Abstract
Aim and background
Reducing inflammation by nutritional supplements may help to reduce the risk of many chronic diseases. Our aim in this meta-analysis was to determine the effect of L-carnitine on inflammatory mediators including C-reactive protein (CRP), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6).
Methods
Our systematic search to find relevant randomized clinical trials (RCTs) was performed up to October 2018 using ISI Web of Science, Google Scholar, PubMed/Medline, and SCOPUS. In this meta-analysis, the weighted mean differences (WMD) with standard errors (SE) were used to pool the data. WMD was calculated by subtracting change-from-baseline mean values in the control group from change-from-baseline mean values in the intervention group in each study. To identify heterogeneity among studies, the I2 statistic was employed. The protocol was registered with PROSPERO (No. CRD42019116695).
Results
Thirteen articles were included in our systematic review and meta-analysis. The results of the meta-analysis indicated that L-carnitine supplementation was significantly associated with lower levels of CRP in comparison to controls (WMD = −1.23 mg/L; 95% CI: −1.73, −0.72 mg/dL; P < 0.0001). Also, a slight but statistically significant decrease was observed in IL-6 and TNF-α levels (WMD = −0.85 pg/dL; 95% CI: −1.38, −0.32 pg/dL; P = 0.002 and WMD = −0.37 pg/dL; 95% CI: −0.68, −0.06 pg/dL; P = 0.018, respectively).
Conclusion
Our results indicate that L-carnitine reduced inflammatory mediators, especially in studies with a duration of more than 12 weeks. Further studies with different doses and intervention durations and separately in men and women are necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past decade, the relationship between atherosclerosis and inflammation has been widely studied (Table 1). A large body of evidence suggests that the process of atherosclerotic disease is correlated with inflammatory mediator levels. Higher inflammation status can cause development of coronary artery disease (CAD) [1, 2]. In human studies, the levels of C-reactive protein (CRP), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) have been used to predict the risk for CAD [3,4,5]. Reducing inflammation through nutritional supplements may be able to lower the risk of many chronic diseases [6,7,8].
L-carnitine (β-hydroxy-γ-trimethyl-amino-butyric acid, LC) is an essential compound which is synthesized in the kidneys and liver from lysine and methionine or supplied to body by dietary sources such as meat and dairy products [9]. LC is also a necessary cofactor for fatty acid β-oxidation and facilitates long-chain fatty acid transportation across the inner membrane of mitochondria. Therefore, lack of LC impairs the use of fatty acids as fuel [10, 11].
LC is also used as a dietary supplement and has gained popularity with recent reporting of suggested anti-inflammatory properties [12,13,14,15]. LC might be able to reduce inflammation by modulating the function of inflammatory cells [16, 17]. A recent in vitro study has shown that LC might mitigate inflammation by controlling TNF-α and nuclear factor-kappa B (NF-κB) production [17].
There are many randomized clinical trials (RCTs) on the effects of oral and intravenous administration of LC among adult healthy and unhealthy populations, as well as its effects on inflammatory mediators, but their results are inconsistent. Some RCTs suggested that oral LC has a lowering effect on inflammatory mediators, while a few of them did not indicate any effect. However, a meta-analysis in 2015 on six RCTs suggested a lowering effect of LC on CRP [18]. In this meta-analysis, other important inflammatory mediators such as IL-6 and TNF-α were not included, while several new RCTs are available which have either used higher doses of LC or prescribed it for longer duration. Therefore, since there is no systematic review assessing the effect of oral intake of LC on IL-6 and TNF-α in humans, we aimed to perform a systematic review to summarize the effect of oral LC on these inflammatory mediators among healthy and unhealthy adult populations and update its effect on CRP using new published articles in this regard.
Materials and methods
We used systematic search options to search electronic databases including ISI Web of Science, Google Scholar, PubMed/Medline, and SCOPUS to find articles measuring the effect of LC on inflammatory mediators up to October 2018. To search electronic databases, the following MeSH and non-MeSH key words were used: ‘TNF alpha’, ‘Tumor Necrosis Factor-alpha’, ‘Tumor Necrosis Factor alpha’, ‘TNF-alpha’, ‘Tumor Necrosis Factor’, ‘C-Reactive Protein’, ‘Protein, C-Reactive’, ‘C Reactive Protein’, ‘CRP’, ‘Interleukin 6’, ‘IL6’, ‘IL-6’, ‘Interleukin-6’, ‘carnitine’, ‘L-carnitine’, ‘Acetyl Carnitine’, ‘Carnitine, Acetyl’, ‘acetyl-L-carnitine’, ‘propionyl-L-carnitine’. To design a systematic search strategy, quotation marks, parentheses, asterisks, and Boolean operators were used. We used quotation marks to search for the exact term, parentheses to search for a group search term, and asterisks to search for all words derived from one keyword. EndNote software (reference manager software, version X6) was used to import all articles found by systematic search method and to read titles and abstracts. Two authors (MH, FH) read titles and abstracts separately, and all discrepancies were resolved by group discussion. In addition, a list of references of relevant RCTs was searched to find additional articles. We did not impose any restriction on publication date or study design. We emailed corresponding authors in cases of any unclear data. The protocol was registered with PROSPERO (No. CRD42019116695).
Inclusion criteria
Articles with the following criteria were considered in the meta-analysis: 1) original articles; 2) articles with randomized controlled trial design; 3) human studies; 4) use of LC for intervention; 5) taking LC in oral form; 6) not taking any other food supplement in intervention or control groups; 7) if LC group had received any intervention alongside LC supplements, that intervention was also received in the placebo group; 8) articles which assessed CRP or IL-6 or TNF-α as the outcome variables; 9) articles that clearly reported the concentration of CRP or IL-6 or TNF-α at the beginning and end of intervention; and 10) English language articles.
Exclusion criteria
Articles with the following criteria were excluded: 1) reporting unclear data in figures and tables; 2) lack of clear inclusion and exclusion criteria; 3) not having control group; 4) taking other food supplements or diet alongside LC in the intervention group but not in the control group; 5) using intravenous form of LC; and 6) recruiting subjects with inflammatory diseases such as arthritis, hepatitis C, or inflammatory bowel disease.
Data extraction
Two authors (MH and FH) assessed eligible articles in order to extract the following information: 1) first author’s last name; 2) country in which study was performed; 3) publication year; 4) study design (parallel or cross-over); 5) number of participants in control and intervention groups; 6) mean and standard deviation of IL-6, TNF-α, and CRP; 7) age; 8) body mass index (BMI); 9) health status; 10) LC dose; 11) type of placebo; and 12) study duration.
Quality assessment
A Delphi checklist was used for assessing the quality of articles [19]. Delphi scores range from zero (very poor) to 9 (rigorous) where the included items are: I) standard randomization; II) blinding the participants; III) blinding the researchers; IV) blinding outcome analyzer; V) defining inclusion and exclusion criteria; VI) concealment of intervention allocation; VII) similarity between participants in placebo and intervention group at the beginning; VIII) intention-to-treat analysis; and IX) presenting variability of the outcome.
Statistical analysis
In this meta-analysis, the weighted mean differences (WMD) with standard errors (SE) were used to pool data. WMD was calculated by subtracting change-from-baseline mean values in the control group from change-from-baseline mean values in the intervention group in each study. To identify the heterogeneity among studies, the I2 statistic was employed. When I2 was >50%, the heterogeneity was considered to be statistically significant. In the presence of considerable heterogeneity, random-effects model was used to estimate the pooled effect of treatment; otherwise, a fixed effect model was utilized. Subgroup analysis was also performed to identify the potential sources of heterogeneity. Sensitivity analysis was conducted to assess the robustness of our findings by removing one study at a time. Publication bias was evaluated by Egger’s test and visual inspection of funnel plots when the effect sizes were greater than 10. P < 0.1 for Egger’s test presents a significant publication bias. In the case of bias, trim and fill analysis was conducted to detect the contribution of the bias to the overall effect. All statistical analyses were performed using STATA version 11 software (StataCorp, College Station, TX, USA).
Results
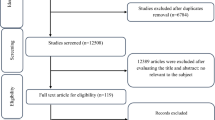
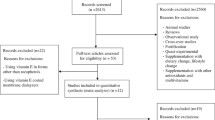
A search of the above-mentioned electronic databases yielded 1105 articles. After removing duplicate articles, 571 remained. Through reading the title and abstract, 546 articles were deleted and 25 articles were assessed for consideration of inclusion or exclusion criteria. According to our inclusion and exclusion criteria, 12 articles were excluded, while 13 articles [12,13,14,15, 20,21,22,23,24,25,26,27,28] were included in our systematic review and meta-analysis (Table 1). Exclusion was for the following reasons (for 17 articles): not having control group (n = 4), unclear tables (n = 2), conducting other interventions alongside taking LC in the intervention group but not the in control group (n = 1), using intravenous form of LC (n = 3), subjects younger than 18 years (n = 2) (Fig. 1). A total of 1108 participants were included in the present analysis. LC dose ranged from 1500 mg to 4000 mg, and intervention duration ranged from 5 days to 48 weeks. Both men and women were included in all but three articles: one included only men [14] and two only women [12, 28]. We did not include unpublished data in this meta-analysis; no additional information obtained by contacting authors is included in this manuscript.
In a study by Rafraf [28], there were three intervention groups (LC, LC + aerobic training, aerobic training + placebo) and one placebo group. We considered the result of the LC and placebo groups as one study and the result of the LC + aerobic training and placebo + aerobic training groups as another study.
Overall, 10 articles measured CRP [12, 14, 15, 21,22,23, 25, 27,28,29], four articles measured IL-6 [14, 15, 20, 28], and seven articles measured TNF-α [13,14,15, 20, 24, 26, 27].
Findings from the meta-analysis
Nine studies involving 11 comparisons with 455 subjects in the intervention group and 446 subjects in the control group assessed the effect of L-carnitine supplementation on serum CRP levels (Fig. 2). L-carnitine supplementation was significantly associated with lower levels of CRP as compared to the control group (WMD = −1.23 mg/L; 95% CI: −1.73, −0.72 mg/L; P < 0.0001). Significant heterogeneity was found among included studies (I2 = 95.0%). Although subgroup analysis identified L-carnitine dosage and gender as potential sources of heterogeneity (P between subgroups <0.05), none of them could eliminate heterogeneity (Table 2).
Five comparisons from four studies with 86 subjects in the intervention group and 83 subjects in the control group reported the effect of L-carnitine on serum IL-6 changes (Fig. 3). The pooled effect demonstrated a slight but statistically significant decline in IL-6 levels following L-carnitine supplementation (WMD = −0.85 pg/dL; 95% CI: −1.38, −0.32 pg/dL; P = 0.002) with significant heterogeneity among included studies (I2 = 77.9%). Subgroup analysis based on L-carnitine dosage and study duration could not explain heterogeneity (Table 2).
Seven comparisons from six studies including 337 subjects in the intervention group and 335 subjects in the control group measured serum changes in TNF-α following L-carnitine supplementation (Fig. 4). L-carnitine slightly reduced TNF-α concentrations significantly in comparison with the control group (WMD = −0.37 pg/dL; 95% CI: −0.68, −0.06 pg/dL; P = 0.018). Significant heterogeneity was observed among the studies (I2 = 91.3%). Subgroup analysis based on L-carnitine dosage and study duration could not explain heterogeneity (Table 2), though study duration was identified as a potential source of heterogeneity (P between subgroups < 0.05).
Sensitivity analysis and publication bias
Sensitivity analysis was conducted to determine whether the effect size of any individual trial would alter the pooled effect size. No significant change was observed in the overall effects of L-carnitine on CRP and IL-6, but results for TNF-α revealed that removal of different studies led to non-significant changes in TNF-α levels [13, 14, 27, 29]. Both funnel plot and Egger’s regression test suggested evidence of publication bias (P < 0.1). However, based on the trim and fill algorithm, the adjusted value did not differ considerably from unadjusted values (WMD = −1.23, 95% CI: −1.73, −0.72). In spite of visual asymmetry in the funnel plot of TNF-α, Egger’s regression test suggested no evidence of publication bias (P = 0.560).
Discussion
This meta-analysis measured the effect of LC on inflammatory mediators. The results revealed that LC decreased IL-6, TNF-α, and CRP. According to our subgroup analysis, LC was more effective in reducing inflammation in studies with a duration of more than 12 weeks. Furthermore, LC with a dose greater than 2000 mg/day was more effective in TNF-α reduction. Our systematic search method indicated that our meta-analysis is the first article summarizing the effects of LC on IL-6 and TNF-α. On the other hand, one meta-analysis on six articles assessed the LC effect on CRP in 2015. Our meta-analysis confirms the results of Sahebkar’s meta-analysis regarding the effects of LC on CRP [18].
Recent evidence has indicated that LC prevents oxidative damage in conditions such as cardiovascular disease by reducing lipid peroxidation, increasing antioxidant defense systems such as antioxidant enzymes, and chelating transition metal ions [30,31,32]. It is well known that reactive oxygen species (ROS) enhance inflammation [33,34,35]. ROS can increase the expression of pro-inflammatory mediators and then up-regulate the NF-κB pathway [36]. NF-κB is a transcription factor and regulates the expression of numerous genes involved in inflammatory and immune responses [37].
Furthermore, LC was able to up-regulate peroxisome proliferator-activated receptor (PPAR)γ [38], which is a key factor in the regulation of oxidative stress and liver inflammation [39, 40]. This evidence suggests that LC might be able to improve liver inflammatory response through regulation of CPT I-dependent PPARγ signaling pathway.
Our results indicated that LC at doses higher than 2000 mg/day was more effective in inflammation reduction. Other meta-analysis articles also indicated that LC with a dose greater than 2000 mg/day is more effective in improving health outcomes [41, 42]. New evidence suggests that lower doses of LC are unlikely to provide absorbable levels of carnitine sufficient to have a beneficial effect. Pharmacokinetic studies have reported that LC has poor oral bioavailability and that with a single oral dose of LC, only 5–16% is absorbed; therefore, higher doses might be more effective [43, 44]. According to our subgroup analysis, LC was more protective in studies longer than 12 weeks. A meta-analysis demonstrated that taking LC for longer periods is more effective in LDL-c reduction [45]; therefore, by reducing atherogenic factors, LC might decrease inflammatory mediators.
There is new evidence regarding the effect of LC on health outcomes such as glucose metabolism and lipid profile [46, 47]. Two recent meta-analyses indicated that LC could improve insulin resistance [46, 47]. LC might improve the glycemic index by reducing inflammation, especially CRP [48]. High levels of CRP may cause glucose intolerance and insulin resistance [49]; therefore, LC can improve insulin resistance by reducing CRP concentration.
Strengths
For our article, several strengths should be considered: First, our results indicated that LC could decrease inflammatory mediators which are considered risk factors for metabolic diseases; therefore, our results will help inform clinicians regarding a consensus of the effects of LC on metabolic disease risk factors. Second, we did not impose any limitation on publication time or article language. Further, we indicated the effects of sex, LC dose, and intervention duration on the effect of LC on inflammatory mediators by subgroup analysis. Also, systematic search results indicated that this meta-analysis is the first to examine the effects of LC on inflammatory mediators. Next, we excluded studies which used nutrients other than LC in the intervention group, and we could therefore exclude the confounding effects of other nutrients. Finally, lack of significant asymmetry in funnel plots was evidence of no publication bias.
Limitations
The following can be considered as limitations of this meta-analysis: 1) The most important limitation was the small number of studies included in the quantitative data synthesis. We had five effect sizes for IL-6, 11 effect sizes for CRP, and seven effect sizes for TNF-α. 2) Our meta-analysis could not identify any correlation between the reduction in inflammatory mediator levels and participants’ clinical outcomes. 3) Since changes in body composition can influence the concentration of inflammatory mediators, subgroup analysis based on changes in fat mass and fat-free mass would be more informative, but most articles did not include information related to changes in body composition. 4) Heterogeneity was high in our analysis. 5) Articles conducted separately on men and women were limited; therefore, gender differences in the effect of LC on inflammation remain unknown. 6) Some confounding factors such as dietary intake of participants, smoking, and physical activity were not considered in the analysis, as no article had information related to them. 7) There was considerable heterogeneity among studies, and we could not eliminate it by statistical methods. Therefore, the results of this meta-analysis should be interpreted with caution.
Conclusion
In conclusion, our results indicate that LC reduced inflammatory mediators, especially in studies with a duration of more than 12 weeks. Additional studies with different doses and intervention duration, and separately on men and women, are necessary. Changes in body composition, nutrient intake, physical activity, and smoking should be considered as confounding factors in future studies. Since inflammatory markers are surrogate parameters, future studies should indicate whether reducing inflammation is associated with improved clinical outcomes.
References
Tracy RP (2002) Inflammation in cardiovascular disease: cart, horse or both--revisited. Arterioscler Thromb Vasc Biol 22(10):1514–1515
Ross R (1999) Atherosclerosis is an inflammatory disease. Am Heart J 138(5 Pt 2):S419–S420
Ridker PM, Rifai N, Stampfer MJ, Hennekens CH (2000) Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 101(15):1767–1772
Gurrola-Diaz CM, Sanchez-Enriquez S, Oregon-Romero E, Garcia-Lopez PM, Garzon de la Mora P, Bastidas-Ramirez BE et al (2009) Establishment of a cut-point value of serum TNF-alpha levels in the metabolic syndrome. J Clin Lab Anal 23(1):51–56
Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M et al (2003) Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107(3):499–511
Haghighatdoost F, Hariri M (2018) Can resveratrol supplement change inflammatory mediators? A systematic review and meta-analysis on randomized clinical trials. Eur J Clin Nutr 73:345
Azimi P, Ghiasvand R, Feizi A, Hosseinzadeh J, Bahreynian M, Hariri M et al (2016) Effect of cinnamon, cardamom, saffron and ginger consumption on blood pressure and a marker of endothelial function in patients with type 2 diabetes mellitus: a randomized controlled clinical trial. Blood Press 25(3):133–140
Feizollahzadeh S, Ghiasvand R, Rezaei A, Khanahmad H, Sadeghi A, Hariri M (2017) Effect of probiotic soy milk on serum levels of adiponectin, inflammatory mediators, lipid profile, and fasting blood glucose among patients with type II diabetes mellitus. Probiotics Antimicrob Proteins 9(1):41–47
Pekala J, Patkowska-Sokola B, Bodkowski R, Jamroz D, Nowakowski P, Lochynski S et al (2011) L-carnitine--metabolic functions and meaning in humans life. Curr Drug Metab 12(7):667–678
Uziel G, Garavaglia B, Di Donato S (1988) Carnitine stimulation of pyruvate dehydrogenase complex (PDHC) in isolated human skeletal muscle mitochondria. Muscle Nerve 11(7):720–724
Amat di San Filippo C, Taylor MR, Mestroni L, Botto LD, Longo N (2008) Cardiomyopathy and carnitine deficiency. Mol Genet Metab 94(2):162–166
Malek Mahdavi A, Mahdavi R, Kolahi S (2016) Effects of l-carnitine supplementation on serum inflammatory factors and matrix metalloproteinase enzymes in females with knee osteoarthritis: a randomized, double-blind, placebo-controlled pilot study. J Am Coll Nutr 35(7):597–603
Derosa G, Maffioli P, Ferrari I, D’Angelo A, Fogari E, Palumbo I et al (2011) Comparison between orlistat plus l-carnitine and orlistat alone on inflammation parameters in obese diabetic patients. Fundam Clin Pharmacol 25(5):642–651
Lee BJ, Lin JS, Lin YC, Lin PT (2015) Antiinflammatory effects of L-carnitine supplementation (1000 mg/d) in coronary artery disease patients. Nutrition (Burbank, Los Angeles County, Calif) 31(3):475–479
Shakeri A, Tabibi H, Hedayati M (2010) Effects of L-carnitine supplement on serum inflammatory cytokines, C-reactive protein, lipoprotein (a), and oxidative stress in hemodialysis patients with Lp (a) hyperlipoproteinemia. Hemodial Int Int Symp Home Hemodial 14(4):498–504
Moeinian M, Farnaz Ghasemi-Niri S, Mozaffari S, Abdollahi M (2013) Synergistic effect of probiotics, butyrate and l-carnitine in treatment of IBD. J Med Hypotheses Ideas 7(2):50–53
Koc A, Ozkan T, Karabay AZ, Sunguroglu A, Aktan F (2011) Effect of L-carnitine on the synthesis of nitric oxide in RAW 264.7 murine macrophage cell line. Cell Biochem Funct 29(8):679–685
Sahebkar A (2015) Effect of L-carnitine supplementation on circulating C-reactive protein levels: a systematic review and meta-analysis. J Med Biochem 34(2):151–159
Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM et al (1998) The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 51(12):1235–1241
Badrasawi M, Shahar S, Zahara AM, Nor Fadilah R, Singh DK (2016) Efficacy of L-carnitine supplementation on frailty status and its biomarkers, nutritional status, and physical and cognitive function among prefrail older adults: a double-blind, randomized, placebo-controlled clinical trial. Clin Interv Aging 11:1675–1686
Bloomer RJ, Fisher-Wellman KH, Tucker PS (2009) Effect of oral acetyl L-carnitine arginate on resting and postprandial blood biomarkers in pre-diabetics. Nutr Metab 6:25
Dastan F, Talasaz AH, Mojtahedzadeh M, Karimi A, Salehiomran A, Bina P et al (2018) Randomized trial of carnitine for the prevention of perioperative atrial fibrillation. Phytothe Res PTR 30(1):7–13
Derosa G, Maffioli P, Ferrari I, D’Angelo A, Fogari E, Palumbo I et al (2010) Orlistat and L-carnitine compared to orlistat alone on insulin resistance in obese diabetic patients. Endocr J 57(9):777–786
Derosa G, Maffioli P, Salvadeo SA, Ferrari I, Gravina A, Mereu R et al (2011) Effects of combination of sibutramine and L-carnitine compared with sibutramine monotherapy on inflammatory parameters in diabetic patients. Metab Clin Exp 60(3):421–429
Hakeshzadeh F, Tabibi H, Ahmadinejad M, Malakoutian T, Hedayati M (2010) Effects of L-carnitine supplement on plasma coagulation and anticoagulation factors in hemodialysis patients. Ren Fail 32(9):1109–1114
Jirillo E, Altamura M, Munno I, Pellegrino NM, Sabato R, Di Fabio S et al (1991) Effects of acetyl-L-carnitine oral administration on lymphocyte antibacterial activity and TNF-alpha levels in patients with active pulmonary tuberculosis. A randomized double blind versus placebo study. Immunopharmacol Immunotoxicol 13(1–2):135–146
Malaguarnera M, Gargante MP, Russo C, Antic T, Vacante M, Malaguarnera M et al (2010) L-carnitine supplementation to diet: a new tool in treatment of nonalcoholic steatohepatitis--a randomized and controlled clinical trial. Am J Gastroenterol 105(6):1338–1345
Rafraf M, Karimi M, Jafari A (2015) Effect of L-carnitine supplementation in comparison with moderate aerobic training on serum inflammatory parameters in healthy obese women. J Sports Med Phys Fit 55(11):1363–1370
Derosa G, Maffioli P, Salvadeo SA, Ferrari I, Gravina A, Mereu R et al (2010) Sibutramine and L-carnitine compared to sibutramine alone on insulin resistance in diabetic patients. Intern Med (Tokyo, Japan) 49(16):1717–1725
Lysiak W, Lilly K, DiLisa F, Toth PP, Bieber LL (1988) Quantitation of the effect of L-carnitine on the levels of acid-soluble short-chain acyl-CoA and CoASH in rat heart and liver mitochondria. J Biol Chem 263(3):1151–1156
Broderick TL, Quinney HA, Lopaschuk GD (1992) Carnitine stimulation of glucose oxidation in the fatty acid perfused isolated working rat heart. J Biol Chem 267(6):3758–3763
Gulcin I (2006) Antioxidant and antiradical activities of L-carnitine. Life Sci 78(8):803–811
Binienda ZK, Ali SF (2001) Neuroprotective role of L-carnitine in the 3-nitropropionic acid induced neurotoxicity. Toxicol Lett 125(1–3):67–73
Augustyniak A, Skrzydlewska E (2010) The influence of L-carnitine suplementation on the antioxidative abilities of serum and the central nervous system of ethanol-induced rats. Metab Brain Dis 25(4):381–389
Miguel-Carrasco JL, Monserrat MT, Mate A, Vazquez CM (2010) Comparative effects of captopril and l-carnitine on blood pressure and antioxidant enzyme gene expression in the heart of spontaneously hypertensive rats. Eur J Pharmacol 632(1–3):65–72
Setia S, Sanyal SN (2012) Nuclear factor kappa B: a pro-inflammatory, transcription factor-mediated signalling pathway in lung carcinogenesis and its inhibition by nonsteroidal anti-inflammatory drugs. J Environ Pathol Toxicol Oncol Off Organ Int Soc Environ Toxicol Cancer 31(1):27–37
Siomek A (2012) NF-kappaB signaling pathway and free radical impact. Acta Biochim Pol 59(3):323–331
Zambrano S, Blanca AJ, Ruiz-Armenta MV, Miguel-Carrasco JL, Arevalo M, Vazquez MJ et al (2013) L-carnitine protects against arterial hypertension-related cardiac fibrosis through modulation of PPAR-gamma expression. Biochem Pharmacol 85(7):937–944
El-Sheikh AA, Rifaai RA (2014) Peroxisome proliferator activator receptor (PPAR)-gamma ligand, but not PPAR-alpha, ameliorates cyclophosphamide-induced oxidative stress and inflammation in rat liver. PPAR Res 2014:626319
Chen K, Li J, Wang J, Xia Y, Dai W, Wang F et al (2014) 15-deoxy-gamma 12,14-prostaglandin J2 reduces liver impairment in a model of ConA-induced acute hepatic inflammation by activation of PPAR gamma and reduction in NF-kappa B activity. PPAR Res 2014:215631
Pooyandjoo M, Nouhi M, Shab-Bidar S, Djafarian K, Olyaeemanesh A (2016) The effect of (L-)carnitine on weight loss in adults: a systematic review and meta-analysis of randomized controlled trials. Obes Rev Off J Int Assoc Study Obes 17(10):970–976
Nazary-Vannani A, Ghaedi E, Mousavi SM, Teymouri A, Rahmani J, Varkaneh HK (2018) The effect of L-carnitine supplementation on serum leptin concentrations: a systematic review and meta-analysis of randomized controlled trials. Endocrine 60(3):386–394
Harper P, Elwin CE, Cederblad G (1988) Pharmacokinetics of bolus intravenous and oral doses of L-carnitine in healthy subjects. Eur J Clin Pharmacol 35(1):69–75
Bain MA, Milne RW, Evans AM (2006) Disposition and metabolite kinetics of oral L-carnitine in humans. J Clin Pharmacol 46(10):1163–1170
Huang H, Song L, Zhang H, Zhang H, Zhang J, Zhao W (2013) Influence of L-carnitine supplementation on serum lipid profile in hemodialysis patients: a systematic review and meta-analysis. Kidney Blood Press Res 38(1):31–41
Xu Y, Jiang W, Chen G, Zhu W, Ding W, Ge Z et al (2017) L-carnitine treatment of insulin resistance: a systematic review and meta-analysis. Adv Clin Exp Med Off Organ Wroclaw Med Univ 26(2):333–338
Vidal-Casariego A, Burgos-Pelaez R, Martinez-Faedo C, Calvo-Gracia F, Valero-Zanuy MA, Luengo-Perez LM et al (2013) Metabolic effects of L-carnitine on type 2 diabetes mellitus: systematic review and meta-analysis. Exp Clin Endocrinol Diabetes Off J Ger Soc Endocrinol Ger Diabetes Assoc 121(4):234–238
Shoelson SE, Lee J, Goldfine AB (2006) Inflammation and insulin resistance. J Clin Invest 116(7):1793–1801
Wu T, Dorn JP, Donahue RP, Sempos CT, Trevisan M (2002) Associations of serum C-reactive protein with fasting insulin, glucose, and glycosylated hemoglobin: the third National Health and nutrition examination survey, 1988-1994. Am J Epidemiol 155(1):65–71
Author contributions
MH found keywords and conducted database searches. FH, MH, and MJ found relevant RCTs, excluded irrelevant RCTs, read full text of articles, and extracted data. FH conducted statistical analysis. MH wrote the first version of article, FH corrected the first version of the paper, and MJ performed final editing. Discrepancies in any part of the work were resolved through group discussions. MJ rewrote some parts of the manuscript according to reviewers’ comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Haghighatdoost, F., Jabbari, M. & Hariri, M. The effect of L-carnitine on inflammatory mediators: a systematic review and meta-analysis of randomized clinical trials. Eur J Clin Pharmacol 75, 1037–1046 (2019). https://doi.org/10.1007/s00228-019-02666-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-019-02666-5