Abstract
Objective
To assess the effect of chronic hepatic impairment on rosuvastatin disposition, pharmacodynamic activity and tolerability.
Methods
This was an open-label, non-randomised, parallel-group trial. Six subjects were enrolled in each of three hepatic-function strata: Child-Pugh class A (CP-A, mild impairment), Child-Pugh class B (CP-B, moderate impairment) and normal hepatic function; the latter two strata were age, weight, race, sex and smoking history matched. All subjects were given rosuvastatin 10 mg for 14 days.
Results
In subjects with CP-A, and in four of six subjects with CP-B, rosuvastatin steady-state AUC(0–24) and Cmax were similar to subjects with normal hepatic function (geometric mean values 60.7 ng h/ml and 6.02 ng/ml, respectively). Two of six subjects with CP-B who had the highest CP scores (i.e. the highest degrees of hepatic impairment) had the highest AUC(0–24) (128 ng h/ml and 242 ng h/ml) and Cmax (23.4 ng/ml and 96.7 ng/ml) values. Low-density lipoprotein cholesterol (LDL-C) was decreased in all strata, but the response was more variable in the CP-B group. Rosuvastatin was well tolerated, and the safety profile was similar in subjects with hepatic impairment and normal hepatic function.
Conclusion
In most subjects with mild-to-moderate hepatic impairment, the steady-state pharmacokinetics of rosuvastatin were similar to subjects with normal hepatic function (more extensive hepatic impairment may increase systemic exposure to rosuvastatin), and most had LDL-C reductions similar to subjects with normal hepatic function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rosuvastatin* Footnote 1 (Crestor) is a highly effective inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase for the treatment of patients with dyslipidaemia. In clinical trials, 1-mg to 80-mg doses of rosuvastatin produced reductions in low-density lipoprotein cholesterol (LDL-C) (up to 65%), total cholesterol and triglycerides, and increases in high-density lipoprotein cholesterol (HDL-C) [1, 2].
The pharmacokinetic profile of rosuvastatin following single- and multiple-dose administration to healthy volunteers has been investigated in a number of trials [3, 4, 5, 6]: systemic exposure was dose proportional over the dose range 1080 mg, and the elimination half-life of rosuvastatin was approximately 20 h. In addition, absolute oral bioavailability was estimated as 20.1%, which together with an estimated hepatic-extraction ratio of 0.63 implies that absorption was greater than 20% (data on file, AstraZeneca, 2002).
Rosuvastatin is eliminated mainly via the liver: non-renal (hepatic) clearance represents approximately 70% of total plasma clearance (data on file, AstraZeneca, 2002). In a trial involving healthy volunteers [7], 90% of an oral dose of [14C]-radiolabelled rosuvastatin was recovered in faeces primarily as unchanged drug; this result is consistent with metabolism being a minor route of clearance for rosuvastatin. In-vitro studies with human hepatic microsomes and hepatocytes also demonstrated limited metabolism of rosuvastatin (which was attributed mainly to CYP2C9 and CYP2C19) [8]. Studies with rats demonstrated selective hepatic uptake of rosuvastatin by an active transport process [9, 10]. An organic anion transport protein (OATP) present in the basolateral membrane of human hepatocytes has been shown to be a means by which rosuvastatin (and other HMG-CoA reductase inhibitors) is transported into liver cells [11, 12]. There may also be active transport from liver to bile. In addition, the liver is the target organ for the lipid-regulating effect of rosuvastatin. Thus, hepatic impairment may alter the pharmacokinetics and pharmacodynamic profile of rosuvastatin.
The purpose of this trial was to assess the effect of hepatic impairment on rosuvastatin disposition, pharmacodynamic activity and tolerability.
Materials and methods
This open-label, non-randomised, parallel-group, single-centre trial was designed and monitored in accordance with the ethics principles of Good Clinical Practice and the Declaration of Helsinki. An institutional review board approved the protocol before the trial started, and all participants gave written informed consent.
Subjects
Participants were male or female subjects aged over 18 years with normal hepatic function, or mild or moderate chronic hepatic impairment. Hepatic impairment was classified using the Child-Pugh (CP) scheme [13, 14]. The protocol specified that subjects with mild hepatic impairment (CP-A) were to be recruited prior to subjects with moderate hepatic impairment (CP-B). Subjects in the normal hepatic function and moderate hepatic impairment strata were matched with respect to age, weight, race, sex and smoking history. Female subjects were required to use acceptable forms of contraception (surgical sterilisation or double-barrier contraception) or to be postmenopausal. Subjects with hepatic impairment were required to have serum levels of alanine aminotransferase and aspartate aminotransferase within two times the upper limit of normal; creatine kinase levels had to be normal.
A re-classification of the subjects with hepatic impairment was carried out using the Maddrey discriminant function [15, 16] (although none of the subjects in this trial had acute hepatitis) as follows:
-
Maddrey discriminant function =4.6 × prothrombin time (s) + serum total bilirubin (mg/dl)
-
Maddrey scores ≤54 were graded as 'not severe', and scores from 55 to 92 were graded as 'severe'
Rosuvastatin administration
The trial consisted of one 18-day period during which all subjects were given a daily oral dose of rosuvastatin (1×10-mg capsule) at 0700 hours for 14 consecutive days (day 2 through day 15). Subjects fasted for 2 h before and 3 h after their doses.
During the trial period, subjects remained in the clinical research centre. Subjects were required to abstain from alcohol consumption during the trial (evaluated by breath test at a screening and each trial visit), and from smoking on the morning of each trial assessment. Concomitant medications were not permitted except for spironolactone, furosemide, multivitamins, lactulose and acetaminophen (although these were withheld on the mornings when blood samples were collected for pharmacokinetic evaluation).
Evaluation of rosuvastatin pharmacokinetics
Blood samples for rosuvastatin assay were collected before the dose of rosuvastatin on days 2, 3, 8, 9, 12, 13, 14 and 15 and 0.5, 1, 2, 3, 4, 6, 8, 9, 10, 12, 18, 24, 48 and 72 h after the dose of rosuvastatin on day 15. Samples were collected, prepared and analysed as described previously [5, 17]. Due to the dilution of samples, the effective limit of quantification (LoQ) for rosuvastatin in plasma was 0.2 ng/ml. Correlation coefficients for rosuvastatin calibration curves were 0.997 to 0.999. The highest rosuvastatin concentration measured in plasma was 250 ng/ml. Mean imprecision values and inaccuracy levels for rosuvastatin quality control samples were <6% and ≤3% (at all concentrations), respectively.
Urine samples for rosuvastatin assay were collected 0–6, 6–12 and 12–24 h after the dose of rosuvastatin on day 15. Samples were collected into containers containing 100 ml sodium acetate buffer 0.5 M (pH 4.0). At the end of the collection period, an aliquot of the sample was retained and stored at −70 C until assay. Samples were prepared by dilution and analysed using the same method as for plasma samples. The LoQ for rosuvastatin in urine was 10 ng/ml. Correlation coefficients for rosuvastatin calibration curves were 0.997 to 0.999. The highest rosuvastatin concentration measured in urine was 3750 ng/ml. Mean imprecision values and inaccuracy levels for rosuvastatin quality control samples were <6% and ≤5% (at all concentrations), respectively.
The following rosuvastatin pharmacokinetic parameters were determined: area under the plasma concentration–time curve from time zero to 24 h [AUC(0–24); determined using the linear trapezoidal rule]; maximum observed plasma drug concentration (Cmax); time of Cmax (tmax); minimum observed plasma drug concentration (Cmin); terminal elimination half-life (t1/2; calculated as 0.693/λz, where λz is the terminal elimination rate constant derived from log-linear regression of the terminal portion of the plasma concentration–time profile); renal clearance of drug from plasma (CLR; calculated as Ae(0–24)/AUC(0–24), where Ae(0–24) is the amount of rosuvastatin recovered in the urine within 24 h); and the fraction of rosuvastatin excreted in the urine as unchanged drug [Fe; calculated as Ae(0–24)/rosuvastatin dose].
Evaluation of lipid levels
Fasting blood samples for lipid assay were collected before the dose of rosuvastatin on days 1 and 8, and on day 16. Plasma from these blood samples was analysed at a laboratory certified for the standardisation of lipid analysis as specified by the Standardization Program of the Center for Disease Control and Prevention and the National Heart, Lung and Blood Institute. LDL-C was estimated using the Friedewald formula for subjects who had triglyceride concentrations ≤3.38 mmol/l (300 mg/dl), and using the beta-quantification method for subjects who had triglyceride concentrations >3.38 mmol/l (300 mg/dl).
The percentage change from baseline (defined as the mean value from samples collected at the screening visit and on day 1) to day 16 in fasting LDL-C, HDL-C, triglycerides and total cholesterol was evaluated.
Statistical methods
The plasma steady-state AUC(0–24) and Cmax of rosuvastatin were compared between subjects with hepatic impairment and subjects with normal hepatic function using an analysis of variance model that allowed for the effects of hepatic-function stratum and matching. Hepatic impairment effects were presented as the ratios of the geometric means (gmeans) for hepatic impairment/normal groups, and 90% confidence intervals were constructed to assess differences between groups. Predictors of rosuvastatin systemic exposure in subjects with hepatic impairment were explored using a multiple linear regression analysis to describe the relationship between albumin, prothrombin time and total bilirubin measures on day 1 and log-transformed pharmacokinetic parameters on day 15. Changes from baseline in lipid levels were analysed using a paired t-test.
Safety evaluation
Safety assessments included adverse-event reports, clinical laboratory tests, electrocardiograms (ECGs) and physical examinations.
Results
Demographics and aetiology of hepatic impairment
Demographic and baseline hepatic characteristics are shown in Table 1. The aetiology of hepatic impairment in all subjects with CP-A and CP-B was alcohol-induced cirrhosis of the liver. Eight subjects (four in each stratum) had biopsy-confirmed cirrhosis; in the remaining four subjects, the diagnosis was substantiated by ultrasound, liver-spleen scan and medical history. All subjects had abstained from alcohol use during the trial, and none had evidence of acute hepatitis. Three CP-A and two CP-B subjects were hepatitis-C antibody positive.
Rosuvastatin pharmacokinetics
The summary pharmacokinetic parameters of rosuvastatin are presented by hepatic-function stratum in Table 2. There were no statistically significant differences in rosuvastatin steady-state AUC(0–24) and Cmax values between subjects with hepatic impairment and subjects with normal hepatic function (Table 2).
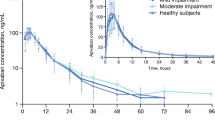
The highest CP scores occurred in two subjects who exhibited larger increases in systemic exposure than other subjects with hepatic impairment (subjects 109 and 110 in Fig. 1 and Fig. 2 with AUC(0–24) values of 128 ng h/ml and 242 ng h/ml and Cmax values of 23.4 ng/ml and 96.7 ng/ml, respectively). When a re-classification of the subjects with hepatic impairment was carried out using the Maddrey discriminant function, these two subjects were the only ones to be classified as Maddrey 'severe' (Fig. 3 and Fig. 4). The Maddrey 'not severe' group showed pharmacokinetic parameters that were similar to healthy volunteers and had similar variability (Fig. 3 and Fig. 4).
A multiple linear regression model identified prothrombin time as the most important predictor of AUC(0–24) and Cmax in the subjects with hepatic impairment in this trial. However, no single parameter was a good predictor of increased systemic exposure (which is not surprising since only two subjects showed a notable increase in exposure and there were no particularly unique laboratory values for either subject).
Rosuvastatin AUC(0–24) and Cmax did not appear to be affected by hepatitis C antibody status (Fig. 1 and Fig. 2).
Median tmax was reached slightly earlier in subjects with hepatic impairment than in subjects with normal hepatic function (Table 2); steady state was reached after 7 days of dosing in all strata and mean trough concentrations were similar in all strata (Table 2). It was not possible to calculate the terminal elimination half-life in many subjects, but there was no evidence that the decline in plasma concentrations was different in subjects with hepatic impairment than in subjects with normal hepatic function.
The gmean fraction of rosuvastatin excreted unchanged in the urine was approximately 5% of the administered dose in all strata (Table 2), with a range approximating 4-fold in subjects with normal hepatic function and CP-A (3.4–11.9% and 2.6–10.6%, respectively), and a range approximating 40-fold in subjects with CP-B (0.45–19.9%).
Lipid levels
Mean baseline lipid levels and percentage changes from baseline to day 16 are presented in Table 3. LDL-C was decreased in all strata. The greatest reductions occurred in healthy volunteers. The responses for subjects with CP-B were much more variable, with three subjects showing reductions in LDL-C and three subjects showing no change or an increase in LDL-C. The CP-B subjects with typical responses to a 10-mg dose of rosuvastatin [1, 2] had baseline LDL-C values of 3.18 mmol/l and 4.00 mmol/l (LDL-C reductions of −41.5% and −51.5%, respectively). The other CP-B subjects had low baseline LDL-C values of 1.34, 1.44, 1.86 and 2.12 mmol/l (a mean LDL-C change of 7%); the lowest baseline values belonged to the two subjects with the Maddrey 'severe' classification.
HDL-C responses were also more variable for subjects with hepatic impairment. The CP-A and CP-B subjects in this trial had baseline HDL-C values that were nearly 30% higher than those of the healthy volunteers (Table 3).
Safety
Rosuvastatin was well tolerated by all subjects. The safety profile in subjects with hepatic impairment was similar to that in subjects with normal hepatic function. There were no serious adverse events, no withdrawals, and no clinically significant changes in clinical laboratory parameters, ECGs or physical examinations.
Discussion
The purpose of this trial was to assess rosuvastatin disposition, pharmacodynamic activity and tolerability in subjects with chronic hepatic impairment, as this population may receive treatment for hypercholesterolaemia in clinical practice. Even though metabolism is a minor route of clearance for rosuvastatin [7, 8], hepatic impairment may alter rosuvastatin systemic exposure. The hepatic organic anion transporter OATP-C has been shown to be a transport protein for rosuvastatin [11], and, despite limited data regarding the effect of changes in transport proteins in liver disease on hepatic uptake of drugs, it is possible that changes in transport proteins in subjects with hepatic impairment could alter rosuvastatin hepatic uptake and therefore systemic exposure.
The trial results showed that in subjects with mild-to-moderate hepatic impairment, rosuvastatin steady-state AUC(0–24) and Cmax were similar to those in subjects with normal hepatic function. Two of six subjects with CP-B (who had the highest CP scores and were the only subjects to be classified as Maddrey 'severe') had the highest AUC(0–24) and Cmax values, suggesting that more extensive hepatic impairment may increase systemic exposure to rosuvastatin following oral administration. These results suggest that in subjects with mild-to-moderate hepatic impairment the rosuvastatin hepatic transport process is not greatly reduced but that in subjects with more extensive impairment hepatic uptake may be reduced.
Thus, the higher systemic exposures seen in the two subjects with the highest degrees of hepatic impairment could be interpreted as increased bioavailability due to reduced first-pass uptake in the liver. Renal clearance accounts for approximately 28% of the total plasma clearance of rosuvastatin when the compound is given intravenously to healthy volunteers (data on file, AstraZeneca, 2002). Subject 110 with the highest Cmax also had the highest proportion of the dose recovered in the urine (19.9%) but a renal clearance (132 ml/min) similar to subjects with normal hepatic function (Table 2); so the higher systemic exposure in this subject was associated with a greater renal elimination as a fraction of the administered dose. It is unlikely that the increased exposure could be attributed to a reduced metabolic capacity because rosuvastatin undergoes limited cytochrome P 450-mediated metabolism in healthy volunteers [7, 8].
The CP scale is the most commonly used method for classifying subjects with hepatic impairment in clinical trials. However, this scale is often a crude predictor of hepatic function and its effect on drug pharmacokinetics [18, 19]. The Maddrey discriminant function was explored in this trial to assess its utility in predicting systemic drug exposure, and it identified successfully those subjects with increased exposure to rosuvastatin. The Maddrey system, which was designed for grading acute alcoholic hepatitis, is a function of prothrombin time and total bilirubin, and is easy to calculate from a clinical perspective. Wider experience will determine the utility of the Maddrey discriminant function as a tool for drug assessment and characterisation in subjects with non-acute hepatic impairment.
Five subjects with hepatic impairment had evidence of hepatitis-C antibodies. However, there was no apparent impact of this on rosuvastatin pharmacokinetics in this trial. Further study of such subjects may be important as the epidemiology of hepatitis C becomes more prevalent.
A multiple-dose (14 day) design was used in order to assess lipid outcome data. Approximately 90% of the lipid response is achieved within the first 2 weeks of treatment with rosuvastatin [1]. In most subjects with mild-to-moderate hepatic impairment, the lipid responses to rosuvastatin were similar to those in subjects with normal hepatic function. The responses for subjects with CP-B disease were more variable, although poor response may have been related in part to low baseline LDL-C values. Thus, although baseline LDL-C values do not appear to be important in predicting the response to HMG-CoA reductase inhibitors in healthy volunteers and subjects with dyslipidaemia, they may be important in subjects with chronic hepatic impairment. (Similarly, HDL-C response may be related in part to baseline HDL-C levels.) Larger trials are required to characterise fully the lipid-regulating properties of rosuvastatin in the population of patients with hepatic impairment.
Conclusions
In most subjects with mild-to-moderate hepatic impairment, the steady-state pharmacokinetics of rosuvastatin were similar to subjects with normal hepatic function (more extensive hepatic impairment may increase systemic exposure to rosuvastatin), and most had LDL-C reductions similar to subjects with normal hepatic function.
Notes
*Rosuvastatin is licensed from Shionogi and Co. Ltd, Osaka, Japan.
References
Olsson AG, Pears J, McKellar J, Mizan J, Raza A (2001) Effect of rosuvastatin on low-density lipoprotein cholesterol in patients with hypercholesterolemia. Am J Cardiol 88:504–508
Davidson M, Ma P, Stein EA, Gotto AM Jr, Raza A, Chitra R, Hutchinson H (2002) Comparison of effects on low-density lipoprotein cholesterol and high-density lipoprotein cholesterol with rosuvastatin versus atorvastatin in patients with type IIa or IIb hypercholesterolemia. Am J Cardiol 89:268–275
Warwick MJ, Dane AL, Raza A, Schneck DW (2000) Single- and multiple-dose pharmacokinetics and safety of the new HMG-CoA reductase inhibitor ZD4522 (abstract MoP19:W6). Atherosclerosis 151:39
Martin PD, Dane AL, Nwose OM, Schneck DW, Warwick MJ (2002) No effect of age or gender on the pharmacokinetics of rosuvastatin a new HMG-CoA reductase inhibitor. J Clin Pharmacol 42:1116–1121
Cooper KJ, Martin PD, Dane AL, Warwick MJ, Schneck DW, Cantarini MV (2002) The effect of fluconazole on the pharmacokinetics of rosuvastatin. Eur J Clin Pharmacol 58:527–531
Cooper KJ, Martin PD, Dane AL, Warwick MJ, Schneck DW (2002) Lack of effect of ketoconazole on the pharmacokinetics of rosuvastatin. Br J Clin Pharmacol (in press)
Martin PD, Dane AL, Schneck DW, Warwick MJ (2000) Disposition of new HMG-CoA reductase inhibitor ZD4522 following dosing in healthy subjects (abstract 48). J Clin Pharmacol 40:1056
McCormick AD, McKillop D, Butters CJ, Miles GS, Baba T, Touchi A, Yamaguchi Y (2000) ZD4522 an HMG-CoA reductase inhibitor free of metabolically mediated drug interactions: metabolic studies in human in vitro systems (abstract 46). J Clin Pharmacol 40:1055
Nezasa K, Higaki K, Hasegawa H, Inazawa K, Takeuchi M, Yukawa T, McTaggart F, Nakano M (2000) Uptake of HMG-CoA reductase inhibitor ZD4522 into hepatocytes and distribution into liver and other tissues of the rat (abstract MoP21:W6). Atherosclerosis 151:39
Nezasa K, Higaki K, Yukawa T, Inazawa K, Hasegawa H, Nakano M (2002) Liver-specific distribution of rosuvastatin in rats: comparison with pravastatin and simvastatin. Drug Metab Dispos 30:1158–1163
Brown CDA, Windass A, Bleasby K, Lauffart B (2001) Rosuvastatin is a high affinity substrate of hepatic organic anion transporter OATP-C (abstract P174). Atherosclerosis Suppl 2:90
Hsiang B, Zhu Y, Wang Z, Wu Y, Sasseville V, Yang WP, Kirchgessner TG (1999) A novel human hepatic organic anion transporting polypeptide (OATP2). Identification of a liver-specific human organic anion transporting polypeptide and identification of rat and human hydroxymethylglutaryl-CoA reductase inhibitor transporters. J Biol Chem 274:37161–37168
Child CG, Turcotte JG (1964) Surgery and portal hypertension. In: Child CG (ed) The liver in portal hypertension. Saunders, Philadelphia, pp 5052
Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R (1973) Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 60:646–649
Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr (1978) Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 75:193–199
Carithers RL Jr, Herlong HF, Diehl AM, Shaw EW, Combes B, Fallon HJ, Maddrey WC (1989) Methylprednisolone therapy in patients with severe alcoholic hepatitis. A randomized multicenter trial. Ann Intern Med 110:685–690
Hull CK, Penman AD, Smith CK, Martin PD (2002) Quantification of rosuvastatin in human plasma by automated solid-phase extraction using tandem mass-spectrometric detection. J Chromatogr B Biomed Appl 772:219–228
Bergquist C, Lindegård J, Salmonson T (1999) Dosing recommendations in liver disease (letter). Clin Pharm Ther 66:201–204
Guidance for Industry (1999) Pharmacokinetics in patients with impaired hepatic function: study design, data analysis, and impact on dosing and labelling (Draft Guidance). US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research 1999 Nov. Guidance No.: \\CDS018\REGAFF\!GUIDANC\2629dft.doc.
Acknowledgements
The authors thank Elizabeth Eaton, PhD, for assistance with manuscript preparation. The trial described here was conducted at Clinical Pharmacology Associates (2060 NW 22nd Avenue, Miami, Florida 33142, USA) and complied with the current laws of the USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Simonson, S.G., Martin, P.D., Mitchell, P. et al. Pharmacokinetics and pharmacodynamics of rosuvastatin in subjects with hepatic impairment. Eur J Clin Pharmacol 58, 669–675 (2003). https://doi.org/10.1007/s00228-002-0541-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-002-0541-7