Abstract
Anthropogenic disturbances are having strong, negative effects on aquatic systems globally, altering ecological communities and potentially creating vacant niches for both native and non-native species (NNS). Globalization and new trade routes have amplified the spread and establishment of NNS by connecting disturbed areas worldwide. In this study, we conducted a comparative assessment of seasonal variations in amphipod communities at three southeastern Baltic Sea locations – two anthropogenically impacted and one protected habitat – to determine if native and NNS diversity differed among these habitats. Our study revealed nine amphipod species - of which two were NNS - across all three habitats. The impacted habitats had significantly higher native species richness and lower NNS abundance. Grandidierella japonica was the only NNS found at the impacted habitas. In the case of the protected habitat, NNS Gammarus tigrinus was dominant for most of the year. In autumn, dominance shifted in favour of the native Gammarus locusta and Microdeutopus cf. gryllotalpa. Grandidierella japonica was not detected there. Although anthropogenically impacted habitats may be under higher invasion risk, other environmental factors, such as salinity and temperature, may be driving the establishment pattern of NNS and the resulting community structures. Furthermore, undisturbed and/or protected habitats may be highly vulnerable to invasions due to more tolerable environmental conditions, robust NNS populations and naïve native species to newcomers. Seasonality is an important aspect of ecological studies and must be taken into account, as omissions could potentially distort our understanding of the dynamics of ecosystems and prevent the detection of NNS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Marine habitats are facing strong, negative impacts due to anthropogenic disturbances, with coastal zones particularly threatened (Halpern et al. 2008). These regions receive land-derived pollutants from a variety of sources, including agriculture and livestock farming, urban development, tourism and industry. Ocean-based pollutants, on the other hand, arise from activities like shipping, fisheries, aquaculture, exploitation for fossil fuels (Halpern et al. 2008; Nogales et al. 2011), and – among others – the introduction of non-native species (NNS; Simberloff et al. 2013; Linders et al. 2019; Pyšek et al. 2020; Soto et al. 2024). The impacts of associated noise and light pollution are also being increasingly recognised (Davies et al. 2014; Di Franco et al. 2020). Such disturbances alter the composition of communities and may lead to vacant niches and habitats (Wilkinson 2002; Lockwood et al. 2013), potentially creating opportunities for both native species and NNS (Daehler 2003).
The majority of NNS are found in areas affected by human disturbances, and several instances showed that such disturbances foster their establishment (Smith and Knapp 1999; Minchinton 2002; Lockwood et al. 2013). This can be attributed to three main reasons. The first is high propagule pressure (i.e., introduction effort) between many anthropogenically disturbed areas due to strong interconnectivity (Briski et al. 2011, 2012a; Lockwood et al. 2013). For example, shipping ports are not only exposed to high levels of heavy metals, noise and light pollution, and contain huge amounts of artificial structures, such as pontoons, wharfs and buoys, but they also receive huge amounts of ballast water containing diverse taxa from viruses and bacteria to macroinvertebrates and fishes (Briski et al. 2012b, 2013; Lin et al. 2022). Furthermore, the equipment used during the construction of those ports is typically moved from site to site, often without biosecurity protocols to prevent the spread of “hitchhiking” species (Lockwood et al. 2013). The second reason is connected to the high environmental tolerance of NNS to diverse stressors when compared to species without an invasion record (Briski et al. 2018; Paiva et al. 2018, 2020; Casties et al. 2019; Martinez Reyes et al. 2024). This possibly stems from selection (i.e. survival of only prior-adapted individuals for particular environmental conditions) before and during the invasion process (Hufbauer et al. 2012; Briski et al. 2018). Finally, NNS may alter invaded habitats, causing an additional disturbance, and facilitate the establishment of more NNS. This phenomenon creates a positive feedback loop – known as “invasional meltdown” (Simberloff and Von Holle 1999; Light and Marchetti 2007; Lockwood et al. 2013).
The Baltic Sea is exposed to many anthropogenic stressors, including strong shipping activities (Kaluza et al. 2010; Knebel 2021), introduction of NNS (Casties et al. 2016), and increases in temperature and pCO2 levels exceeding the global average seven times (Nikulina et al. 2008; Belkin 2009; Jutterström et al. 2014; Pachauri et al. 2014; Reusch et al. 2018). It is a large semi-enclosed brackish water system characterized by a strong salinity gradient ranging between 2 and 24 ppt (Casties et al. 2016). Seasonality in the system also strongly influences community compositions by affecting species behaviour, feeding, reproduction cycle, and even taxon survival (Fretwell 1972; Theurich et al. 2024). Yet, seasonality is rarely considered in ecological studies, particularly in connection to other anthropogenic activities and/or introductions of NNS (Wahl et al. 2020; White and Hastings 2020), and its omission could distort our understanding of ecosystem dynamics.
Amphipods are a globally distributed, diverse and widespread taxonomic group, spanning various aquatic habitats from freshwater to fully marine environments (Cuthbert et al. 2020). They are highly abundant, playing important roles in various ecosystem functions including the detritus cycle, the microbial loop, and as prey in the diet of many fish species (Grabowska and Grabowski 2005; Gerhardt et al. 2011). Due to their tolerance of environmental stressors, and in particular wide ranges of salinity, accompanied by foraging plasticity, migration ability and tendency to drift, they are also very successful and often notorious invaders (Grabowski et al. 2007; Gerhardt et al. 2011; Paiva et al. 2018; Cuthbert et al. 2020). Some well-know gammarid invaders include species such as Dikerogammarus villosus, Gammarus tigrinus, Echinogammarus ischnus and Pontogammarus robustoides that have spread throughout European and North American brackish and freshwater ecosystems (Grabowski et al. 2007; Cuthbert et al. 2020; Soto et al. 2022). Non-native species can outnumber and sometimes replace native species, exhibiting rapid growth, early maturation and high fecundity (Dermott et al. 1998; Grabowski et al. 2007; Soto et al. 2023).
With the Baltic Sea as our chosen study system, we therefore used amphipod taxa – an important taxonomic group in Baltic Sea ecosystems (Zettler and Zettler 2017) – to explore the effects of diverse environmental conditions on the biological communities. We conducted a comparative assessment of seasonal variation in amphipod communities from three Baltic habitats - two anthropogenically impacted and one protected, characterized by different salinity and temperature conditions. We expect that in impacted habitats native diversity will be lower than in protected ones, assuming native species unable to withstand disturbances have previously retreated to less affected areas. We also expect anthropogenic activities to provide more chances for NNS to be introduced into impacted habitats, and therefore we anticipate more NNS in these habitats than in the protected site. The three null hypotheses that we tested were: (i) there is no difference in native diversity between impacted and protected habitats; (ii) there is no difference in NNS diversity between impacted and protected habitats; and (iii) there is no difference in gammarid communities across seasons.
Methods
Sample collection
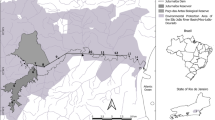
From April 2022 until March 2023, amphipod taxa were collected monthly at three locations in Schleswig-Holstein, Germany. Two locations were anthropogenically impacted habitats: (i) Falckenstein beach (54°39’04” N 10°19’24” E) and (ii) downtown Kiel (54°32’97” N 10°14’93” E), and one location was a protected area: (iii) Dassower See (i.e. Dassow Bay, 53°90’10” N 10°90’69"E; Supplementary Figure S1). Due to its proximity to the Nord-Ostsee-Kanal (i.e. North-Baltic Canal), one of the busiest canals in the world (Knebel 2021), Falckenstein beach is exposed to strong shipping activities and ballasting operations throughout the year, as well as being subjected to tourism activities during summer months (Fig. 1). Meanwhile, downtown Kiel faces numerous urban disturbances from light, noise, tourism and sailing activities to commercial shipping, ferry and shipyard activities. Both of these locations are also highly polluted by heavy metals, such as copper, zinc, tin and lead (Nikulina et al. 2008). Conversely, Dassower See has been a protected habitat since 1983, and is one of the largest bird reserves in Germany (Landkreis Nordwestmecklenburg, FD Bauordnung/Umwelt). However, it is also linked to the Trave River which connects the Baltic Sea with the Port of Lübeck; the Trave River catchment is polluted by diverse antifouling biocides (Mavchate et al. 2021). In addition, salinity conditions at Falckenstein beach and downtown Kiel fluctuate between 11 and 22 ppt, while those at Dassower See between 6 and 15 ppt (Supplementary Table S1). Temperature is similar throughout the year across locations (around 5 °C in winter and 20 °C in summer), except for Falckenstein beach where the temperature can rise to 30 °C during summer months (Supplementary Table S1).
Samples were collected during the first week of each month by intensive sampling of one person for an hour (April-July), or two persons for half an hour (August-March). At Falckenstein beach, Fucus spp. was collected using a 26 × 26 cm 500 μm mesh size landing net and shaken until the amphipods detached from the algae. In downtown Kiel, blue mussels, Mytilus sp., were scratched from the dock pilings with 16 × 11 cm 500 μm mesh size net and shaken to collect the amphipods hiding in between them. For Dassower See, a 26 × 26 cm 500 μm landing net was used to collect plant debris and algae from the shallower parts of the lake near bulrushes and other vegetation. This debris was spread out on a sampling table allowing for amphipods to be collected. Slightly different sampling methods were used due to varying local conditions. At all three locations, only animals bigger than 2 mm in size were collected, as smaller individuals cannot be identified morphologically to the species level using conventional methods. Additionally, at each sampling location, salinity, pH, temperature, and oxygen were measured using WTW ProfiLine Cond 3110 m with TetraCon 325 probe and WTW ProfiLine pH 3110 m for salinity and pH, respectively, and WTW ProfiLine Oxi 3310 IDS meter for temperature and oxygen. Collected individuals were transferred in source water to GEOMAR in Kiel, preserved in 99% ethanol and stored at 4 °C until identification. All animals were identified morphologically following Zettler and Zettler (2017). At least five individuals of each morphologically identified species were further identified molecularly to confirm morphological identification (see below). Individuals that could not be visually identified to the species level were grouped morphologically, and again at least five individuals per group were taken for molecular identification.
Molecular species identification
Total genomic DNA was extracted from the telson using the DNeasy Blood and Tissue kit (QIAGEN, Germany) following the manufacturer’s protocol. Partial sequences of cytochrome c oxidase subunit 1 (COI) gene were amplified using several sets of primers to be able to obtain sequences for different gammarid species: LCO1490 and HCO2198 (Folmer et al. 1994), UCOIF and UCOIR (Costa et al. 2009), LoboF1 and LoboR1 (Lobo et al. 2013) and G. tigrinus species-specific primers (Kelly et al. 2006a). PCR reactions were performed in 20 µl volume including 9.8 µl of nuclease-free water, 2 µl of 10X PCR buffer (Invitrogen, USA), dNTPs, forward and reverse primer (5mM concentration) and 2 µl template DNA, and 0.2 µl of DreamTaq DNA polymerase (Invitrogen, USA). The amplifications using LCO1490 and HCO2198 and UCOIF and UCOIR sets of primers were performed with a denaturation step for 5 min at 94 °C, followed by 33 cycles of denaturation at 94 °C (35 s), annealing at 47 °C (45 s), extension at 69 °C (45 s) and a final extension step of 69 °C for 10 min. In the case of LoboF1 and LoboR1 primers, denaturation was performed at 94 °C for 1 min, followed by 5 cycles of denaturation at 94 °C (30 s), annealing at 45 °C (1 min 30 s) and extension at 72 °C (1 min), then by 44 cycles of denaturation at 94 °C (30 s), annealing at 54 °C (1 min 30 s) and extension at 72 °C (1 min), and finally ended with a final extension of 5 min at 72 °C. For G. tigrinus species-specific primers, the protocol of Kelly et al. (2006a) was followed. The PCR products were sequenced on Sanger sequencing platform (Applied Biosystems, USA) at Eurofins Genomics (Kiel, Germany). Raw COI sequences were assembled and trimmed using CodonCode Aligner v 3.7.1 (Codon Code Corporation). Each sequence was blasted on NCBI (https://www.ncbi.nlm.nih.gov/). Sequences with ≥ 98% similarity were deemed a species-level identification, while those with < 98% were grouped as unidentified; sequences with ≥ 98% similarity are shown in Supplementary Table S2.
Statistical analyses
To compare species diversity among three sampling locations (i.e. Falckenstein beach, downtown Kiel, and Dassower See), Simpson’s Diversity Index (D) was calculated for each month (i.e. from April 2022 to March 2023), for each location, following the equation:
whereby ni was the number of amphipods that belong to species i, and N was the total number of amphipods. The value of D spans from 0 to 1, whereby 1 represents maximal diversity and 0 represents no diversity. For months where no species were collected from sampling, an index score of zero was assigned. To determine the effect of site on diversity, a non-parametric Kruskal-Wallis test was conducted, with a post-hoc Dunn’s test to determine the pairwise differences between locations. A Benjamini and Hochberg p-adjustment was used to control the rate of false discoveries (Benjamini and Hochberg 1995).
To assess the variation in amphipod abundance and richness over a 12-month period across different locations we employed Generalized Additive Models (GAMs) at three locations due to non-linear relationship and non-constant variance. To account for the temporal patterns in the data across different locations, we first transformed the sampling dates into a continuous numeric variable, representing the number of days since the start of our sampling period. This transformation facilitated the use of GAMs to investigate temporal trends across locations. Subsequently, we applied a non-cyclic cubic spline (i.e., cs spline) with a maximum of five knots to avoid overfitting but allowing flexible modeling. We then evaluated the model by examining the distribution of the residuals using histograms, and determined that the negative binomial distribution with a log-link was the most appropriate distribution to use. Afterwards, we explored the population dynamic for native and non-native amphipod abundances across sites. However, due to the small number of NNS records at Falckenstein beach (n = 5) and downtown Kiel (n = 1), we were not able to create reliable models for these two locations, but only for Dassower See (n = 11).
Finally, we analyzed the influence of the four environmental predictors (i.e. pH, salinity, water temperature, and dissolved oxygen) on the temporal abundance of amphipod communities. For this purpose, we fitted a series of GAMs with individual predictors because the low number of sample points (n = 12 per location, i.e. one per month) generated the risk of overfitting across each site. Due to the multiple statistical tests conducted for each predictor across each location, we applied Holm’s method for p-value adjustment to control the family-wise error rate. After visually inspecting the residual distribution of all models, we selected negative binomial distribution for all models. Additionally, Redundancy Analysis (RDA) was performed using the rda function of the vegan R package (Oksanen 2012). To assess the significance of the environmental variables in the RDA model, we conduced a permutation test using the anova.cca function with 999 permutations. The reproducible code and data used in this study are stored at https://github.com/IsmaSA.
Results
Absolute and relative abundance and richness of amphipod communities
Across the three sampling locations, a total of nine species of amphipods were identified: G. salinus, G. oceanicus, G. locusta, G. zaddachi, G. tigrinus, Calliopius laeviusculus, Monocorophium insidiosum, Grandidierella japonica, and Microdeutopus cf. gryllotalpa (Figs. 1 and 2). All species except M. cf. gryllotalpa were identified both morphologically and molecularly; Microdeutopus cf. gryllotalpa was identified only morphologically due to the lack of the corresponding record of the species in GenBank (https://www.ncbi.nlm.nih.gov/nuccore/; accessed on March 20th, 2024). At all three locations, there were very few numbers of unidentified individuals (Supplementary Tables S1). Grandidierella japonica and G. tigrinus are NNS at the Baltic Sea, native to the North-West Pacific and the North-West Atlantic, respectively (Chapman and Dorman 1975; Kelly et al. 2006a, b; Soors et al. 2022).
At Falckenstein beach, the amphipod community was the most diverse and abundant with eight species present: G. salinus, G. oceanicus, G. locusta, G. zaddachi, C. laeviusculus, M. cf. insidiosum, and G. japonica. Gammarus salinus was the most abundant in spring, from April to June, when G. locusta took over (Figs. 1 and 2). Grandidierella japonica was present at low abundance. At downtown Kiel, six species were identified: G. salinus, G. oceanicus, C. laeviusculus, M. insidiosum, M. cf. gryllotalpa, and G. japonica. Gammarus salinus, G. oceanicus and M. cf. gryllotalpa were abundant species (Figs. 1 and 2). At Dassower See, three amphipod species were present: M. cf. gryllotalpa, G. locusta, and G. tigrinus, with G. tigrinus being the most abundant (Figs. 1 and 2). Indeed, G. tigrinus was dominant throughout most of the year, except November and December, when M. cf. gryllotalpa took over (Figs. 2 and 3). There was a significant effect of study location on Simpson’s Diversity Index (χ2 = 12.764, df = 2, p < 0.005). Both Falckenstein beach and downtown Kiel exhibited significantly greater diversity in comparison to Dassower See (Z = 3.186, p < 0.005; Z = 2.994, p < 0.005: Fig. 4).
Seasonal effects on amphipod communities
Our models did not indicate any significant seasonal change in amphipod abundance over time across the three locations, though Falckenstein beach demonstrated slight seasonality but not significantly (all p > 0.09; Fig. 5; Table 1). The observed temporal patterns showcased considerable fluctuations among the period studied, with Falckenstein beach having the highest abundance of amphipods on average (135.1 and 9.1 mean and standard error, respectively), followed by downtown Kiel (60.3 and 5.0, respectively), and lastly, Dassower See (43.4 and 5.3, respectively). The maximum abundance was recorded in Falckenstein beach during April with 351 individuals. Similar to abundance trends, we did not find any significant changes in the species richness over time across the three locations (all p > 0.12; Fig. 5; Table 2). Model responses suggested high variability in species richness over time (all p > 0.12), with Falckenstein beach displaying highest species richness variability (ranging from 3 to 8 species). On the other hand, Dassower See exhibited the least variability, as well as the lowest amount of species richness (ranging from 1 to 3 species).
Following the above analyses, the abundance of native amphipods did not change over time in Falckenstein beach and downtown Kiel (both p > 0.15; Fig. 6; Table 3). Though, in Falckenstein beach, slight decrease in abundance was noticeable during the winter months (Fig. 6). However, we found a significant change in Dassower See (p < 0.003), with peak abundance during the fall months (i.e. October and November), followed by a decline in the subsequent months. A significant change was also observed in the abundance of NNS in Dassower See (p < 0.001; i.e. G. tigrinus), with a peak during the summer months (i.e. June and July) followed by a sharp decrease until the end of the year, before a slight increase at the beginning of 2023 (January, February, and March).
Environmental effects on amphipod abundance
Over the study period, pH varied between 8.1 and 8.9, 7.8 and 8.2, and 7.9 and 9.1 at Falckenstein beach, downtown Kiel, and Dassower See, respectively (Supplementary Table S1). Salinity ranged from 11.1 to 20.4 ppt, 12.1 to 20 ppt, and from 6.2 to 15.1 ppt, respectively, while temperature ranged from 5.5 to 29.6, 4.6 to 20.9, and 4.2 to 20.8 °C. Dissolved oxygen measures were from 98.4 to 167.3, 84.0 to 107.2, and 93.1 to 122.4%, respectively (Supplementary Table S1). Our analyses did not reveal significant impacts of pH or dissolved oxygen on amphipod abundance across the three locations (Supplementary Table S3). In the case of salinity, we observed a notable effect on the overall amphipod abundance at Falckenstein beach (p < 0.001; Fig. 7), while no significant effect was found for downtown Kiel nor Dassower See (p > 0.55); interestingly, an increase in salinity at Dassower See in November and December was associated with a decrease in abundance of G. tigrinus and increase of M. cf. gryllotalpa. Lastly, water temperature exerted a positive influence on the overall amphipod abundance in Dassower See (p = 0.04) (Supplementary Table S3). The RDA plot revealed distinct clustering of samples based on location, indicating that species composition varied among the three studied locations. Salinity had an opposite effect on amphipod community than pH, oxygen and temperature (Fig. 8; Supplementary Table S4).
Redundancy Analysis (RDA) plot showing the relationship between environmental variables and species composition across three locations: Falckenstein beach, downtown Kiel, and Dassower See. The marginal histograms on the top and right sides show the distribution of RDA1 and PC1 scores from each location
Discussion
Anthropogenic disturbance is a major threat to ecosystem biodiversity, with NNS often establishing in habitats subject to high levels of human activity (Smith and Knapp 1999; Minchinton 2002; Halpern et al. 2008; Nogales et al. 2011; Lockwood et al. 2013; Linders et al. 2019; Haubrock et al. 2021). To assess the impacts of such activities on the diversity of native, as well as NNS, we monitored amphipod communities in two anthropogenically impacted and one protected habitat in the Baltic Sea for a whole year. Despite the higher invasion risk in the two anthropogenically impacted habitats, they experienced significantly higher native species richness, as well as lower NNS abundance, when compared to the protected Dassower See. Out of nine amphipod species identified, only two were NNS, with G. japonica found strictly in the anthropogenically impacted habitats, and G. tigrinus in the protected habitat. Interestingly, G. japonica was also consistently recorded at low abundances, while Dassower See was dominated by G. tigrinus for the majority of the year. Similar results were also observed by Lin et al. (2022), where several NNS were highly abundant at an unpolluted oyster aquaculture site compared to a heavily polluted nearby shipping port.
Recent studies across a variety of taxa have revealed that species can adapt quickly in response to anthropogenically impacted environments (Johnson and Munshi-South 2017; Thompson et al. 2018; Santangelo et al. 2018; Borden and Flory 2021). The two anthropogenically impacted habitats in our study have already been affected by humans for centuries, and therefore the adaptation of native communities to numerous and diverse stressors may have already occurred or may currently be ongoing. However, we emphasize that our study does not provide evidence that adaptation of native taxa to anthropogenic conditions is the driver of differences of species abundances across sites. We also point out that newly arrived NNS might fail to establish, or persist only at low abundances, due to suboptimal environmental conditions. These NNS populations may be “sleeper populations” (Spear et al. 2021). Such populations can remain at low abundance for years, or even decades, until their population growth is triggered by a change in environmental factors, freshly introduced genetic material by newly arrived individuals from native or other non-native regions, or selection acting on the established population (Bock et al. 2015; Colautti and Lau 2015; Dlugosch et al. 2015; Spear et al. 2021). Grandidierella japonica, a NNS recorded at both anthropogenically impacted habitats in our study, was constantly detected at low density. For decades, this species has already been spreading throughout North America and Northern Europe, where it has been reported at both low and high densities (Pilgrim et al. 2013; Soors et al. 2022). Though this species can colonise a variety of habitats, including harbours, marinas, and areas near sewage treatment plants (Chapman and Dorman 1975), it is sensitive to high metal concentrations (Lee et al. 2005). With both of the locations where we recorded the species being highly polluted by heavy metals (Nikulina et al. 2008), the heavy metal concentrations may prevent the species from becoming abundant.
Although the three locations sampled during this study are in close proximity, the highly invasive NNS G. tigrinus was observed only in Dassower See. One explanation for this could be the susceptibility of G. tigrinus to disturbances and habitat structural characteristics (Kotta et al. 2014), making the anthropogenically impacted Falckenstein beach and downtown Kiel environments possibly too disturbed for the species. Kotta et al. (2014) also reported that various forms of disturbance, such as the removal of plants and the mixing or removal of the sediment surface layer, can significantly affect its biomass in the Northeastern Baltic Sea. They suggested that while native species have already adapted to these disturbances, characteristic of the area, G. tigrinus has not. Another reason could stem from Dassower See having lower salinity compared to Falckenstein beach and downtown Kiel. Though G. tigrinus’ North American native-range salinity varies from 4 to 30 ppt, it has invaded only freshwater and brackish habitats (Sexton and Cooper 1939; Jazdzewski et al. 2002; Kelly et al. 2006b). Paiva et al. (2018) demonstrated that populations of gammarids from different regions have distinct salinity tolerances, suggesting local adaptation of populations to environmental conditions. Multiple introduction events of G. tigrinus to Europe promoted population admixture, increasing its genetic diversity (Kelly et al. 2006b). However, all established populations originated from freshwater and estuarine environments (Kelly et al. 2006b). Gammarus tigrinus established in European waters may therefore prefer lower saline habitats, as demonstrated for the Dassower See population by Dickey et al. (2021). Finally, a shift in the dominating population from G. tigrinus to native M. cf. gryllotalpa when salinity at Dassower See increased, further suggests that salinity may be one of the barriers preventing the spread of current G. tigrinus populations in European waters.
Gammarus tigrinus was the most abundant species in Dassower See, dominating over the native G. locusta and M. cf. gryllotalpa. Furthermore, the previously recorded native G. duebeni was completely absent during our sampling. With sampling in previous years showing similar abundances of G. duebeni and G. tigrinus (Zettler 2001; Briski, personal observation), the native species may have since been replaced by the NNS. Indeed, G. tigrinus has a long invasion history in Europe and has been identified as one of the most widespread and aggressive invaders currently in the Baltic Sea (Ojaveer and Kotta 2015). Its success may stem from traits such as higher fecundity, earlier sexual maturity and wider salinity tolerance when compared to native gammarid taxa (Pinkster et al. 1977; Grabowski et al. 2007; Orav-Kotta et al. 2009; Jänes et al. 2015; Paiva et al. 2018). The impact of this NNS is further enhanced by its aggressive and predatory behavior towards native amphipods and the propensity to attack and wound prey without consumption. This not only depletes potential food sources, but also introduces additional stress and vulnerability to native populations (Dick 1996; Dickey et al. 2021). Despite native gammarids occupying a wider ecological niche, they are limited by their attack and predation rates relative to G. tigrinus (Herkül et al. 2016; Cuthbert et al. 2022), which may also contribute to this NNS successfully outcompeting and replacing native species (Pinkster 1975; Pinkster et al. 1992; Szaniawska et al. 2003; Grabowski et al. 2006). After the decline of G. tigrinus abundance in late autumn, the abundance of native species increased, suggesting that G. tigrinus exerts strong competitive pressure on the native G. locusta and M. cf. gryllotalpa.
Though our model did not find significant seasonal differences for abundance nor species richness at Falckenstein beach when all gammarids were taken into account, the study demonstrated a clear shift in dominance from G. salinus to G. locusta between spring and summer. There was also a significant change at Dassower See from summer to autumn, with the native G. locusta and M. cf. gryllotalpa replacing the non-native G. tigrinus as the most dominant taxa. Seasonality is rarely considered in ecology, but several recent studies have demonstrated its importance (Fuhrman et al. 2015; Needham and Fuhrman 2016; Wahl et al. 2020; White and Hastings 2020; Theurich et al. 2024). For instance, Fuhrman et al. (2015), Needham and Fuhrman (2016), Needham et al. (2017) determined prokaryotic and eukaryotic phytoplankton bloom species successions among seasons, while Theurich et al. (2024) revealed fluctuating feeding impacts of the non-native Hemigrapsus takanoi towards Mytilus sp. prey, with seasonality also mediating feeding responses between sexes of the predator. Our study correlates with these, further emphasizing the importance of seasonality in ecological studies, and in our case, its omission would have failed to recognize the seasonal succession. Further, failing to account for seasonality could lead to NNS being unrecorded from a study location should sampling be conducted when the NNS is at low abundance or absent.
Consequently, our study demonstrated that anthropogenically impacted habitats may be under higher invasion risk – serving as gateways for NNS (Smith and Knapp 1999; Minchinton 2002; Lockwood et al. 2013), but at the same time, they may not be the best environments for them to establish and flourish. In contrast, nearby undisturbed and/or protected habitats, such as Dassower See, may be highly vulnerable to invasions due to more optimal environmental conditions, naïve populations, and robust, prior adapted populations of NNS (Kotta et al. 2014; Bock et al. 2015; Colautti and Lau 2015; Dlugosch et al. 2015; Briski et al. 2018; Lin et al. 2022). Although other factors, such as salinity, temperature, heavy metal concentrations, as well as stochasticity and randomness, may be affecting the establishment success of NNS and subsequently the community structures of the invaded habitats (Lockwood et al. 2013; Paiva et al. 2018; Casties et al. 2019; Briski et al. 2024; Martinez Reyes et al. 2024). Finally, our results show that seasonality is an important aspect of ecological studies and must be taken into account, as omissions could potentially distort our understanding of the dynamics of ecosystems, as well as lead to the unsuccessful detection of NNS (Wahl et al. 2020; White and Hastings 2020; Theurich et al. 2024).
Data availability
The reproducible code and data used in this study are stored at https://github.com/IsmaSA/Amphipods_Kiel.
References
Belkin IM (2009) Rapid warming of large marine ecosystems. Prog Oceanogr 81(1–4):207–213
Benjamini Y, Hochberg Y (1995) Controlling the false Discovery rate: a practical and powerful Approach to multiple testing. J R Stat Soc Ser B (Methodological) 57(1):289–300
Bock D, Caseys C, Cousens RD, Hahn MA, Heredia SM, Hübner S, Turner KG, Whitney KD, Rieseberg LH (2015) What we still do not know about invasion genetics. Mol Ecol 24:2277–2297
Borden JB, Flory L (2021) Urban evolution of invasive species. Front Ecol Environ 19(3):184–191
Briski E, Bailey SA, MacIsaac HJ (2011) Invertebrates and their dormant eggs transported in ballast sediments of ships arriving to the Canadian coasts and the Laurentian Great Lakes. Limnol Oceanogr 56(5):1929–1939
Briski E, Wiley CJ, Bailey SA (2012a) Role of domestic shipping in the introduction or secondary spread of nonindigenous species: biological invasions within the Laurentian Great Lakes. J Appl Ecol 49:1124–1130
Briski E, Ghabooli S, Bailey SA, MacIsaac HJ (2012b) Invasion risk posed by macroinvertebrates transported in ships’ ballast tanks. Biol Invasions 14:1843–1850
Briski E, Bailey SA, Casas-Monroy O, DiBacco C, Kaczmarska I, Lawrence JE, Leichsenring J, Levings C, MacGillivary ML, McKindsey CW, Nasmith LE, Parenteau M, Piercey GE, Rivkin RB, Rochon A, Roy S, Simard N, Sun B, Way C, Weise AM, MacIsaac HJ (2013) Taxon- and vector-specific variation in species richness and abundance during the transport stage of biological invasions. Limn Ocean 58(4):1361–1372
Briski E, Chan FT, Darling JA, Lauringson V, MacIsaac HJ, Zhan A, Bailey SA (2018) Beyond propagule pressure: importance of selection during the transport stage of biological invasions. Front Ecol Environ 16(6):345–353
Briski E, Kotronaki SG, Cuthbert NR, Bortolus A, Campbell ML, Dick JTA, Fofonoff P, Galil BS, Hewitt CL, Lockwood JL, MacIsaac HJ, Ricciardi A, Ruiz G, Schwindt E, Sommer U, Zhan A, Carlton JT (2024) Does non-native diversity mirror Earth’s biodiversity? Glob Ecol Biogeogr 33:48–62
Casties I, Seebens H, Briski E (2016) Importance of geographic origin for invasion success: a case study of the North and Baltic seas versus the Great Lakes-St. Lawrence River region. Ecol Evol 6:8318–8329
Casties I, Clemmesen C, Briski E (2019) Environmental tolerance of three gammarid species with and without invasion record under current and future global warming scenarios. Divers Distrib 25:603–612
Chapman JW, Dorman JA (1975) Diagnosis, systematics, and notes on Grandidierella japonica (Amphipoda: Gammaridea) and its introduction to the Pacific coast of the United States. Bull South Calif Acad Sci 74:104–108
Colautti RI, Lau JA (2015) Contemporary evolution during invasion: evidence for differentiation, natural selection, and local adaptation. Mol Ecol 24:1999–2017
Costa FO, Henzler CM, Lunt DH, Whiteley NM, Rock J (2009) Probing marine Gammarus (Amphipoda) taxonomy with DNA barcodes. Syst Biodivers 7(4):365–379
Cuthbert RN, Kotronaki SG, Dick JT, Briski E (2020) Salinity tolerance and geographical origin predict global alien amphipod invasions. Biol Lett 16(9):20200354
Cuthbert RN, Kotronaki SG, Hütt JC, Renk E, Warlo N, Briski E (2022) Do alternative resources dampen functional responses of native but not alien gammarids? Ecol Evol 12(9):e9262
Daehler CC (2003) Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration. Annu Rev Ecol Evol Syst 34(1):183–211
Davies TW, Duffy JP, Bennie J, Gaston KJ (2014) The nature, extent, and ecological implications of marine light pollution. Front Ecol Environ 12:347–355
Dermott R, Witt J, Um YM, Gonzalez M (1998) Distribution of the Ponto-Caspian amphipod Echinogammarus ischnus in the Great Lakes and replacement of native Gammarus fasciatus. J Gt Lakes Res 24(3):442–452
Di Franco E, Pierson P, Di Iorio L, Calò A, Cottalorda JM, Derijard B, Di Franco A, Galvé A, Guibbolini M, Lebrun J, Micheli F, Priouzeau F, Risso-de Faverney C, Rossi F, Sabourault C, Spennato G, Verrando P, Guidetti P (2020) Effects of marine noise pollution on Mediterranean fishes and invertebrates: a review. Mar Pollut Bull 159:111450
Dick JTA (1996) Post-invasion amphipod communities of Lough Neagh, Northern Ireland: influences of habitat selection and mutual predation. J Anim Ecol 65(6):756–767
Dickey JWE, Cuthbert RN, Steffen GT, Dick JTA, Briski E (2021) Sea freshening may drive the ecological impacts of emerging and existing invasive non-native species. Divers Distrib 27:144–156
Dlugosch KM, Anderson SR, Braasch J, Cang FA, Gillette H (2015) The devil is in the details: genetic variation in introduced populations and its contributions to invasion. Mol Ecol 24:2095–2111
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–229
Fretwell SD (1972) Populations in a seasonal environment. Monogr Popul Biology. https://doi.org/10.12987/9780691209647
Fuhrman JA, Cram JA, Needham DM (2015) Marine microbial community dynamics and their ecological interpretation. Nat Rev Microbiol 13(3):133–146
Gerhardt A, Bloor M, Mills CL (2011) Gammarus: important taxon in freshwater and marine changing environments. Int J Zool 2011:2–4
Grabowska J, Grabowski M (2005) Diel-feeding activity in early summer of race goby Neogobius gymnotrachelus (Gobiidae): a new invader in the Baltic Basin. J Appl Ichthyol 21:282–286
Grabowski M, Konopacka A, Jazdzewski K, Janowska E (2006) Invasions of alien gammarid species and retreat of natives in the Vistula Lagoon (Baltic Sea, Poland). Helgol Mar Res 60:90–97
Grabowski M, Bacela K, Konopacka A (2007) How to be an invasive gammarid (Amphipoda: Gammaroidea)–comparison of life history traits. Hydrobiologia 590:75–84
Halpern BS, Walbridge S, Selkoe KA, Kappel CV, Micheli F, D’Agrosa C, Bruno JF, Casey KS, Ebert C, Fox HE, Fujita R, Heinemann D, Lenihan HS, Madin EMP, Perry MT, Selig ER, Spalding M, Steneck R, Watson R (2008) A global map of human impact on marine ecosystems. Science 319:948–952
Haubrock PJ, Pilotto F, Innocenti G, Cianfanelli S, Haase P (2021) Two centuries for an almost complete community turnover from native to non-native species in a riverine ecosystem. Glob Change Biol 27(3):606–623
Herkül K, Lauringson V, Kotta J (2016) Specialization among amphipods: the invasive gammarus tigrinus has narrower niche space compared to native gammarids. Ecosphere 7(6):e01306
Hufbauer RA, Facon B, Ravigné V, Turgeon J, Foucaud J, Lee CE, Rey O, Estoup A (2012) Anthropogenically induced adaptation to invade (AIAI): contemporary adaptation to human-altered habitats within the native range can promote invasions. Evol Appl 5(1):89–101
Jänes H, Kotta J, Herkül K (2015) High fecundity and predation pressure of the invasive Gammarus tigrinus cause decline of indigenous gammarids. Estuar Coast Shelf Sci 165:185–189
Jazdzewski K, Konopacka A, Grabowski M (2002) Four Ponto-Caspian and one American gammarid species (Crustacea, Amphipoda) recently invading Polish waters, Contrib Zool. 71(4):115–122
Johnson MTJ, Munshi-South J (2017) Evolution of life in urban environments. Science 358:eaam8327
Jutterström S, Andersson HC, Omstedt A, Malmaeus JM (2014) Multiple stressors threatening the future of the Baltic Sea–kattegat marine ecosystem: implications for policy and management actions. Mar Pollut Bull 86(1–2):468–480
Kaluza P, Kölzsch A, Gastner MT, Blasius B (2010) The complex network of global cargo ship movement. J R Soc Interface 7:1093–1103
Kelly DW, MacIsaac HJ, Heath DD (2006a) Vicariance and dispersal effects on phylogeographic structure and speciation in a widespread estuarine invertebrate. Evolution 60:257–267
Kelly DW, Muirhead JR, Heath DD, Macisaac HJ (2006b) Contrasting patterns in genetic diversity following multiple invasions of fresh and brackish waters. Mol Ecol 15(12):3641–3653
Knebel D (2021) Column: History of world’s busiest canal, Current Publishing. [online] https://www.youarecurrent.com/2021/04/12/column-history-of-worlds-busiest-canal/ [Accessed 15 Apr. 2023]
Kotta J, Torn K, Reisalu G, Veber T (2014) Relationships between mechanical disturbance and biomass of the invasive amphipod Gammarus tigrinus within a charophyte-dominated macrophyte community. Mar Ecol 35:11–18
Lee JS, Lee KT, Park GS (2005) Acute toxicity of heavy metals, tributyltin, ammonia and polycyclic aromatic hydrocarbons to benthic amphipod Grandidierella japonica. Ocean Sci J 40:61–66
Light T, Marchetti MP (2007) Distinguishing between invasions and habitat changes as drivers of diversity loss among California’s freshwater fishes. Conser Biol 21(2):434–446
Lin Y, Vidjak O, Ezgeta-Balić D, Bojanić Varezić D, Šegvić-Bubić T, Stagličić N, Zhan A, Briski E (2022) Plankton diversity in anthropocene: Shipping vs. aquaculture along the eastern Adriatic coast assessed through DNA metabarcoding. Sci Tot Environ 807:151043
Linders TEW, Schaffner U, Eschen R, Abebe A, Choge SK, Nigatu L, Mbaabu PR, Shiferaw H, Allan E (2019) Direct and indirect effects of invasive species: Biodiversity loss is a major mechanism by which an invasive tree affects ecosystem functioning. J Ecol 107:2660–2672
Lobo J, Costa PM, Teixeira MA, Ferreira MS, Costa MH, Costa FO (2013) Enhanced primers for amplification of DNA barcodes from a broad range of marine metazoans. BMC Ecol 13(1):34
Lockwood JL, Hoopes MF, Marchetti MP (2013) Invasion Ecology, 2nd edn. New York Academy of Sciences Ser. Chichester, West Sussex, UK: Wiley-Blackwell
Martinez Reyes C, Cuthbert RN, Langrehr L, Briski E (2024) Warming, not acidification, favours survival of non-indigenous over native gammarid species. Biol Invasions 26:591–604
Minchinton TE (2002) Disturbance by wrack facilitates spread of Phragmites australis in a coastal marsh. J Exp Mar Biol Ecol 281(1–2):89–107
Needham D, Fuhrman J (2016) Pronounced daily succession of phytoplankton, archaea and bacteria following a spring bloom. Nat Microbiol 1:16005
Needham DM, Sachdeva R, Fuhrman JA (2017) Ecological dynamics and co-occurrence among marine phytoplankton, bacteria and myoviruses shows microdiversity matters. ISME 11:1614–1629
Nikulina A, Polovodova I, Schönfeld J (2008) Foraminiferal response to environmental changes in Kiel Fjord, SW Baltic Sea. eEarth 3(1):37–49
Nogales B, Lanfranconi MP, Piña-Villalonga JM, Bosch R (2011) Anthropogenic perturbations in marine microbial communities. FEMS Microbiol Rev 35(2):275–298
Ojaveer H, Kotta J (2015) Ecosystem impacts of the widespread non-indigenous species in the Baltic Sea: literature survey evidences major limitations in knowledge. Hydrobiologia 750:171–185
Oksanen J (2012) Constrained ordination: tutorial with R and vegan. R-packace Vegan 1(10):1–9
Orav-Kotta H, Kotta J, Herkül K, Kotta T, Paalme T (2009) Seasonal variability in the grazing potential of the invasive amphipod Gammarus tigrinus and the native amphipod Gammarus salinus (Amphipoda: Crustacea) in the northern Baltic Sea. Biol Invasions 11:597–608
Pachauri RK, Allen MR, Barros VR, Broome J, Cramer W, Christ R, Church JA, Clarke L, Dahe Q, Dasgupta P, Dubash NK, Edenhofer O, Elgizouli I, Field CB, Forster P, Friedlingstein P, Fuglestvedt J, Gomez-Echeverri L, Hallegatte S, Hegerl G, Howden M, Jiang K, Jimenez Cisneroz B, Kattsov V, Lee H, Mach KJ, Marotzke J, Mastrandrea MD, Meyer L, Minx J, Mulugetta Y, O’Brien K, Oppenheimer M, Pereira JJ, Pichs-Madruga R, Plattner GK, Pörtner HO, Power SB, Preston B, Ravindranath NH, Reisinger A, Riahi K, Rusticucci M, Scholes R, Seyboth K, Sokona Y, Stavins R, Stocker TF, Tschakert P, van Vuuren D, van Ypserle JP (2014) Climate change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the fifth assessment report of the Intergovernmental Panel on Climate Change. IPCC 151
Paiva F, Barco A, Chen Y, Mirzajani A, Chan F, Lauringson V, Baltazar-Soares M, Zhan A, Bailey S, Javidpour J, Briski E (2018) Is salinity an obstacle for biological invasions? Glob Chang Biol 24:2708–2720
Paiva F, Pauli NC, Briski E (2020) Are juveniles as tolerant to salinity stress as adults? A case study of northern European, Ponto-Caspian and North American species. Divers Distrib 26:1627–1641
Pilgrim EM, Blum MJ, Reusser DA, Lee H, Darling JA (2013) Geographic range and structure of cryptic genetic diversity among Pacific North American populations of the non-native amphipod Grandidierella japonica. Biol Invasions 15:2415–2428
Pinkster S (1975) The introduction of the alien amphipodGammarus Tigrinus Sexton, 1939 (Crustacea, Amphipoda) in the Netherlands and its competition with indigenous species. Hydrobioll Bull 9:131–138
Pinkster S, Smit H, Brandse-de Jong N (1977) The introduction of the alien amphipod Gammarus Tigrinus Sexton, 1939, in the Netherlands and its competition with indigenous species. Crustaceana Suppl 4:91–105
Pinkster S, Scheepmaker M, Platvoet D, Broodbakker N (1992) Drastic changes in the amphipod fauna (Crustacea) of Dutch inland waters during the last 25 years. Bijdr Dierkd 61(4):193–204
Pyšek P, Hulme PE, Simberloff D, Bacher S, Blackburn TM, Carlton JT, Dawson W, Essl F, Foxcroft LC, Genovesi P, Jeschke JM, Kühn I, Liebhold AM, Mandrak NE, Meyerson LA, Pauchard A, Pergl J, Roy HE, Seebens H, van Kleunen M, Vilà M, Wingfield MJ, Richardson DM (2020) Scientists’ warning on invasive alien species. Biol Rev 95(6):1511–1534
Reusch TB, Dierking J, Andersson HC, Bonsdorff E, Carstensen J, Casini M, Czajkowski M, Hasler B, Hinsby K, Hyytiäinen K, Johannesson K, Jomaa S, Jormalainen V, Kuosa H, Kurland S, Laikre L, MacKenzie BR, Margonski P, Melzner F, Oesterwind D, Ojaveer H, Refsgaard JC, Sandström, Schwarz G, Tonderski K, Winder M, Zandersen M (2018) The Baltic Sea as a time machine for the future Coastal Ocean. Sci Adv 4(5):eaar8195
Santangelo JS, Rivkin LR, Johnson MT (2018) The evolution of city life. Proc R Soc B: Biol Sci 285:20181529
Sexton E, Cooper L (1939) On a new species of Gammarus (G. Tigrinus) from Droitwich District. J Mar Biol Assoc 23(2):543–551
Simberloff D, VonHolle B (1999) Positive interactions of nonindigenous species: invasional meltdown? Biol Invasions 1:21–32
Simberloff D, Martin JL, Genovesi P, Maris V, Wardle DA, Aronson J, Courchamp F, Galil B, García-Berthou E, Pascal M, Pyšek P, Sousa R, Tabacchi E, Vila M (2013) Impacts of biological invasions: what’s what and the way forward. Trends Ecol Evol 28(1):58–66
Smith M, Knapp A (1999) Exotic plant species in a C4-dominated grassland: invasibility, disturbance, and community structure. Oecologia 120:605–612
Soors J, de Beukelaer J, Bezdenjesnji O, Buerms D (2022) Two new alien crustacean invaders Grandidierella japonica (Stephensen, 1938) and Neomysis americana (SI Smith, 1873) in Belgium. Bioinvasions Rec 11(3):747–757
Soto I, Cuthbert RN, Ahmed DA, Kouba A, Domisch S, Marquez JRG, Beidas A, Amatulli G, Kiesel J, Shen LQ, Florencio M, Lima H, Briski E, Altermatt F, Archambaud-Suard G, Borza P, Csabai Z, Datry T, Floury M, Forcellini M, Fruget JF, Leitner P, Lizée MH, Maire A, Ricciardi A, Schäfer RB, Stubbington R, Van der Lee GH, Vannevel R, Várbíró G, Verdonschot RCM, Haase P, Haubrock PJ (2022) Tracking a killer shrimp: Dikerogammarus Villosus invasion dynamics across Europe. Divers Distrib 29:157–172
Soto I, Ahmed DA, Balzani P, Cuthbert RN, Haubrock PJ (2023) Sigmoidal curves reflect impacts and dynamics of aquatic invasive species. Sci Tot Environ 872:161818
Soto I, Balzani P, Carneiro L, Cuthbert RN, Macêdo R, Serhan Tarkan A, … and, Haubrock PJ (2024) Taming the terminological tempest in invasion science. Biol Rev. https://doi.org/10.1111/brv.13071
Spear MJ, Walsh JR, Ricciardi A, Vander Zanden MJ (2021) The invasion ecology of sleeper populations: prevalence, persistence, and abrupt shifts. Bioscience 71(4):357–369
Szaniawska A, Lapucki T, Normant M (2003) The invasive amphipod Gammarus tigrinus Sexton, 1939, in Puck Bay. Oceanologia 45(3):507–510
Theurich N, Cuthbert RN, Briski E (2024) Warming effects on a non-indigenous predator are not conserved across seasons. Limnol Oceanogr (in revision)
Thompson KA, Rieseberg LH, Schluter D (2018) Speciation and the city. Trends Ecol Evol 33(11):815–826
Wahl M, Werner FJ, Buchholz B, Raddatz S, Graiff A, Matthiessen B, Karsten U, Hiebenthal C, Hamer J, Ito M, Gülzow E, Rilov G, Guy-Haim T (2020) Season affects strength and direction of the interactive impacts of ocean warming and biotic stress in a coastal seaweed ecosystem. Limnol Oceanogr 65:807–827
White ER, Hastings A (2020) Seasonality in ecology: Progress and prospects in theory. Ecol Complex 44:100867
Wilkinson DM (2002) Ecology before ecology: biogeography and ecology in Lyell’s ‘Principles’. J Biogeogr 29:1109–1115
Zettler ML (2001) Some malacostracan crustacean assemblages in the southern and western Baltic Sea. Rostock Meeresbiol Beitr 9:127–143
Zettler ML, Zettler A (2017) Marine and freshwater Amphipoda from the Baltic Sea and adjacent territories. Conch Bookr, Harxheim, Germanywood
Funding
JWED was funded by a Humboldt Postdoctoral Fellowship from the Alexander von Humboldt Foundation.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
EK: Sample collection, Data curation, Formal analysis, Visualisation, Writing – original draft, Writing – review & editing. JWED: Formal analysis, Visualisation, Writing – original draft, Writing – review & editing. IS: Formal analysis, Visualisation, Writing – original draft, Writing – review & editing. PJH: Formal analysis, Visualisation, Writing – original draft, Writing – review & editing. AK: Writing – review & editing. RB: Formal analysis, Visualisation, Writing – review & editing. GS: Sample collection, Writing – review & editing. EB: Conceptualisation, Data curation, Formal analysis, Visualisation, Writing – original draft, Writing – review & editing.
Corresponding author
Ethics declarations
Ethics approval
Ethical approval was not required for the nature of this work.
Conflict of interest
Elizabeta Briski is an associate editor of Marine Biology. The other authors declare no conflicts of interest.
Additional information
Communicated by A. Zhan.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kazanavičiūtė, E., Dickey, J.W.E., Soto, I. et al. Seasonal changes in biodiversity of native and non-native amphipod taxa under diverse environmental contexts. Mar Biol 171, 156 (2024). https://doi.org/10.1007/s00227-024-04477-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-024-04477-4