect during a surgery; however, interaction between pregabalin and an intravenous (IV) anesthetic that mainly exerts a postsynaptic inhibitory action may result in additive/synergistic sedative effects.
In this study, we investigated the effects of pregabalin on IV sedation (IVS) using the IV anesthetic propofol. We analyzed the pregabalin-induced differences in the target blood concentration (C T) of propofol at the adequate sedation level, which was determined using bispectral index (BIS) value and a sedation score. Furthermore, we also examined pregabalin-induced changes in circulation and respiration during IVS and any complications.
Methods
Study design and participants
After the approval of the Research Ethics Committee of Kyushu University Hospital and the registration of the study protocol in the clinical trials database (UMIN000015674), ten adult volunteers with American Society of Anesthesiologists (ASA) physical status 1, 20–39 years of age, were recruited for this study. After written informed consent was obtained, a blood test and electrocardiography were performed to assess the volunteers’ physical condition. Volunteers were excluded from recruitment if they were allergic to egg or soy (raw materials for propofol formulation) or antibiotics or analgesics, had chronic neuralgia, received γ-aminobutyric acid (GABA) derivatives, or had any pain-causing conditions.
Healthy volunteers without any abnormalities in the preanesthetic examination were administered intravenous propofol using a Diprifusor target-controlled infusion (TCI) system (Marsh pharmacokinetic model; Terufusion TCI-pump, TE-371; Terumo, Japan) for 1 h. IVS was administered three times per subject on separate days: the first IVS was without pregabalin as a control, the second IVS was with pregabalin 100 mg, and the third IVS with pregabalin 200 mg. To prevent residual effects of the drug, the time interval between each examination was set as 1 week or more.
Data collection
The subjects were required to refrain from eating and drinking after midnight on the day of the study. They were asked to visit the examination room at 8 a.m. on the day of the study, i.e., 1 h before starting IVS. The BIS value and vital signs, namely, body temperature, pulse rate, blood pressure, respiratory rate, and oxygen saturation (SpO2) level, were measured using a flexible multi-parameter bedside monitor (Life Scope TR, BSM-6301; Nihon Kohden, Japan). The study protocol was canceled if the subject’s vital signs were abnormal (hypo- or hypertension, hypo- or hyperthermia, tachycardia, or bradycardia) or if the subject had any poor physical conditions such as a common cold, nausea, headache, or stomachache. At the second and third IVS, the subjects received each dose of pregabalin orally and rested in the examination room for 1 h while being monitored and observed by the study operators.
An IV line with a 22-gauge IV cannula (Jelco Plus; Smiths Medical, USA) and IV fluid (SOLDEM 1; Terumo, Japan) at a rate of 60 ml/h was secured to the dorsum of each volunteer’s right hand. Oxygen (3 l/min) was supplied via a nose cannula. Because pregabalin is rapidly absorbed with peak blood concentration within 1 h in healthy volunteers [1], the IVS was started at 1 h after pregabalin administration, with a starting propofol C T of 0.5 µg/ml. BIS value was constantly recorded, and vital signs were measured every 5 min under IVS. At 10 min after the start of IVS, propofol C T was increased to 1.0 µg/ml. After the propofol C T reached 1.0 µg/ml, the propofol was increased by 0.2 µg/ml every 10 min until the BIS value reached 60. The BIS value was recorded at each propofol C T. Propofol C T was evaluated when the BIS value reached 80, 70, and 60, respectively. Ramsay’s sedation score (RSS) [19] was determined at each propofol C T by the same researcher (the first author) throughout the three times of sedation. RSS 1–3 shows awake levels: 1, patient anxious, agitated, or restless; 2, patient co-operative, oriented, and tranquil; 3, patient responds to commands only. RSS 4–6 shows asleep levels, which are dependent on the patient’s response to a light glabellar tap or loud auditory stimulus: 4, a brisk response; 5, a sluggish response; 6, no response. The time duration of the IVS was about 1 h. After the IVS, the subject remained lying on the examination bed under monitoring and observation without any stimulus until arousal.
Dizziness, blurred vision, and nausea, which might occur as complications of pregabalin, were evaluated at 30 min after the oral administration of pregabalin and just before starting the IVS, and at 30 min, 1 h, and 5 h after the termination of IVS. Dizziness, blurred vision, and nausea were evaluated by a 11-point numerical rating scale (NRS) [6, 8, 9, 13]. The subjects were asked to evaluate their symptoms on a scale from 0 to 10, where 0 represents ‘no symptoms’ and 10 represents ‘the worst possible,’ using whole numbers (11 integers including 0). Metoclopramide 10 mg was administered as a rescue antiemetic medication if the subjects complained of moderate nausea (NRS of 5 or more) or displayed vomiting. After evaluation of complications at 5 h after IVS, the subjects were discharged from the examination room.
Study endpoints
The primary endpoint of the study was the pregabalin-induced difference in the propofol C T at the adequate sedation level. Secondary endpoints were the pregabalin-induced changes in circulation and respiration during IVS and any complications.
Statistical analysis
Data are presented as mean (SD), median [IQR (range)] or number of subjects (proportion). Statistical analysis was performed using SPSS version 20.0 (IBM, USA). The data were analyzed by multilevel modeling using one-way repeated-measures analysis of variance, followed by multiple comparisons with Bonferroni correction. A p value <0.05 was considered significant. Because this trial involved the measurement of new items, no preliminary study was available for power analysis to estimate the sample size at the time of design. Therefore, the sample size was set as ten volunteers, taking into consideration feasibility and cost to obtain primary exploratory data.
Results
Ten volunteers, five male and five female, participated in this study. The mean age of the volunteers was 29.2 years (range, 23–32 years). Mean height and weight were 165 (range, 8.2) cm and 58.8 (range, 6.5) kg, respectively. No subject had developed any allergy to drugs. The study protocol was performed without discontinuation during the trial.
BIS value
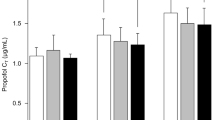
Figure 1 shows the propofol C T at BIS values of 60–80 in the groups with or without premedication of pregabalin. In the no pregabalin (control) group, the mean propofol C T at BIS values of 60, 70, and 80 was 1.70 (0.445), 1.46 (0.417), and 1.09 (0.281), respectively. The mean propofol C T at BIS values of 60 and 70 was significantly reduced in both premedicated groups [pregabalin 100 mg: 1.44 (0.227), p = 0.043, and 1.21 (0.179), p = 0.041; pregabalin 200 mg: 1.34 (0.313), p = 0.004, and 1.17 (0.271), p = 0.016, respectively], compared to the corresponding values in the control group. However, there was no significant difference between the two premedicated groups. The mean propofol C T in the pregabalin 200 mg group [0.69 (0.345)], but not in the pregabalin 100 mg group [0.97 (0.157)], was significantly less at a BIS value of 80 (p < 0.001).
Propofol target blood concentration (C T) in groups without pregabalin (control, white) and with pregabalin (100 mg, shaded; 200 mg, black) when the bispectral index (BIS) value reached 60–80. Data are expressed as mean (SD). *p < 0.05 and **p < 0.01 represent significant differences among groups. C T propofol target blood concentration
Sedation score
Compared to the control group, the pregabalin 100 and 200 mg groups showed significant decrease of propofol C T at RSS 4–6 (RSS 4: p = 0.047; RSS 5: p = 0.007; RSS 6: p = 0.014) and at RSS 3–6 (RSS 3–5: p < 0.001; RSS 6: p = 0.002), respectively (Fig. 2). The data of the propofol C T at RSS 1 and 2 could not be evaluated because the sedation levels of some or all of the pregabalin-premedicated subjects had already reached RSS 2 (100 mg, 10 subjects; 200 mg, 4 subjects) and RSS 3 (200 mg, 6 subjects) before starting the propofol infusion for sedation.
Changes in circulation and respiration
Figure 3 shows blood pressure from the time of premedication to the end of the IVS in the groups with or without premedication of pregabalin. From the time of premedication to the end of IVS, there were no significant differences in systolic blood pressure (SBP) and diastolic blood pressure (DBP) among the three groups, except for low SBP in the pregabalin 200 mg group at the end of IVS (p = 0.020). Immediately before premedication, mean blood pressure (MBP) showed significant differences in the pregabalin 100 mg group compared with the control group (p = 0.036). At a BIS value of 60, MBP was significantly low in the pregabalin 200 mg group compared with the control group (p = 0.009). No group showed abnormal blood pressure values during the examination, provided that abnormal blood pressure in this study was defined as SBP ≥140 mmHg or DBP ≥90 mmHg [20], or a decline of at least 30% of the subject’s baseline MBP measured before premedication [21]. Pulse rates significantly decreased at the start of IVS (p < 0.001), at a BIS value of 80 (p = 0.002), at a BIS value of 70 (p = 0.021), and at the end of IVS (p = 0.007) in the pregabalin 200 mg group (Fig. 4). There were no significant differences in SpO2 level (95–100%) and respiratory rate (12–24) among the three groups (data not shown).
Blood pressure (BP) from the time of premedication to the end of the intravenous sedation (IVS) in groups without pregabalin (control, white dotted lines) and with pregabalin (100 mg, shaded dashed lines; 200 mg, black dashed lines). T1 immediately before premedication; T2 30 min after premedication; T3 at the start of IVS; T4 at BIS value of 80; T5 at BIS value of 70; T6 at BIS value of 60; T7 end of IVS. Results are expressed as mean (SD). *p < 0.05 and **p < 0.01 represent significant differences compared with control group
Pulse rate (PR) from the time of premedication to the end of IVS in groups without pregabalin (control, white dotted lines) and with pregabalin (100 mg, shaded dashed lines; 200 mg, black dashed lines). T1 immediately before premedication; T2 30 min after premedication; T3 at the start of IVS; T4 at BIS value of 80; T5 at BIS value of 70; T6 at BIS value of 60; T7 end of IVS. Results are expressed as mean (SD). *p < 0.05 and **p < 0.01 represent significant differences compared with control group
Complications
The incidences of blurred vision significantly increased in the pregabalin 200 mg group at 1 and 5 h after the end of IVS (p = 0.005 and 0.029, respectively). One subject in the pregabalin 100 mg group and three in the pregabalin 200 mg group asked for and were administered rescue antiemetics; however, there was no significant difference in the incidence of nausea among the three groups (Table 1).
Discussion
In this study, we investigated the sedative effects of oral pregabalin premedication on IVS using propofol TCI. Oral premedication of 100 and 200 mg pregabalin significantly reduced the propofol C T to obtain an adequate sedation level, estimated on the basis of the BIS value and RSS.
It has been reported that preoperative administration of pregabalin 150 mg does not reduce the propofol requirement for induction of general anesthesia [12]. White et al. have reported that premedication with oral pregabalin at 300 mg increases the level of sedation before induction of anesthesia and in the early postoperative period [9]. One systematic review has suggested that pregabalin improves postoperative analgesia at the expense of increased sedation and visual disturbance [11]. Thus, the sedative effect of pregabalin during anesthesia or sedation has been left unclear. From the results of this study, however, it is considered that oral administration of pregabalin augments the sedative effect on IVS using propofol. As already mentioned, Moreau-Bussière et al. have reported that preoperative pregabalin does not reduce the propofol requirement for induction of general anesthesia. In their study, entropy values were measured between 1 and 3 min post induction with propofol, and the necessary dose of propofol for the achievement of a targeted anesthetic depth was determined [12]. In this present study, the propofol C T was gradually increased every 10 min until the BIS value reached 60, and BIS value and RSS were observed for 10 min per 1 C T of propofol. The discrepant results between the two studies may be attributable to the differences in the duration of propofol administration and the requirement of propofol to reach the required anesthetic depth (i.e., induction of general anesthesia vs. sedation). In recent years, there are several reports on the presynaptic effects of general anesthetics [22,23,24,25,26]. The regulation of neurotransmitter release from presynaptic nerve terminals may be related to anesthetic action in the CNS. Pregabalin inhibits the release of excitatory neurotransmitters from presynaptic terminals; therefore, it is probably reasonable that pregabalin affects anesthetic action.
Premedication with pregabalin contributes to decreases in heart rate and mean arterial pressure at airway instrumentation [27]. Gupta et al. have reported that oral pregabalin premedication decreases mean arterial pressure, but not heart rate, after propofol induction [28]. In our study, pregabalin administration did not result in any evident differences in respiration. The administration of 100 mg pregabalin did not cause a significant difference in circulation. However, the pregabalin 200 mg group showed significant declines of SBP at the end of IVS and MBP at a BIS value of 60 (Fig. 3), and significant reduction of pulse rate during IVS compared with control group (Fig. 4). The sedative effect of pregabalin may lead to the reduction of pulse rate, although the effect of the interaction of pregabalin and propofol on the cardiovascular system remains unknown.
Dose-dependent effects of pregabalin were only observed at light levels of sedation (BIS value, 80: Fig. 1; RSS 3 and RSS 4: Fig. 2). It is difficult to explain why there were no significant differences between the 100 and 200 mg pregabalin groups at deep sedation levels. The 200 mg dose of pregabalin is not the maximum dose of one medication. In fact, in many reports on the analgesic effects of pregabalin, 300 mg was used as a high dose of pregabalin, and postanesthetic complications, such as dizziness and drowsiness, were often observed in the patients administered 300 mg of pregabalin [2, 3, 5,6,7, 11]. In our study, to prevent complications caused by oral pregabalin 300 mg, 200 mg was used as the high dose. However, even 200 mg pregabalin induced complications after IVS (Table 1). The high incidences of complications after IVS may be related to the long elimination half-life of pregabalin, ranging from 5.5 to 6.7 h [1]. Pregabalin premedication seems not to be suitable for day surgery for an outpatient because of its long elimination half-life. In addition, in using pregabalin as the premedication for anesthesia/sedation, it is necessary to take precautionary measures against postanesthetic complications.
In this study, two doses of pregabalin were used to investigate the sedative effects on the IVS using propofol: 100 mg pregabalin has nearly the same effect as 200 mg at BIS values of 60 and 70 and at RSS 5 and 6; therefore, 100 mg is considered to be the adequate dose of pregabalin in deep sedation level. On the other hand, 200 mg pregabalin is more effective than 100 mg at BIS value 80 and RSS 3 and 4 (Figs. 1, 2). The required sedation level may be different depending on each case. If the patient is required to respond briskly to commands or waking stimuli, 200 mg pregabalin seems to be the efficacious dose. However, taking into consideration the avoidance of risks of side effects and fluctuations in circulation, 100 mg may be the safe dose of pregabalin for premedication of IVS.
In addition to the augmentation of sedative effects, pregabalin has analgesic effects on postoperative pain [3,4,5,6,7,8]; therefore, pregabalin may be useful as a premedication. However, the preoperative use of pregabalin for premedication is unapproved. Hence, the off-label use of pregabalin is not immediately recommended for premedication. It will be necessary to verify the effectiveness of pregabalin for premedication in comparison with other sedative premedication drugs. On the other hand, it may be rather important to focus on the anesthetic/sedative effects of pregabalin as an inhibitor of neurotransmitter release, because the action mechanism of pregabalin is completely different from the principal mechanisms of existing anesthetics. A more detailed study on the anesthetic effects caused by inhibiting excitatory neurotransmitter release from presynaptic nerve terminals in the CNS appears to be useful.
Our study has some limitations. First, this study was a nonrandomized and nonblinded design; therefore, we cannot deny the possibility of bias. Immediately before premedication, for example, blood pressure and pulse rate were slightly high in the pregabalin 100 mg group compared with control group (Figs. 3, 4). These differences in the circulatory kinetics between two groups may be caused by the subjects’ stress and anxiety about taking pregabalin. This nonrandomized and nonblinded study design is based on the advice of the Ethics Committee as follows. There are no sufficient preliminary studies demonstrating the interaction between pregabalin and propofol in IVS. Hence, randomization or blinding was not suitable because of the need to secure the safety of the volunteers. Second, our results are applicable only to a healthy young population with no systematic disease. Three cases of postoperative respiratory depression have been reported in elderly patients who received pregabalin [29]. The results of our study do not clarify the possible effects of the interaction between propofol and pregabalin in elderly or medically compromised subjects. Third, some subjects showed reduction of BIS value and increase of the sedation score at the starting of the IVS. Pregabalin alone may have some sedative effect. Therefore, a group given only pregabalin would have been desirable in our study. Fourth, there is a correlation between electromyogram (EMG) and BIS [30]. Although EMG activity is often present during IVS, we failed to record EMG data during IVS. Therefore, we cannot examine the potential effects of EMG on BIS.
In conclusion, oral premedication of pregabalin reduced the amount of propofol required to achieve an adequate level of sedation. To confirm our findings, further randomized controlled trials are needed. In addition, it seems necessary to investigate the anesthetic effects of inhibiting excitatory neurotransmitter release from presynaptic terminals in the CNS.
References
Gajraj NM. Pregabalin for pain management. Pain Pract. 2005;5:95–102.
Moore RA, Straube S, Wiffen PJ, Derry S, McQuary HJ. Pregabalin for acute and chronic pain in adults. Cochrane Database Syst Rev. 2009;3:CD007076.
Yucel A, Ozturk E, Aydoan MS, Durmus M, Colak C, Ersoy MO. Effects of 2 different doses of pregabalin on morphine consumption and pain after abdominal hysterectomy: a randomized, double-blind clinical trial. Curr Ther Res Clin Exp. 2011;72:173–83.
Mathiesen O, Jørgensen DG, Hilsted KL, Trolle W, Stjernholm P, Christiansen H, Hjortsø NC, Dahl JB. Pregabalin and dexamethasone improves post-operative pain treatment after tonsillectomy. Acta Anaesthesiol Scand. 2011;55:297–305.
Peng PW, Li C, Farcas E, Haley A, Wong W, Bender J, Chung F. Use of low-dose pregabalin in patients undergoing laparoscopic cholecystectomy. Br J Anaesth. 2010;105:155–61.
Bornemann-Cimenti H, Lederer AJ, Wejbora M, Michaeli K, Kern-Pirsch C, Archan S, Rumpold-Seitlinger G, Zigeuner R, Sandner-Kiesling A. Preoperative pregabalin administration significantly reduces postoperative opioid consumption and mechanical hyperalgesia after transperitoneal nephrectomy. Br J Anaesth. 2012;108:845–9.
Hill CM, Balkennohl M, Thomas DW, Walker R, Mathe H, Murray G. Pregabalin in patients with postoperative dental pain. Eur J Pain. 2001;5:119–24.
Jokela R, Ahonen J, Tallgren M, Haanpaa M, Korttila K. A randomized controlled trial of perioperative administration of pregabalin for pain after laparoscopic hysterectomy. Pain. 2008;134:106–12.
White PF, Tufanogullari B, Taylor J, Klein K. The effect of pregabalin on postoperative anxiety and sedation levels: a dose-ranging study. Anesth Analg. 2009;108:1140–5.
Ghai A, Gupta M, Rana N, Wadhera R. The effect of pregabalin and gabapentin on preoperative anxiety and sedation: a double blind study. Anaesth Pain Intensive Care. 2012;16:257–61.
Mishriky BM, Waldron NH, Habib AS. Impact of pregabalin on acute and persistent postoperative pain: a systematic review and meta-analysis. Br J Anaesth. 2014;114:10–31.
Moreau-Bussière F, Gaulin J, Gagnon V, Sansoucy Y, Médicis E. Preoperative pregabalin does not reduce propofol ED50: a randomized controlled trial. Can J Anaesth. 2013;60:364–9.
Baidya DK, Agarwal A, Khanna P, Arora MK. Pregabalin for acute and chronic pain. J Anaesthesiol Clin Pharmacol. 2011;27:307–14.
Bauer CS, Nieto-Rostro M, Rahman W, Tran-Van-Minh A, Ferron L, Douglas L, Kadurin I, Sri Ranjan Y, Fernandez-Alacid L, Millar NS, Dickenson AH, Lujan R, Dolphin AC. The increased trafficking of the calcium channel subunit α2δ-1 to presynaptic terminals in neuropathic pain is inhibited by the α2δ ligand pregabalin. J Neurosci. 2009;29:4076–88.
Fink K, Dooley DJ, Meder WP, Suman-Chauhan N, Duffy S, Clusmann H, Göthert M. Inhibition of neuronal Ca2+ influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology. 2002;42:229–36.
Tanabe M, Takasu K, Takeuchi Y, Ono H. Pain relief by gabapentin and pregabalin via supraspinal mechanism after peripheral nerve injury. J Neurosci Res. 2008;86:3258–64.
Bee LA, Dickenson AH. Descending facilitation from the brainstem determines behavioural and neuronal hypersensitivity following nerve injury and efficacy of pregabalin. Pain. 2008;140:209–23.
Sills GJ. The mechanism of action of gabapentin and pregabalin. Curr Opin Pharmacol. 2006;6:108–13.
Ramsay MAE, Savege TM, Simpson BRJ, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–9.
James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E. Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–20.
Taffe P, Sicard N, Pittet V, Pichard S, Burnand B. The occurrence of intra-operative hypotension varies between hospitals: observational analysis of more than 147,000 anaesthesia. Acta Anaesthesiol Scand. 2009;53:995–1005.
Ito S, Sugiyama H, Kitahara S, Ikemoto Y, Yokoyama T. Effects of propofol and pentobarbital on calcium concentration in presynaptic boutons on a rat hippocampal neuron. J Anesth. 2011;25:727–33.
Ito S, Karube N, Sugiyama H, Hirokawa J, Kitahara S, Yokoyama T. Effects of propofol on glutamate-induced calcium mobilization in presynaptic boutons of rat hippocampal neurons. Open J Anesthesiol. 2016;6:27–36.
Herring BE, McMillan K, Pike CM, Marks J, Fox AP, Xie Z. Etomidate and propofol inhibit the neurotransmitter release machinery at different sites. J Physiol. 2011;589:1103–15.
Xie Z, McMillan K, Pike CM, Cahill AL, Herring BE, Wang Q, Fox AP. Interaction of anesthetics with neurotransmitter release machinery proteins. J Neurophysiol. 2013;109:758–67.
Wakita M, Kotani N, Nonaka K, Shin M, Akaike N. Effects of propofol on GABAergic and glutamatergic transmission in isolated hippocampal single nerve-synapse preparations. Eur J Pharmacol. 2013;718:63–73.
Bhawna R, Gupta K, Gupta PK, Agarwal S, Jain M, Chauhan H. Oral pregabalin premedication for attenuation of haemodynamic pressor response of airway instrumentation during general anaesthesia: a dose response study. Indian J Anaesth. 2012;56:49–54.
Gupta K, Bansal P, Gupta PK, Singh YP. Pregabalin premedication—a new treatment option for hemodynamic stability during general anesthesia: a prospective study. Anesth Essays Res. 2011;5:57–62.
Eipe N, Penning J. Postoperative depression associated with pregabalin: a case series and a preoperative decision algorithm. Pain Res Manag. 2011;16:353–6.
Panousis P, Heller AR, Burghardt M, Bley MJ, Koch T. The effects of electromyographic activity on the accuracy of the Narcotrend® monitor compared with the Bispectral Index during combined anaesthesia. Anaesthesia. 2007;62:868–74.
Acknowledgements
This work was supported by the Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research (24593058) and the Academic Enrichment Fund allotted to the Department of Dental Anesthesiology, Faculty of Dental Science, Kyushu University.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Karube, N., Ito, S., Sako, S. et al. Sedative effects of oral pregabalin premedication on intravenous sedation using propofol target-controlled infusion. J Anesth 31, 586–592 (2017). https://doi.org/10.1007/s00540-017-2366-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-017-2366-7