Abstract
Background
Lipoproteins are closely associated with the atherosclerotic vascular process. Elevated levels of high-density lipoprotein cholesterol (HDL-C) and apolipoprotein AI (apo AI) in plasma indicate a low probability of coronary heart disease (CHD) together with enhanced longevity, and elevated levels of low-density lipoprotein-cholesterol (LDL-C) and apo B indicate an increased risk of CHD and death. Studies linking gene activation and the induction of cytochrome P450 with elevated plasma levels of apo AI and HDL-C and lowered plasma levels of LDL-C presented a new potential approach to prevent and treat atherosclerotic disease.
Objective and methods
This is a review aimed at clarifying the effects of P450-enzymes and gene activation on cholesterol homeostasis, the atherosclerotic vascular process, prevention and regression of atherosclerosis and the manifestation of atherosclerotic disease, particularly CHD, the leading cause of death in the world.
Results
P450-enzymes maintain cellular cholesterol homeostasis. They respond to cholesterol accumulation by enhancing the generation of hydroxycholesterols (oxysterols) and activating cholesterol-eliminating mechanisms. The CYP7A1, CYP27A1, CYP46A1 and CYP3A4 enzymes generate major oxysterols that enter the circulation. The oxysterols activate—via nuclear receptors—ATP-binding cassette (ABC) A1 and other genes, leading to the elimination of excess cholesterol and protecting arteries from atherosclerosis. Several drugs and nonpharmacologic compounds are ligands for the liver X receptor, pregnane X receptor and other receptors, activate P450 and other genes involved in cholesterol elimination, prevent or regress atherosclerosis and reduce cardiovascular events.
Conclusions
P450-enzymes are essential in the physiological maintenance of cholesterol balance. They activate mechanisms which eliminate excess cholesterol and counteract the atherosclerotic process. Several drugs and nonpharmacologic compounds induce P450 and other genes, prevent or regress atherosclerosis and reduce the occurrence of non-fatal and fatal CHD and other atherosclerotic diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the 1960s, cytochrome P450 was known as a hepatic enzyme directly involved in the metabolism of drugs and other foreign compounds [1–3]. Studies in the 1970s linked liver microsomal P450-induction with elevated levels of plasma apolipoprotein AI (apo AI) and high-density lipoprotein cholesterol (HDL-C) [4, 5], which then were identified as powerful predictors of coronary heart disease (CHD), cerebrovascular and other atherosclerotic diseases [6, 7]. This was followed by the discovery that plasma levels of LDL cholesterol (LDL-C) decreased with increasing P450 activity in the liver [8, 9]. High plasma HDL-C and apo AI indicate a low probability of CHD together with enhanced longevity, and individuals with high LDL-C or apo B have an increased risk of CHD and death [6, 7, 10]. The results from the original studies on P450, cholesterol fractions, proteins and induction suggested a novel approach to atherosclerosis—i.e. activation of P450 and other genes coding proteins which regulate cholesterol balance in the body— and directed research to new avenues [5, 9, 11].
By 1980, the first P450 isoenzymes were identified [12] and in the following years, dozens of P450 genes were found to code enzymes catalysing diverse metabolic processes in humans [13]. In addition to foreign compounds, P450-enzymes metabolize endogenous substrates, including vitamins, steroid hormones, cholesterol and bile acids [13]. Several P450-enzymes metabolize cholesterol to hydroxycholesterols (oxysterols), which have been identified as ligands for nuclear receptors in the induction of genes involved in cholesterol elimination [14, 15]. A number of drugs and nonpharmacologic compounds also induce P450 and other genes coding for proteins involved in lipid metabolism, including apo AI, the predominant apolipoprotein of HDL. This article reviews studies on P450-enzymes and gene activation and gene-activating compounds acting in cholesterol elimination and the prevention and regression of atherosclerotic cardiovascular disease, particularly CHD, which has been identified as the leading cause of death in the world [16].
Liver P450, lipids and proteins and the fate of cholesterol—effect of gene activation
The first studies on P450, apo AI, HDL and LDL evaluated the effects of gene-activating agents on lipids and proteins in the atherosclerotic vascular process (reviewed in [10]). Persons undergoing therapy with drugs such as phenobarbital, primidone or phenytoin, alone or in combination, showed an increase in protein and phospholipid concentrations and P450-induction in the liver as well as a concurrent and parallel elevation in apo AI and HDL-C levels in the plasma implying an upregulation of apo AI and HDL synthesis [11,17]. Further studies revealed that P450- inducing compounds such as phenobarbital [18], fenofibrate [19] and gemfibrozil [20] induce apo AI synthesis, and P450-inhibitors such as ketoconazole and metyrapone [10,20] prevent it. Studies in transgenic animals demonstrated that an activation of human apo AI gene elevates plasma levels of HDL-C and prevents atherogenesis [21]. Ketoconazole also inhibits cholesterol synthesis, reduces LDL cholesterol level [22], and by counteracting the induction of P450 suppresses the generation of oxysterols and activation of nuclear receptor and transporter genes which are involved in the elimination of cholesterol [23]. In contrast to ketoconazole, a high-dose itraconazole therapy has been found raise plasma HDL cholesterol [24]. The inducing effect of itraconazole on apo AI synthesis in Caco-2 cells in vitro suggested a mechanism for the elevation of HDL-C. From the clinical point of view it is significant, that a deficient P450-hydroxylase activity affects the fate of cholesterol leading to cellular cholesterol accumulation [25], hypercholesterolemia [26], and enhanced manifestation of atherosclerotic disease.
P450-isoenzymes and cholesterol
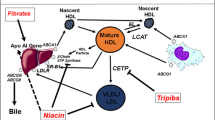
Accumulation of excess cholesterol in cells may have serious consequences; in the arterial wall it can progress to atherosclerotic disease. P450-enzymes are essential in the finely tuned physiological system which controls cell cholesterol balance and takes care of the elimination of excess cholesterol. They are needed in the synthesis of oxysterol and bile acid metabolites of cholesterol and in the activation of the cholesterol-eliminating mechanisms (Fig. 1) [14, 15, 27]. The oxysterols are endogenous signal compounds, which via liver X receptors (LXR) induce genes acting in cholesterol efflux, transport, excretion and absorption [28]. Hydroxylation of cholesterol to oxysterols is necessary for the natural activation of these genes; free cholesterol and cholesterol esters do not have similar ability [29].
From the quantitative point of view, CYP7A1, CYP27A1, CYP46A1 and CYP3A4 are the most important P450-isoenzymes in the formation and metabolism of oxysterols in man [30] (Fig. 1). CYP7A1 is a hepatic key P450-enzyme in maintaining cellular cholesterol balance. It is the ratelimiting enzyme in the most significant pathway for bile acid synthesis. It generates 7α-hydroxycholesterol [31] which is further metabolized to bile acids. CYP8B1 is needed in the synthesis of cholate [32]. CYP27A1, a mitochondrial P450-enzyme, is expressed in most tissues and cell types, including macrophages. It generates 27-hydroxycholesterol which, via the liver X receptor (LXR) activates genes coding transporter proteins that shuttle intracellular cholesterol to outer cell membranes for elimination [33]. A direct secretion of 27-hydroxycholesterol contributes to cholesterol efflux [27]. 27-Hydroxycholesterol is further hydroxylated by oxysterol 7α-hydroxylase, CYP7B1, and also CYP7A1 [34]. CYP46A1 has an active role in cholesterol metabolism in the brain [35]. It generates 24S-hydroxycholesterol, which readily passes the blood brain barrier and thus transports cholesterol from the brain. This metabolite is also a ligand for LXR and may regulate cholesterol balance through transcriptional mechanisms; it may also activate the apo AI-dependent cholesterol efflux from brain endothelial cells [36]. Hepatic CYP39A1 and possibly also CYP7A1 and CYP27A1 convert 24S-hydroxycholesterol to bile acids [35]. CYP3A4, which is expressed particularly in the liver and intestine, is the most common hepatic P450-enzyme. It metabolizes about half of all pharmaceutical agents and also acts on cholesterol and bile acid metabolism. It generates 4β-hydroxycholesterol [30].
CYP51A1 (lanosterol-14α-demethylase) is ubiquitously expressed and is the only P450 enzyme participating in cholesterol synthesis [37]. It demethylates lanosterol to cholesterol in a reaction that produces oxysterols, which inhibit hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase and sterol synthesis [37, 38]. Squalene, an intermediate in cholesterol synthesis, is metabolized to 24S,25 epoxycholesterol in a shunt pathway; this product is an activator of LXR [15] and may act against cholesterol accumulation.
Oxysterol binding protein (OSBP) and 11 related compounds form a cytoplasmic family of OSBP-related-proteins (ORPs) [39]. These compounds bind intracellular oxysterols, which are mainly generated by P450-enzymes. Recent studies suggest that ORPs are lipid sensors in the integration of lipid and sterol metabolism and transport and the regulation of cell signalling [39].
Nuclear receptors, ABC transporters, apolipoproteins and P450-enzymes
Nuclear LXR receptors
LXRα and LXRβ, are cholesterol sensors that mediate the expression of multiple genes involved in the regulation of cellular cholesterol homeostasis [28]. They are activated by physiological concentrations of several oxysterols. LXRα is expressed at high levels in the liver and to lesser extent in the intestine, adipose tissue and macrophages, whereas LXRβ is ubiquitously expressed. The LXR induces transcription of ATP-binding cassette (ABC) transporters, such as ABCA1, G1, G4, G5 and G8, which act in intracellular cholesterol transport, apolipoproteins in the cluster of apo E, CI, CII and CIV, which have been shown to participate in the ABCA1-mediated cholesterol efflux [28], and of apo IV, which may contribute to the latter [40]. In addition, LXR influences the expression of several lipoprotein remodelling enzymes, sterol regulatory binding proteins (SREBPs) and also hepatic scavenger receptor B1 (SR-B1), which selectively uptakes HDL-associated cholesterol esters to the liver [28].
Pregnane X receptor
(PXR), which is expressed predominantly in the human liver and to a lesser extent in the small intestine, is a master regulator of P450 enzymes in the metabolism of xenobiotic compounds and affects the fate of cholesterol [31, 41]. It mediates the induction of CYP3A4 and other P450-enzymes and can be activated by numerous structurally diverse compounds including statins, anticonvulsants and hyperforin, a constituent of the herb St John`s wort [42–45], and bile acid and epoxycholesterol metabolites of cholesterol [46]. The compounds include several PXR agonists [42–45, 47–49] that induce P450 and elevate plasma levels of apo AI and HDL-C [10, 11, 45]. A PXR agonist has been recently found to upregulate CYP27A1 and generate 27-hydroxycholesterol in the intestine, suggesting a LXRα-mediated activation of cholesterol efflux from intestinal cells to apo AI and HDL [50].
Peroxisome proliferator-activated receptors
PPARα, PPARγ and PPARδ, are transcription factors which affect the development of atherosclerosis in many ways. They mediate the induction of apo AI synthesis and co-operate with LXR receptors and ABC transporters in cholesterol elimination [51, 52].
ATP-binding cassette A1
ABCA1, is a key regulator of cellular lipid efflux. It transfers cholesterol and phospholipids to the plasma membrane where apo AI picks up them [53]. Liver ABCA1 has been identified as a main factor [54] and intestinal ABCA1 as a contributory factor [55] in the generation of HDL-C levels in the circulation. Intestinal ABCA1 also controls the absorption of cholesterol by effluxing it from the enterocytes back to the intestinal lumen [56]. A genetic defect in ABCA1 causes Tangier disease, which is characterized by a near or complete absence of HDL, accumulation of cholesterol esters in tissues, and enhanced manifestation of cardiovascular disease [57].
ATP-binding cassette G1
ABCG1, which acts in several tissues, and ABCG4, which acts in the brain, transfer cholesterol to the HDL [53, 58]. The ABCG5 and ABCG8 transporters promote biliary cholesterol secretion and reduce the absorption of dietary cholesterol [59]. Mutations in the ABCG5 or ABCG8 gene cause sitosterolemia, a rare genetic disorder of sterol metabolism characterized by hypercholesterolemia, xanthomas and premature coronary atherosclerosis [60].
Bile acids, nuclear receptors and P450-enzymes
Oxysterols are precursors of bile acids (Fig. 1) which, as ligands for nuclear receptors, such as FXR (farnesoid X receptor), PXR, CAR (constitutive androstane receptor) and VDR (vitamin D3 receptor), activate mechanisms controlling cellular bile acid balance [31, 41]. The FXR has a central role in the regulation on bile acid levels. Accumulation of bile acids stimulates the FXR-mediated suppression of CYP7A1 and CYP8B1, which are key enzymes in the synthesis of bile acids. The FXR also induces the expression of CYP3A4, which in turn detoxifies bile acids by oxidizing them. The PXR and VDR regulate the metabolism of secondary bile acids in the liver and intestine. They mediate the expression of the CYP3A4 gene and the detoxification of bile acids. The PXR also downregulates CYP7A1 in response to the elevation of intracellular bile acid levels. The CAR affects the metabolism of xenobiotic compounds and also detoxifies bile acids.
Drugs and nonpharmacologic compounds, gene activation and the fate of cholesterol
Many drugs and other compounds activate genes that affect the fate of lipids. These include drugs indicated for the treatment of lipid disorders, such as statins, fibrates, cholestyramine and niacin (Table 1), as well as drugs for other indications.
Statins inhibit HMGCoA reductase and cholesterol synthesis and via enzyme-, receptor- and transporter-mediated mechanisms enhance cholesterol elimination, and raise plasma apo AI and HDL-C and reduce apo B and LDL-C. The drugs are ligands for PXR receptor which is a key mediator of the induction of P450 enzymes including CYP3A4 [42–45]. The statins have been identified as P450 inducing agents [10, 42–44, 47–49], and the effects of PXR agonists on HDL-C and apo AI have been linked with their ability to induce CYP3A in rodents [45]. The drugs have been shown to induce PPARα and apo AI synthesis [61, 62] and to activate PPARγ, LXR, ABCA1 and ABCG1 genes in cholesterol-loaded macrophages [63] and ABCA1 in HepG2 cells [64] as well as to increase the cholesterol efflux to apo AI and HDL [63]. Statins can act differently in nonloaded macrophages, inhibiting the synthesis of oxysterol ligand for LXR and downregulating ABCA1 gene and cholesterol efflux [65]. Such effects, however, have not been seen in vivo.
Fibrates are P450-inducing PPARα agonists, which stimulate apo AI synthesis, raise plasma apo AI and HDL-C levels and reduce levels of LDL-C and triglycerides [10, 52, 66, 67]. They activate the LXR and ABCA1 genes and promote cholesterol efflux to apo AI [52]. An inhibition of P450 prevents fibrate-caused oxysterol generation and the induction of genes acting in cholesterol elimination, such as LXRα, PPARα, ABCA1 [23] and apo AI [20].
Cholestyramine induces CYP7A1, the rate-limiting enzyme of bile acid synthesis, leading to a depletion of hepatic cholesterol pool, and consequently to an upregulation of the LDL receptor pathway and lowering of plasma LDL-C level [68]. The drug also induces apo AI synthesis and raises plasma apo AI and HDL-C [69]. Niacin similarly raises apo AI and HDL-C levels and reduces LDL-C [70], but it differs from many other compounds that elevate HDL-C levels in that it does not increase P450 activity and apo AI synthesis. Instead, it has been found to inhibit hepatic uptake of apo AI, activate the PPARγ, LXRα and ABCA1 genes and stimulate HDL-dependent cholesterol efflux from monocytoid cells [70].
Anticonvulsants, such as phenobarbital, phenytoin and carbamazepine, are PXR agonists which upregulate P450-enzymes, including CYP3A4 [42–44], and raise plasma apo AI and HDL-C levels proportionately to P450-induction [10, 11]. Phenobarbital induces hepatic apo AI mRNA [18], and a recent study linked the effect of phenobarbital and other PXR agonists on apo AI and HDL-C with their ability to induce CYP3A in mice [45]. Anticonvulsants also enhance the generation of 4β-hydroxycholesterol [71], a LXR agonist which may—via transcriptional mechanisms—increase cholesterol efflux and raise HDL-C. The inverse relation of LDL-C level to the induction [8, 9] probably reflects the inhibiting effect of oxysterols on cholesterol synthesis and enhanced LDL-C elimination via the upregulated LDL receptor pathway.
Glitazones are PPARγ and PXR agonists and P450-inducing agents [43, 44, 72–74] that increase apo AI synthesis, raise plasma HDL-C levels [75] and, via activation of the LXRα, ABCA1, ABCG1, apo E and SR-B1 genes, promote cholesterol efflux from macrophages [51, 52].
Alcohol enhances P450 activity [10, 76] and apo AI synthesis [10, 77], and persons using alcohol regularly show an elevation of plasma apo AI and HDL-C [76–79] levels that is proportional to the increase in P450 [78]. Alcohol also promotes ABCA1- and cAMP-mediated cholesterol efflux from macrophages [80].
Vitamin A derivates—retinoids—upregulate several genes involved in reverse cholesterol transport, such as CYP27A1, LXRα, PPARγ, ABCA1, ABCG1 and the apolipoproteins CI, CII, CIV and E [73, 81]. Hypertriglyceridemia and other adverse effects have limited the clinical usage of retinoids. Calcium channel blockers also have the potential to induce P450 [44]. Verapamil, nifedipine and nicardapine were found to induce ABCA1 expression and increase apo AI-dependent cholesterol efflux from macrophages [82]. Telmisartan, an angiotensin receptor blocker, has been shown to enhance both apo AI- and HDL-mediated cholesterol efflux from macrophages by increasing ABCA1, ABCG1 and SR-B1 expression via PPARγ-dependent and LXR-dependent/-independent pathways [83]. Angiotensin-converting enzyme (ACE) inhibitors can also affect the fate of cholesterol [84, 85]. Adenosine A2A receptor agonists have been found to upregulate CYP27A1 and ABCA1 expression and prevent the formation of foam cells [86] and biphosphonate, to inhibit foam cell formation [87], to induce ABCA1 transcription and to stimulate cholesterol efflux from monocytoid cells [88].
Drugs and other compounds against atherosclerosis
Several drugs prevent or retard the progression, or even regress, atherosclerosis, as assessed by angiography, ultrasonography or histological/ biochemical analysis. These include statins, such as rosuvastatin [89], atorvastatin [90] and simvastatin [91], which have been shown to regress coronary atherosclerosis. Positive results have also been obtained with lovastatin, fluvastatin, gemfibrozil, fenofibrate [92], bezafibrate [93] and cholestyramine [10]. Niacin was recently found to reduce carotic intimal media thickness in persons with metabolic syndrome [94].
The recent ASTEROID trial demonstrated that rosuvastatin therapy, which effectively reduced LDL-C and apo B and raised HDL-C and apo AI, resulted in a significant regression of atherosclerosis in coronary arteries of CHD patients [89]. A post-hoc analysis combining data from four prospective intravascular ultrasonography–statin trials revealed, in particular, that patients whose HDL-C levels increased by more than 7.5% in addition to effective LDL-C lowering exhibited the most profound regression of atherosclerosis [95]. The increases in HDL-C levels were found to be an independent predictor of a beneficial outcome with statin therapy.
Many compounds used for other purposes than dyslipidemia also have antiatherogenic effects. Phenobarbital prevents cholesterol accumulation and the formation of atherosclerotic lesion in arterial wall [10], and those persons using alcohol moderately show less carotic [96] and coronary [97] atherosclerosis. Pioglitazone and etidronate have been found to reduce carotic intima-media thickness in type 2 diabetic subjects [98] and those with osteopenic type 2 diabetes, respectively [99].
Drugs and other compounds and cardiovascular events
Several trials have evaluated the effects of statins and other compounds on the occurrence of cardiovascular events (Table 1). Statins reduce coronary events, strokes and all-cause mortality [100, 101]. A meta-analysis of data on more than 90,000 participants revealed that the change in total mortality reflects a decrease in coronary mortality [101]. A recent atorvastatin study found that a relatively small increase (mean 7%) in HDL-C level independently of LDL-C lowering is linked with a reduced risk of major coronary events and stroke [102]. An analysis of 18-years of follow-up data from the Helsinki Heart Study revealed that gemfibrozil reduces both CHD mortality and total mortality in the subgroup of persons with dyslipidemia related to the metabolic syndrome [103]. In type 2 diabetic subjects, bezafibrate has been shown to reduce the incidence of coronary events [93], niacin, that of myocardial infarctions [92] and fenofibrate, that of non-fatal myocardial infarctions [92]. A follow- up survey conducted 9 years after the completion of a niacin trial, showed a 11% lower total mortality in patients originally treated with niacin compared with placebo-treated patients [70]. Cholestyramine reduces the risk of CHD death and/or non-fatal myocardial infarction [92], a reduced death rate from CHD has been reported also for people undergoing anticonvulsant therapy [104], and population studies show that moderate alcohol consumption reduces CHD morbidity and mortality, and also total mortality [105].
Pioglitzone has been found to reduce cardiovascular events in type 2 diabetics [106], whereas a meta-analysis reported an increase in the risk of myocardial infarction and an increase cardiovascular death of borderline significance with rosiglitazone [107]. This controversy led to the warning stating that rosiglitazone is not recommended in CHD and/or peripheral artery disease, and that it is contraindicated in acute coronary syndrome [EMEA, European Medicines Agency].
Mutation and inhibition of P450, enhanced atherogenesis and cardiovascular events
A mutation in the P450 gene affects the fate of cholesterol and enhances the atherosclerotic process. A defect in the CYP27A1 gene causes cerebrotendinous xanthomatosis, CTX, a genetic disease characterized by xanthoma formation and premature atherosclerosis [25], and a mutation in the CYP7A1 gene can lead to hypercholesterolemia and enhance atherogenesis [26]. A low P450 activity could also impede oxysterol generation and LXR-mediated activation of the proteins participating in cholesterol transport, such as ABC transporters, apolipoproteins, lipoprotein-modifying enzymes, SREBPs and SR-B1.
An exogenous inhibition of P450 can similarly promote cholesterol accumulation. Carbon monoxide (CO) binds to ferrous heme of P450, consequently blocks oxidative reactions [3], and promotes cholesterol accumulation and formation of atherosclerotic lesions in arterial walls [108]. The effect of CO on P450 could contribute to the increased incidence of cardiovascular events and deaths observed with increasing CO exposure in smokers [109]. Early studies with interferon showed a decrease in plasma HDL-C and apo AI level [110] and, later on, interferon γ was found to reduce P450 and ABCA1 activity, impede reverse cholesterol transport and promote atherogenesis [111, 112]. Ketoconazole prevents P450-induction, and consequently oxysterol generation and the activation of genes involved in reverse cholesterol transport, such as LXRα, PPARα, ABCA1 [23].
Discussion and conclusions
Advanced methods in molecular biology and genomics have identified diverse biological and clinical roles for isoenzymes of P450, which was once believed to be one enzyme in the hepatic detoxification system. P450s hydroxylate both endogenous and foreign substances and affect multiple metabolic processes and clinical outcomes. P450-enzymes are essential for the physiological maintenance of cholesterol homeostasis, responding to elevated cholesterol by activating mechanisms which efflux cellular cholesterol and raise plasma HDL-C and suppress cholesterol synthesis, thereby reducing LDL-C. Correspondingly, a naturally low P450 activity or a genetic defect in P450 can promote cholesterol accumulation and atherogenesis. A mutation in a transporter gene, such as ABCA1, G5, G8, or apo AI, can impede the response to activation and also enhance the atherosclerotic process.
Several drugs and nonpharmacologic compounds also induce genes with similar effects as the endogenous gene activation on lipid and proteins, and prevent or regress atherosclerosis, thereby reducing cardiovascular events. These compounds can produce a lipoprotein pattern comparable with that in familial hyper-HDL-emia, which is characterized by a low risk of CHD and enhanced longevity [10]. A recent statin study revealed that individuals who show increases in HDL-C level greater than the mean percentage change together with an effective LDL-C reduction experience the greatest degree of atheroma regression [95]. Statins increase HDL-C level up to 15% and can regress atherosclerosis, whereas torcetrapib, an inhibitor of CETP (cholesterol ester transfer protein) which raised HDL-C level up to 60%, failed to slow the progression of coronary plaques [113]. This difference emphasizes the significance of the mechanisms by which the drugs raise HDL-C.
The discovery of LXRs and oxysterols, with the latter functioning as ligands for LXRs and as secretory forms for cellular cholesterol efflux, significantly clarified the cholesterol-lowering mechanisms. The investigations that identified PXR, PPAR, FXR and several other receptors as well as ABC and other transporters—and their functions—further explained important processes in maintaining cholesterol homeostasis. The progress in studies on cholesterol regulation has greatly stimulated the search for new agents with a potential to regress atherosclerosis. Recently, a LXR agonist was found to stimulate reverse cholesterol transport by promoting cholesterol efflux from macrophages and transporting it to liver and further to feces in vivo in mice [114]. Another study showed that LXR agonist increases ABCA1 activity in atherosclerotic lesions and also induces the regression of these lesions in mice [115].
The liver is of critical importance in the metabolism and elimination of endogenous and exogenous compounds. Many natural and foreign compounds upregulate key effectors in cellular cholesterol efflux, including hepatic ABCA1 and apo AI, main factors in the generation of plasma HDL-C levels, and the LDL receptor, which leads to a lowering of LDL-C levels. Liver disease and a change in liver size can also affect the fate of cholesterol [10, 116, 117]. Patients treated with P450-inducing drugs show an elevation of plasma HDL-C and the HDL-C/LDL-C ratio together with an increase of metabolically active liver mass [116], and the 24S-hydroxycholesterol levels reflect the balance between cerebral production capacity and the metabolic capacity in the liver [117].
An atherogenic diet upregulates hepatic P450-enzymes, hydroxycholesterols [38] and ABCA1, thereby supporting a major role for the liver in the dietary modulation of HDL-C levels [118]. In accordance with this, a recent study found that an upregulation of the hepatic LXRα protects animals on a Western diet from atherosclerosis, underlining the potential of selective activators of LXR target genes in the liver as agents against lipid disorders and atherosclerosis [119]. The PXR, a master regulator of hepatic CYP3A4 and other P450 enzymes, also protects against the toxicity caused by a high-cholesterol diet [120], and PXR agonists raise apo AI and HDL-C levels. Interestingly, a recent statin study revealed that the increases in HDL-C levels independently predict the rate of atherosclerosis regression in coronary arteries [95]. Modification of life-style factors, including the Western-type diet, together with or without effective drug therapy are key factors in the fight against atherosclerosis and the epidemic of CHD.
References
Conney AH (1967) Pharmacological implications of microsomal enzyme induction. Pharmacol Rev 19:317–366
Remmer H (1967) Die Induktion arzneimittelabbauender Enzyme im endoplasmatischen Retikulum der Leberzelle durch Pharmaka. Dtsch Med Wochensch 92:2001–2008
Omura T, Sato R, Cooper DY, Rosenthal O, Estabrook RW (1965) Function of cytochrome P450 of microsomes. Fed Proc 24:1181–1189
Luoma PV, Pelkonen RO, Sotaniemi EA (1979) Plasma high-density lipoprotein cholesterol and hepatic drug metabolizing enzyme activity in man. Acta Physiol Scand Suppl 473:71
Luoma PV, Sotaniemi EA, Pelkonen RO, Ehnholm C (1980) Plasma high density lipoprotein and liver microsomal enzyme activity in man. In: Pilli-Sihvola AS, Laaksovirta TH (eds) The Medical Research Council 1977–1979. The Academy of Finland; Helsinki, p 71
Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR (1977) High-density lipoprotein as a protective factor against coronary heart disease. Am J Med 62:707–714
Miller NE, Miller GJ (Eds.) (1984) Clinical and metabolic aspects of high-density lipoproteins. Elsevier, Amsterdam New York Oxford
Luoma PV, Sotaniemi EA, Pelkonen RO (1983) Inverse relation of serum LDL cholesterol and the LDL/HDL cholesterol ratio to liver microsomal induction in man. Res Commun Chem Pathol Pharmacol 42:173–176
Luoma PV, Sotaniemi EA, Arranto AJ (1983) Serum LDL cholesterol and LDL/HDL cholesterol ratio and liver microsomal induction evaluated by antipyrine kinetics. Scan J Clin Lab Invest 43:671–675
Luoma PV (1997) Gene activation, apolipoprotein A-I/ high density lipoprotein, atherosclerosis prevention and longevity. Pharmacol Toxicol 81:57–64
Luoma PV, Sotaniemi EA, Pelkonen RO, Myllylä VV (1980) Plasma high density lipoprotein cholesterol and hepatic cytochrome P-450 concentrations in epileptics undergoing anticonvulsant treatment. Scand J Clin Lab Invest 40:163–167
Lu AYH, West SB (1980) Multiplicity of mammalian microsomal cytochromes P450. Pharmacol Rev 31:277–295
Nebert DW, Russell DW (2002) Clinical importance of the cytochromes P450. Lancet 360:1155–1162
Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ (1996) An oxysterol signalling pathway mediated by the nuclear receptor LXRα. Nature 383:728–731
Lehmann JM, Kliewer SA, Moore LB, Smith-Oliver TA, Oliver BB, Su JL, Sundseth SS, Winegard DA, Blanchard DE, Spencer TA, Willson TM (1997) Activation of the nuclear receptor LXR by hydroxycholesterols defines a new hormone response pathway. J Biol Chem 272:3137–3140
Bonow RO, Smaha LA, Smith SC, Mensah GA, Lennfant C (2002) The international burden of cardiovascular disease: Responding to the emerging global epidemic. Circulation 106:1602–1605
Luoma PV, Savolainen MJ, Sotaniemi EA, Pelkonen RO, Arranto AJ, Ehnholm (1983) Plasma high density lipoprotein and liver lipids and proteins in man. Relation to hepatic histology and microsomal enzyme induction. Acta Med Scand 214:103–109
Chao YU, Pickett CB, Yamin TT, Guo LS, Alberts A, Kroon PA (1985) Phenobarbital induces rat liver apoliprotein A-I mRNA. Mol Pharmacol 27:394–398
Malmendier C, Delcroic C (1985) Effects of fenofibrate on high and low density lipoprotein metabolism in heterozygous familial hypercholesterolemia. Atherosclerosis 55:161–169
Tam SP (1991) Effects of gemfibrozil and ketoconazole on human apolipoprotein A-I and E levels in two hepatoma cell lines HepG2 and HepG3. Atherosclerosis 91:51–61
Rubin EM, Krauss MR, Spangler EA, Verstuyft JG, Clift MS (1991) Inhibition of early atherogenesis in transgenic mice by human apoprotein A-I. Nature 353:265–267
Gylling H, Vanhanen H, Miettinen TA (1993) Effects of ketoconazole on cholesterol precursors and low density lipoprotein kinetics in hypercholesterolemia. J Lipid Res 34:59–67
Guan J-Z, Tamasava N, Murakami H, Matsui J, Yamato K, Suda T (2003) Clofibrate,a peroxisome-proliferator, enhances reverse cholesterol transport through cytochrome P450 activation and hydroxycholesterol generation. Tohoku J Exp Med 201:251–259
Schneider B, Gerdsen R, Plat J, Dullens S, Björkhem I, Dicsfalusy U, Neuvonen P, Biber T, von Bergmann K, Lütjohann D (2007) Effects of high-dose itraconazole treatment on lipoproteins in men. Int J Clin Pharmacol Ther 45:377–384
Cali JJ, Hsieh CL, Francke U, Russell DW (1991) Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie cerebrotendinous xanthomatosis. J Biol Chem 266:7779–7783
Pullinger CR, Eng C, Salen G, Shefer S, Barra AK, Erickson SK, Verhagen A, Rivera CR, Mulvihill SJ, Malloy MJ, Kane JP (2002) Human cholesterol 7α-hydroxylase [CYP7A1] deficiency has a hypercholesterolemic phenotype. J Clin Invest 110:109–117
Björkhem I, Diczfalusy U, Lütjohann D (1999) Removal of cholesterol from extrahepatic sources by oxidative mechanisms. Curr Opin Lipidol 10:161–165
Tontonoz P, Mangelsdorf DJ (2003) Liver X receptor signaling pathways in cardiovascular disease. Mol Endocrinol 17:985–993
Ory DS (2004) Nuclear receptor signalling in the control of cholesterol homeostasis: have the orphans found a home. Circ Res 95:660–670
Björkhem I, Diczfalusy U (2002) Hydroxycholesterols - friends, foes or just fellow passangers. Arterioscler Thromb Vasc Biol 22:734–742
Chiang JYL (2003) Bile acid regulation of hepatic physiology: III. Bile acids and nuclear receptors. Am J Physiol Gastrointest Liver Physiol 284:349–356
Eggertsen G, Olin M, Andersson U, Ishida H, Kubota S, Hellman U, Okuda K-I, Björkhem I (1996) Molecular cloning and expression of rabbit sterol 12α-hydroxylase. J Biol Chem 271:32269–32275
Fu X, Menke JG, Chen Y, Zhou G, MacNaul KL, Wright SD, Sparrow CP, Lund EG (2001) 27-hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol loaded cells. J Biol Chem 276:38378–38387
Norlin M, Andersson U, Björkhem U, Wikvall K (2000) Oxysterol 7α-hydroxylase activity by cholesterol 7α-hydroxylase (CYP7A). J Biol Chem 275:34046–34053
Björkhem I (2006) Crossing the barrier: hydroxycholesterols as cholesterol transporters and metabolic modulators in the brain. J Intern Med 260:493–508
Panzenboeck U, Balazs Z, Sovic A, Hrzenjak A, Levak-Frank S, Wintersperger A, Malle E, Sattler W (2002) ABCA1 and scavenger receptor class B, type I, are modulators of reverse cholesterol transport at an in vitro blood-brain barrier constituted porcine brain capillary endothelial cells. J Biol Chem 277:42781–42789
Gibbons GF (2002) The role of cytochrome P450 in the regulation of cholesterol biosynthesis. Lipids 37:1163–1170
Saucier SE, Kandutsch AA, Gayen AK, Swahn DK, Spencer TA (1989) Hydroxycholesterol regulators of 3-hydroxy-3-methylglutaryl-CoA reductase in liver. Effect of dietary cholesterol. J Biol Chem 264:6863–6869
Yan D, Olkkonen VM (2007) The OSBP-related proteins (ORP)—lipid sensors or transporters? Fut Lipidol 2:85–94
Liang Y, Jiang X-C, Liu R, Liang G, Beyer TP, Gao H, Ryan TP, Li SD, Eacho PI, Cao G (2004) Liver X receptors (LXRs) regulate apolipoprotein AIV—implications of the antiatherosclerotic effect of LXR agonists. Mol Endocrinol 18:2000–2010
Eloranta JJ, Kullak-Ublick GA (2005) Coordinate transcriptional regulation of bile acid homeostasis and drug metabolism. Arch Biochem Biophys 433:397–412
Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA (1998) Human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest 102:1016–1023
El-Sankary W, Gibson GG, Aurton A, Plant N (2001) Use of a reporter gene assay to predict and rank the potency and efficacy of CYP3A4 inducers. Drug Me Dispos 29:1499–1504
Sinz M, Kim S, Zhu Z, Chen T, Anthony M, Dickinson K, Rodrigues AD (2006) Evaluation of 170 xenobiotics as transactivators of human pregnane X receptor (hPXR) and correlation to known CYP3A4 drug interactions. Curr Drug Metab 7:375–388
Bachmann K, Patel H, Batayneh Z, Slama J, White D, Posey J et al (2004) PXR and the regulation of apoA1 and HDL-cholesterol in rodents. Pharmacol Res 50:237–246
Sonoda J, Chong LW, Downes M, Barish GD, Coulter S, Liddle C, Lee CH, Evans RM (2005) Pregnane receptor prevents hepatorenal toxicity from cholesterol metabolites. Proc Natl Acad Sci 102:2198–2203
Schuetz EG, Schuetz JD, Strom SC, Thompson MT, Fisher RA, Molowa DT, Li D, Guzelian PS (1993) Regulation of human liver cytochromes P-450 in family 3A in primary and continuous culture of human hepatocytes. Hepatology 18:1254–1262
Kocarek T, Dahn MS, Cai H, Strom SC, Mercer-Haines NA (2002) Regulation of CYP2B6 and CYP3A expression by hydroxymethylglutaryl coenzyme A inhibitors in primary cultured human hepatocytes. Drug Metab Disp 30:1400–1405
Bertrand-Thiebault C, Masson C, Siest G, Batt AM, Visvikis-Siest S (2007) Effect of MHGCoA reductase inhibitors on cytochrome P450 expression in endothelial cell line. J Cardiovasc Pharmacol 49:306–315
Li T, Chen W Chiang JYL (2007) PXR induces CYP27A1 and regulates metabolism in the intestine. J Lipid Res 48:373–384
Chawla A, Boisvert W, Lee C-H, Laffitte BA, Barak Y, Joseph SB, Liao D, Nagy L, Edwards PA, Curtiss LK, Evans RM, Tontonoz P (2001) A PPARγ–LXR–ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell 7:161–171
Chinetti G, Lestavel S, Bocher V, Remaley AT, Neve B, Torra IP, Teissier E, Minnich A, Jaye M, Duverger N, Brewer HB, Fruchart JC, Clavey V, Staels B (2001) PPARα and PPARγ activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat Med 7:53–58
Linsel-Nitschke P, Tall AR (2005) HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nat Rev 4:193–205
Brewer HB Jr, Remaley AT, Neufeld EB, Basso F, Jouce C (2004) Regulation of plasma high-density lipoprotein levels by the ABCA1 transporter and the emerging role of high-density lipoprotein in the treatment of cardiovascular disease. Arterioscler Thromb Vasc Biol 24:1755–1760
Brunham LR, Kruit KJ, Iqbal J, Fievet C, Timmins JM, Pape T et al (2006) Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J Clin Invest 116:1052–1062
Repa JJ, Turley SD, Lobaccaro JA, Medina J, Li L, Lustig K, Shan B, Heyman RA, Dietschy JM, Mangelsdorf DJ (2000) Regulation of absorption of ABCA1-mediated efflux of cholesterol by RXR heterodimers. Science 289:1524–1529
Oram JF (2002) Molecular basis of cholesterol homeostasis: lessons from Tangier disease and ABCA1. Trends Mol Med 8:168–173
Klucken J, Büchler C, Orsó E, Kaminski WE, Porsch-Özcϋrϋmez M, Liebisch G, Kapinnsky M, Diederich W, Drobnik W, Dean M, Alllikmets R, Schmitz G (2000) ABCG1 [ABC8], the human homolog of the drosophila white gene, is regulator of macrophage cholesterol and phospholipid transport. Proc Natl Acad Sci USA 97:817–822
Yu L, Li-Hawkins J, Hammer RE, Berge KE, Horton JD, Cohen JC, Hobbs HH (2002) Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J Clin Invest 110:671–680
Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovicch P, Shan B, Barnes R, Hobbs HH (2000) Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science 290:1771–1553
Martin G, Duez H, Blanquart C, Berezowski V, Poulain P, Fruchart JC, Najib-Fruchart J, Glineur C, Staels B (2001) Statin-induced inhibition of the Rho-signaling pathway activates PPARα and induces HDL apoA-I. J Clin Invest 107:1423–1432
Fan P, Zhang B, Kuroki S, Saku K (2004) Pitavastatin, a potent hydroxymethylglutaryl Coenzyme A reductase inhibitor, increases 7α-hydroxylase gene expression in HepG2 cells. Circ J 68:1061–1066
Argmann CA, Edwards JY, Sawyez CG, O’Neil CH, Hegele RA, Pickering JG, Huff MW (2005) Regulation of macrophage cholesterol efflux through hydroxymethylglutaryl-CoA reductase inhibition. J Biol Chem 280:22212–22221
Maejima T, Yamazaki H, Aoki T, Tamaki T Sato F, Kitahara M, Saito Y (2004) Effect of pitavastatin on apolipoprotein A-I production in HepG2 cell. Biochem Biophys Res Commun 324:835–839
Ando H, Tsuruoka S, Yamamoto H, Takamura T, Kaneko S, Fujimura A (2004) Effect of pravastatin on ATP-binding cassette transporter A1. J Pharmacol Exper Ther 311:420–425
Gatica A, Aguilera MC, Contador D, Loyola G, Pinto CO, Amigo L, Tichauer JE, Zanlungo S, Bronfman M (2007) P450 CYP2C epoxynase and CYP4A ω-hydroxylase mediate ciprofibrateinduced PPARα-dependent peroxisomal proliferation. J Lipid Res 48:924–934
Prueksaritanont T, Richards KM, Qui Y, Strong-Basalyga K, Miller A, Li C, Eisenhandler R, Carlini EJ (2005) Comparative effects of fibrates on drug metabolizing enzymes in human hepatocytes. Pharmaceut Res 22:71–78
Shepherd J, Packard CJ, Bicker S, Lawrie TD, Morgan HG (1980) Cholestyramine promotes receptor-mediated low-density-lipoprotein catabolism. New Engl J Med 302:1219–1222
Shepherd J (1979) The effect of cholestyramine on high density lipoprotein metabolism. Atherosclerosis 33:433–444
Guyton JR (2007) Niacin in cardiovascular prevention: mechanisms, efficacy and safety. Curr Opin Lipidol 18:415–420
Bodin K, Bretillon L, Aden Y, Bertilsson L, Broome U, Einarsson C, Diczfalusy U (2001) Antiepileptic drugs increase plasma levels of 4β-hydroxycholesterol in humans. J Biol Chem 276:38685–38689
Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Wilson TM, Kliewer SA (1995) An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor PPARγ. J Biol Chem 270:12953–12956
Szanto A, Benko S, Szatmari I, Balint LB, Furtos I, Rϋhl R, Molnar S, Csiba L, Garuti R, Calandra S, Laersson H, Diczfalusy U, Nagy L (2004) Transcriptional regulation of human CYP27 integrates retinoid, peroxisome proliferator-activated receptor, and liver X receptor signalling in macrophages. Mol Cell Biol 24:8154–8166
Quinn CM, Jessup W, Wong J, Kritharides L, Brown AJ (2005) Expression and regulation of sterol 27-hydroxylase [CYP27A1] in human macrophages: a role for RXR and PPARγ ligands. Biochem J 385:823–830
Myers CD, Kashyap ML (2005) Pharmacological augmentation of high-density lipoproteins: mechanisms of currently available and emerging therapies. Curr Opin Cardiol 20:307–312
Lieber CS (1984) To drink (moderately) or not to drink? New Engl J Med 310:846–848
Malmendier CL, Delcroix C (1985) Effect of alcohol intake on high and low density lipoprotein metabolism in healthy volunteers. Clin Chim Acta 152:281–288
Luoma PV Sotaniemi EA, Pelkonen RO, Ehnholm C (1982) High-density lipoproteins and hepatic microsomal enzyme induction in alcohol consumers. Res Commun Chem Pathol Pharmacol 37:91–96
LaPorte R, Valvo-Gerard L, Kuller L, Wanju R, Bates M, Cresanta J, Williams K, Palkin D (1981) The relationship between alcohol consumption, liver enzymes and high-density lipoprotein cholesterol. Circulation 64[Suppl 3]67–72
Beulens JW, Sierksma A, van Tol A, Fournier N, van Gent T, Paul J-L, Hendricks HFJ (2004) Moderate alcohol consumption increases cholesterol efflux mediated by ABCA1. J Lipid Res 45:1716–1723
Costet P, Lalanne F, Gerbod-Gionnone M, Molina JR, Fu X, Lund EG, Gudas LJ, Tall AR (2003) Retinoid acid receptor-mediated induction of ABCA1 in macrophages. Mol Cell Biol 23:7756–7766
Suzuki S, Nishimaki-Mogami T, Tamehiro N, Inoue K, Arakawa R, Abe-Dohmae S, Tanaka AR, Ueda K, Yokoyama S (2004) Verapamil increase apolipoprotein-mediated release of cellular cholesterol by induction of ABCA1 expression via liver X receptor-independent mechanism. Arterioscler Thromb Vasc Biol 24:519–525
Nakaya K, Ayaori M, Hisada T, Sawada S, Tanaka N, Iwamoto N, Ogura M, Yakushji M, Nakamura H, Ohsuzu F (2007) Telmisartan enhances cholesterol efflux from THP-macrophages by activating PPARγ. J Atheroscler Thromb 14:133–141
Zadelaar S, Kleemann R, Verschuren L, de Vries-Van der Weij J, van der Hoorn J, Princen HM, Kooistra T (2007) Mouse models for atherosclerosis and pharmacological modifiers. Arterioscler Thromb Vasc Biol 27:1706–1721
Böhm M (2007) Angiotensin receptor blockers versus angiotensin-converting enzyme inhibitors: where do we stand now? Am J Cardiol 100[Suppl]:38J–44J
Reiss AB, Rahman M, Chan ES, Montesinos C, Awadallah NW, Cronstein BN (2004) Adenosine A2A receptor occupancy stimulates expression of proteins involved in reverse cholesterol transport and inhibits foam cell formation in macrophages. J Leukoc Biol 6:727–734
Ylitalo R (2002) Biophosphonates and atherosclerosis. Gen Pharmacol 35:287–296
Strobach D, Lorenz RL (2003) The biophosphonate ibandronate stimulates reverse cholesterol transport out of monocytoid cells by enhanced ABCA1 transcription. Biochem Biophys Res Commun 307:23–30
Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, Davignon E, Erbel JC, Fruchart JC Tardif JC, Schoenhagen P, Crowe T, Cain V, Wolski K, Goormastic M, Tuzcu EM, for the ASTEROID investigators (2007) Effect of very high-intensity statin therapy on regression of coronay atherosclerosis. The Asteroid Trial. JAMA 295:1556–1565
Okazaki S, Yokoyama T, Miyauchi K, Shimada K, Kurata T, Sato H, Daida H (2004) Early statin treatment in patients with acute coronary syndrome. Demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronay event: the ESTABLISH study. Circulation 110:1061–1068
Okkels Jensen L, Thayssen P, Pedersen KE, Stender S, Haghfelt T (2004) Regression of coronary atherosclerosis by simvastatin. A serial intravascular ultrasound study. Circulation 110:265–270
Brown BG, Hinckley Stukovsky K, Zhao XQ (2006) Simultaneous low-density lipoprotein-C lowering and high-density lipoprotein-C elevation for optimum cardiovascular disease prevention with various drug classes, and their combinations: meta-analysis of 23 randomized trial. Curr Opin Lipidol 17:631–636
Ericsson CG, de Faire U, Grip L, Svane B, Hamsten A, Nilsson J (1996) Angiographic assessment of effects of bezafibrate on progression of coronary artery disease in young male postinfarction patients. Lancet 347:849–853
Thoenes M, Oguchi A, Nagamia S, Vaccari CS, Hammoud R, Umpierrez GE, Khan BV (2007) The effects of extended-release niacin on carotid intimal media thickness, endothelial function and inflammatory markers in patients with the metabolic syndrome. Int J Clin Pract 61:1942–1948
Nicholls SJ, Murat E, Sipahi I, Grasso AW, Schoenhagen P, Hu T, Wolski K, Crowe T, Desai MY, Hazen SI, Kapadia SR, Nissen SE (2007) Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA 297:499–508
Kiehl S, Willeit J, Rungger G, Egger G, Oberhollenzer F, Bonora E, fort the Bruneck Study Group (1998) Alcohol consumption and atherosclerosis: what is the relation. Prospective results from the Bruneck study. Stroke 29:900–907
Femia R, Natali A, L’Abbate A, Ferrannini E (2006) Coronay atherosclerosis and alcohol consumption. Angiographic and mortality data. Arterioscler Thromb Vasc Biol 26:1607–1612
Langenfeld MR, Forst T, Hohberg C, Kann P, Lϋbben G, Konrad T, Füllert SD, Sachara C, Pfützner A (2005) Pioglitazone decreases carotid intima-media thickness independently of glycemic control in patients with type 2 diabetes mellitus. Results from a controlled randomized study. Circulation 111:2525–2531
Koshiyama H, Nakamura Y, Tanaka S, Minamikawa J (2000) Decrease in carotid intima-media thickness after 1-year therapy with etidronate for osteopenia associated with type 2 diabetes. J Clin Endocrinol Metab 85:2793–2796
Cheung BMY, Lauder IJ, Lau C-P, Kumana C (2004) Meta-analysis of large randomised controlled trials to evaluate the impact of statins on cardiovascular outcomes. Brit J Clin Pharmacol 57:640–651
Cholesterol Treatment Trialists’ [CTT] Collaborators (2005) Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet 366:1267–1278
Athyros VG, Mikhailidis DP, Papageorgiou AA, Symeonidis AN, Mercouris BR, Pehlivanidis AN, Bouloukos VI, Elisaf M, for the GREACE Study Collaborative Group (2004) Effect of atorvastatin on high density lipoprotein cholesterol and its relationship with coronary events: a subgroup analysis of the GREek Atorvastatin and Coronary-heart-disease evaluation (GREACE) study. Curr Med Res Opin 20:627–637
Tenkanen L, Mänttäri M, Kovanen PT, Virkkunen H, Manninen V (2006) Gemfibrozil in the treatment of dyslipidemia. An 18-year follow-up of the Helsinki Heart Study. Arch Intern Med 166:743–748
Muuronen A, Kaste M, Nikkilä E, Tolppanen E-M (1985) Mortality from ischaemic heart disease among patients using anticonvulsive drugs: a case-control study. Brit Med J 291:1481–1483
Poikolainen K (1995) Alcohol and mortality: a review. J Clin Epidemiol 48:455–465
Dormandy JA, Charbonnel B, Eckland DAJ, Erdmann E, Massi-Benedetti M, Moules IK, on behalf of the PROactive investigators (2005) Secondary prevention of macrovascular events in patients with type 2 diabetes in the Proactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 366:1279–1289
Nissen SE, Wolski K (2007) Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356:2457–2471
Astrup P, Kjeldsen K, Wanstrup J (1970) Effects of carbon monoxide exposure on the arterial walls. Ann NY Acad Sci 174:294–300
Hedblad B, Ögren M, Engström G, Wollmer P, Janzon L (2005) Heterogeneity of cardiovascular risk among smokers is related to degree of carbon monoxide exposure. Atherosclerosis 179:1771–1783
Ehnholm C, Aho K, Huttunen JK, Kostiainen E, Mattila K, Pikkarainen J, Cantell K (1982) Effect of interferon on plasma lipoproteins and the activity of postheparin plasma lipases. Arteriosclerosis 2:68–74
Reiss AB, Patel CA, Rahman MM, Chan ESL, Hasneen K, Montesinos MM, Trachman JD, Cronstein BN (2004) Interferon-γ impedes reverse cholesterol transport and promotes foam cell transformation in TPH-1 human monocytes/macrophages. Med Sci Monit 10:BR420–BR425
Panousis VG, Zuckerman SH (2000) Interferon-γ induces downregulation of Tangier disease gene [ATP-binding cassette transporter 1] in macrophage derived foam cells. Arterioscler Thromb Vasc Biol 20:1565–1571
Nissen SE, Tardif JC, Nicholls SJ, Revkin JH, Shear CL, et al for the ILLUSTRATE investigators (2007) Effect of torcetrapib on the progression of coronary atherosclerosis. New Engl J Med 356:1304–1316
Naik SU, Wang X, Da Silva JS, Jaye M, Macphee CH, Reilly MR, Billheimer JT, Rothblat GH, Rader DJ (2006) Pharmacological activation of liver X receptors promotes reverse cholesterol transport in vivo. Circulation 113:90–97
Levin N, Bischoff ED, Daige CL, Thomas D, Vu CT, Heyman RA, Tangirala RK, Schulman IG (2005) Macrophage liver X receptor is required for anti-atherogenic activity of LXR agonists. Arterioscler Thromb Vasc Biol 25:135–142
Luoma PV, Sotaniemi EA, Pelkonen RO, Pirttiaho HI (1985) Serum low density and high density lipoprotein cholesterol, and liver size in subjects on drugs inducing hepatic microsomal enzymes. Eur J Clin Pharmacol 28:615–618
Bretillon L, Lütjohann D, Stahle L, Widhe T, Bindl L, Eggertsen G, Diczfalusy U, Björkhem I (2000) Plasma levels of 24S-hydroxycholesterol reflect the balance between cerebral production and hepatic metabolism and are inversely related to body surface. J Lipid Res 41:840–845
Wellington CL, Walker EKY, Suarez A, Kwok A, Bissada N, Singaraja R, Yang YZ, Zhang LH, James E, Wilson JE, Francone O, McManus BM, Hayden MR (2002) ABCA1 mRNA and protein distribution patterns predict multiple different roles and levels of regulation. Lab Invest 82:273–283
Lehrke M, Lebherz C, Millington SC, Guan HP, Millar J, Rader DJ, Wilson JM, Lazar MA (2005) Diet-dependent cardiovascular lipid metabolism controlled by hepatic LXRα. Cell Metab 1:297–308
Kliewer SA (2005) Cholesterol detoxification by nuclear pregnane X receptor. Proc Natl Acad Sci USA 102:2675–2676
Acknowledgements
The author is grateful for the excellent collaboration he received, especially to Eero Sotaniemi (†), Olavi Pelkonen, Markku Savolainen and Vilho Myllylä from the University of Oulu, Finland; Christian Ehnholm from the National Public Health Institute, Helsinki; and Heikki Vapaatalo from the University of Helsinki.
The studies were supported by the Academy of Finland and the Paavo Nurmi Foundation, Finland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luoma, P.V. Cytochrome P450 and gene activation—from pharmacology to cholesterol elimination and regression of atherosclerosis. Eur J Clin Pharmacol 64, 841–850 (2008). https://doi.org/10.1007/s00228-008-0515-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-008-0515-5