Abstract
The White-faced Storm Petrel (WFSP) Pelagodroma marina has a widespread distribution, although virtually nothing is known about their feeding ecology and distributions at-sea. To describe their foraging areas, a total of 77 birds were equipped with 1 g-GPS loggers on Selvagem Grande, Madeira, Portugal (30° 09′ N, 15° 52′ W), during the 2018 and 2019 breeding seasons. We also assessed the diet of WFSP by analysing 17 faecal samples from chicks and 1 regurgitation from an adult using DNA metabarcoding techniques. Additionally, we collected body feathers from ten WFSP chicks to determine mercury concentration. WFSP fed mainly in deep oceanic waters, travelling up to 400 km from the colony, and did not concentrate in any well-defined, population-level foraging hotspots. Some individuals foraged along the edge of the shelf, near the African coast and the Canary Islands, especially during chick rearing. The duration of foraging trips and the total distance travelled, were, on average, 5.1 days and 723 km during the incubation period and 3.0 days and 578 km during chick rearing. The diet of WFSP was dominated by fish and cephalopods (crustacean prey were not detected), with Myctophidae (FO = 71%) representing the main fish family. WFSP often consume mesopelagic fish, in line with their preference for deep oceanic waters and with a small difference in at sea behavior (i.e., travel speed) between the diurnal and nocturnal period. The relatively high concentrations of mercury accumulated in body feathers of WFSP chicks (3.45 ± 1.44 mg kg−1 dry weight; range 1.68–6.01 mg kg−1) support the idea that WFSP raise their chicks mostly on mesopelagic prey from deep pelagic areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Seabirds represent an important component of marine trophic networks worldwide (Fauchald 2009). They are major consumers in marine ecosystems (Furness 1978; Brooke 2004a, b) and use foraging areas ranging from tens to thousands of kilometres from their breeding grounds (Coulson 2002; Brooke 2004a). During the breeding season, seabirds are central place foragers, having to commute regularly between foraging locations and the colony, to attend their eggs or feed their chicks. The at-sea distributions of most seabirds are linked to spatial distribution of prey, their abundance, and availability (Hunt and Schneider 1987; Fauchald et al. 2000; Depot et al. 2020). The diet of seabirds can reflect changes that occur at lower trophic levels, and thus, seabirds can be used as indicators helping to monitor the marine environment (Romero et al. 2021). Information on at-sea behavior and space-use of pelagic seabirds is essential to understand their role in ocean ecosystems and is also increasingly used to inform marine spatial planning (Camphuysen et al. 2012; Oppel et al. 2018).
In recent years, bird-borne GPS devices have provided new insights into the spatial distribution and movement patterns for many large- and medium-sized seabird species at sea (BirdLife 2020; Yoda 2019). Although there has been much progress in the miniaturization of tracking devices, foraging areas for the small procellariform seabirds are still largely unknown (Oro 2014; Rodríguez et al. 2019). However, in the last few years, lightweight (~ 1 g) GPS devices have become available, which now allow tracking even the smallest seabird species over extended periods of time (Hedd et al. 2018; Rotger et al. 2020; Bolton 2021).
The White-faced Storm Petrel Pelagodroma marina is a small burrow-nesting seabird (40–70 g; Marchant and Higgins 1990) of the Hydrobatidae family, comprising six subspecies, found in Atlantic, Pacific, and Indian Oceans in both Hemispheres (del Hoyo et al. 1992). Except for few breeding pairs in the Canary Islands (ca. 50 pairs, Rodríguez et al. 2003), the global population of the endemic European subspecies (Pelagodroma marina hypoleuca) is confined to a small archipelago, the Selvagens Islands, in the North-east Atlantic (Campos and Granadeiro 1999; Silva et al. 2015). Although further studies are required to confirm the actual population in the Selvagens archipelago, in 1996, population was estimated at 61,000 breeding pairs, of which 36,000 were counted in Selvagem Grande for the same year (Campos and Granadeiro 1999).
Most studies on White-faced Storm Petrels have focused on breeding biology (Richdale 1943–1944; Campos and Granadeiro 1999; Menkhorst et al. 1984; Underwood and Bunce 2004). Their at-sea distribution is largely unknown, but ship-based observations report that White-faced Storm Petrels are generally seen foraging over continental shelves (Rankin and Duffey 1948; Warham 1990; Cramp and Simmons 1997; Spear et al. 2007). In contrast, an analysis of stable isotopes of carbon in toe-nails of the Selvagem Grande population suggests that birds probably forage over the deep ocean around the Selvagens, rather than feeding close to the African coast (Furtado et al. 2016).
White-faced Storm Petrels are solitary-feeders and surface foragers, pattering the water with their long legs with out-stretched wings (Watson et al. 1986; Warham 1990; Spear et al. 2007). The diet of White-faced Storm Petrels consists mostly of small fish, pelagic crustaceans, and surface plankton (e.g., Imber 1984; Marchant and Higgins 1990; Brooke 2004a, b; Spear et al. 2007). The species appears to be opportunistic (Spear et al. 2007), feeding both nocturnally and diurnally on a diverse array of Myctophidae fishes, but also pelagic fishes (e.g., Bregmacerotidae), crustaceans (e.g., Hyperiidea, Euphausiid, crab megalops), and other non-cephalopods invertebrates (e.g., water-striders Halobates spp., Janthina sp.). However, information about the feeding ecology of White-faced Storm Petrels from the North Atlantic is very scarce (Waap 2015).
DNA metabarcoding of faecal samples is a non-invasive and robust method for identifying prey taxa (Symondson 2002; De Barba et al. 2014; Buglione et al. 2018). With the development of high-throughput sequencing techniques (HTS) of DNA barcodes, it is now possible to detect DNA sequences from degraded biological material (Valentini et al. 2009; Taberlet et al. 2012), including from faeces. The emergence of such techniques opens promising opportunities to gather information on the diet of these small seabird species, avoiding the use of intrusive methods (Symondson 2002).
Seabirds that rely extensively on mesopelagic prey tend to display high mercury concentrations compared to species with an epipelagic diet (e.g., Carravieri et al. 2018; Furtado et al. 2019, 2021; Monteiro and Furness 1995; Kim et al. 1996). This is due to the higher rate of microbial-mediated methylation of mercury in sub-thermocline low oxygen waters (Choy et al. 2009). Furthermore, pelagic seabirds show higher mercury concentrations as compared to coastal species (Monteiro and Furness 1995; Monteiro et al. 1996). Determination of feather mercury concentration hence allows further insights into the diet of seabirds, as mercury is deposited during feather growth, reflecting accumulation through diet over this period (Monteiro and Furness 2001).
Here, for the first time, we examine the foraging movements of White-faced Storm Petrels in the NE Atlantic using GPS devices, during the incubation and chick rearing periods of 2 consecutive years. We also briefly describe the prey delivered during chick rearing by examining chick faecal samples using DNA metabarcoding. In addition, we report the mercury levels in chick body feathers in Selvagem Grande, reflecting the concentration of this heavy metal in prey obtained in the foraging areas used during chick rearing (Stewart et al. 1997; Furtado et al. 2021).
Methods
Study area
Fieldwork was carried out on the island of Selvagem Grande (30° 09′ N, 15° 52′ W) (Fig. 1), the largest (245 ha) of the three islands which constitute the Selvagens Nature Reserve. Selvagem Grande is located in the North-east Atlantic, ca. 300 km south of the Island of Madeira (Portugal) and consists of a flat plateau surrounded by cliffs. The White-faced Storm Petrel breeds in burrows located in two well-defined areas of sandy soil in the plateau.
GPS tracking
In 2018 (April to May) and 2019 (April to June), a total of 65 White-faced Storm Petrels were captured at their nests during incubation, and equipped with 0.95 g GPS devices (Pathtrack Ltd., UK). Between May and June 2019, another 12 individuals were captured after feeding their chicks, using drop-traps set at the entrance of their burrows. Individuals were only tracked once, during the course of the study and 41% recorded more than one trip.
The GPS devices were attached to the base of the four central tail feathers using three narrow strips of TESA tape. The devices and attaching material weighed 1.1 g, corresponding to 1.9–2.4% of the body mass of tagged birds, below the 3% threshold above which it is believed the behavior of birds may be affected (Phillips et al. 2003). Total handling time was kept to a minimum (less than 10 min) and birds were returned to their burrows immediately afterwards to resume incubation or until leaving the chick.
During the first deployments in 2018 (incubation period, n = 23 deployments), GPS devices were set to record a location every 3 h, but subsequently we increased the frequency of locations to two fixes per hour (n = 15). In 2019, still during the incubation period, GPS devices (n = 27) were programmed to take fixes every hour. Shorter trips were expected during chick rearing, and therefore, we programmed 8 GPS devices to record a location every hour and four GPS devices were set to record a location every half hour. Prior to GPS deployment, a set of birds in nests were marked with a small white patch in the head. Nests were then inspected daily to avoid fitting GPS devices on individuals who had recently returned from foraging trips. In addition, a group of control nests (n = 30) was chosen and also monitored regularly to evaluate any impact of GPS on behavior by comparing duration of absences from the nest (i.e., trip duration) with those of deployed birds. We only checked controls during the incubation period. We tested for differences in trip duration between tagged birds and control group, using a generalized linear model (GLM) with a Poisson distribution.
GPS data processing
All GPS data were informally scanned for the presence of unrealistic fixes, identified as very large displacements associated with sudden changes in direction. These points (< 10) were eliminated before any analysis. All tracks from a single individual were split into individual trips, setting the start of the trip as the first fix obtained at more than 10 km from the colony, and the end if a fix was within 20 km from the colony (without any subsequent point further away from the colony). Several trips (25 out of 55) were not complete, due to battery failure (often in the end of the trip) or to other unknown cause (in this case, creating some gaps in the trips). Based on the degree of completeness of the trips, and on our daily attendance records at each nest, some of the trips were used to calculate trip metrics (last GPS fixes missing only when returning birds were very close to the colony, n = 55 trips). Incomplete trips were also used to calculate kernel utilization distributions when areas of intensive use could be clearly identifiable (n = 48) or to calculate other trip metrics (e.g., maximum trip distances) when tracking data clearly indicated that birds were clearly on their return to the colony (n = 46). We considered complete trips (n = 17 for incubation; n = 13 for chick rearing) to calculate total distance travelled (km), maximum distance (km) from the colony and trip duration (h). In addition, we also used nearly complete trips based on the quality of the trips for each trip characteristic parameter [total distance travelled (n = 10 for incubation; n = 5 for chick rearing), maximum distance (n = 11 for incubation; n = 5 for chick rearing), and trip duration (n = 6 for incubation; n = 4 for chick rearing)]. Finally, for the identification of foraging areas and to calculate the depth at foraging areas, we used data from complete tracks (n = 17 for incubation; n = 13 for chick rearing) and nearly complete trips (n = 14 for incubation; n = 4 for chick rearing).
Kernel utilization distributions were calculated for the incubation and chick rearing period on projected coordinates (UTM zone 28N, datum of Selvagens, EPSG = 2943) linearly interpolated at 1 h, using adehabitatHR package (Calenge 2006), setting the smoothing parameter (h) at 10,000 m (close to the average step length recorded per hour, see “Results”). We excluded from this analysis all interpolated points, whenever their time difference was ≥ 4 h. All fixes were then interpolated at 1 h intervals for subsequent analysis. To assess the differences in speed (hence total extent) estimated at different sampling intervals, we resampled all trips obtained at 0.5–1 h and 2 h intervals, and calculated the mean speeds in each case. As expected, shorter sampling intervals delivered higher estimates of speed (0.5 h = 9.4 ± 5.5 km h−1) than those estimated at longer periods (1 h = 8.9 ± 5.3 km h−1; 2 h = 8.4 ± 5.4 km h−1), but the differences were small, particularly between 1 and 2 h (ca. 5.8%). Since all trips were interpolated at 1 h (hence eliminating biases due to 0.5 h sampling intervals), we did not undertake any correction to deal with differences in fix intervals. All fixes were classified as diurnal or nocturnal according to the civil twilight, i.e., setting them as nocturnal whenever the fix was obtained when the sun was – 6° or less below the local horizon and diurnal otherwise [function crepuscle in maptools R package (Bivand and Lewin-Koh 2020)]. To quantify any difference in sea-floor depth between incubation and chick rearing, we intersected the 50% kernel utilization distribution (UD) of each individual with the bathymetric data obtained from ETOPO1 Global Relief Model (at 1 arc-min resolution, https://www.ngdc.noaa.gov/mgg/global), from which we calculated an average value of depth per individual. We also identified all fixes lying within the 50% UD of all individuals during incubation and chick rearing, to quantify the proportion of fixes in these areas during the day and during the night in each period.
Chick diet determination with DNA metabarcoding
DNA isolation and sequencing
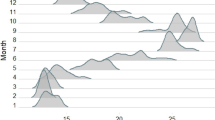
In 2019, we collected faecal samples from 28 chicks at their nests. The mean age of the chicks sampled was 19.6 ± 9.4 days (range 3–33). A tinfoil sheet was placed at the bottom of the nest chamber each morning (after the adult had left the nest), where chicks would defecate naturally. The nest chamber was inspected regularly during the morning period until the faecal samples were collected. If during that period, the chick had not defecated, the tinfoil was retrieved and sampling would be resumed the following day. Faecal samples were collected with a plastic spatula and stored in 2-mL tubes with absolute ethanol and stored at – 20 °C. One spontaneous regurgitate from an adult about to feed its chick was also included in diet analysis. DNA was isolated from each sample with a Norgen Stool DNA isolation kit (cat#27600, Norgen Biotek, Canada). The tubes were centrifuged for 1 min at 13,000 rpm and ethanol was carefully removed by aspiration with a micropipette before transferring the solid phase into the bead tubes. Samples were incubated in lysis buffer with gentle vortex for 1–2 h at room temperature before horizontal bead beating in a vortex at full speed. DNA was eluted in 65 µL of elution buffer at the final step of the protocol. The elution buffer was preheated at 70 °C and allowed to incubate at room temperature for 30 min before centrifuging. DNA samples were evaporated with SpeedVac to a final volume of 20 µL, using then Qubit 2.0 [Invitrogen with Qubit dsDNA HS Assay kit (Thermo Fisher Scientific)] to measure final DNA concentration. Samples with less than 1 ng/µL were discarded (8 samples) and the remaining (20 samples) were used for DNA metabarcoding. Libraries were prepared by AllGenetics and Biology SL (http://www.allgenetics.eu), aiming to target the main prey groups with the 16S gene: fishes/cephalopods and crustaceans. Primers and the blocking primer used as well as conditions for DNA amplification by polymerase chain reaction (PCR) are provided in the Online Resource 1. All samples that produced a PCR product were sequenced in NovaSeq PE250 (Illumina). Amplifications with the crustacean primers were successful for the regurgitate sample but failed for the faecal samples, despite several rounds of optimization. The DNA concentrations obtained from the regurgitate sample were high (24.2 ng/μL), but it was very low for several faecal samples (mean 7.5 ng/μL, range 1.03–30.4 ng/μL), which likely added difficulties to obtain successful amplification of DNA fragments. DNA obtained from faeces is expected to present higher degradation as compared to stomach content due to longer digestion time of prey tissues (Sousa et al. 2019).
Sequence analysis and taxonomic assignment
Sequence data were processed under Qiime2-2021.4 pipeline (Bolyen et al. 2019) with the DADA2 plugin (Callahan et al. 2016) to trim primer sequences, filter reads by quality (Phred ≥ 20), merge paired-end reads (setting a minimum overlap of 50 bp for pairing forward and reverse reads), and collapse them into a list of unique Amplicon Sequence Variants (ASVs). ASVs were then classified with Qiime2 classify-consensus-vsearch (Rognes et al. 2016; Bokulich et al. 2018) with the 16S Midori UNIQ-NUC_GB244 database as reference (Machida et al. 2017), setting 0.8 as minimum identity and 0.7 as minimum cover (full list of commands are provided in Online Resource 1). Taxa assignments by vsearch were confirmed by querying ASVs against GenBank with NCBI BLASTn (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Assignments to species level were confirmed if ASVs had a 98–100% match to the best hit in blastN or adjusted to the least common ancestor whenever other taxa were assigned with similar identity or no occurrence of the species in the North Atlantic was documented. Unassigned and non-target contaminant sequences were discarded from further analyses.
Mercury analysis
We collected eight-to-ten back body feathers from ten White-faced Storm Petrel chicks during the 2019 breeding season for quantification of total mercury. The mean age of the chicks sampled was 33.2 days (SD 2.44, range 30–36 days). These ten chicks were also included in the diet determination using DNA metabarcoding. The difference between faecal and feather sampling was 3.7 days (range 2–6 days). Feathers were clipped at the superior umbilicus of the feather, excluding the calamus, and stored in polyethylene bags. Samples were weighed on a Sartorius M5P micro balance (Sartorius AG, Goettingen) (mass between 0.441 and 3.020 mg, mean 1.23 ± 0.59 mg) and analysed according to the method described in Furtado et al. (2021). Total mercury in the body feathers was quantified by atomic absorption spectrophotometry with thermal decomposition (Costley et al. 2000) in LECO AMA-254 with a detection limit of 0.01 ng of mercury. At least two aliquotes of each sample were analysed, until the standard deviation was < 10%. Subsequently, the mean of the repeated mercury measurements was used for statistical analysis. Blanks were systematically run between samples (two procedural blanks). The mercury concentrations in procedural blanks were always below the detection limit of the equipment (0.01 ng of mercury). Precision and accuracy of the analytical method were evaluated by analysis of certified reference material (lobster hepatopancreas TORT-3; NRC, Canada). Reference values were of 0.292 ± 0.022 mg kg−1 dry weight (dw), and the mean determinations ± SD were 0.280 ± 0.176 mg kg−1 dw (n = 8). Thus, the recovery of the Certified Reference Material (CRM) was 95.9%. Results were corrected using the daily recovery efficiency of CRM.
Statistical analysis
Comparisons of trip metrics between individuals tracked during incubation and chick rearing were carried out using general linear models. We also compared the distance travelled by birds during the day and night in both reproductive phases, used linear mixed models (LMM), assuming a Gaussian error distribution. To do this, we calculated the distance travelled between consecutive points for each bird [i.e., travel speed (km h−1)] using time of day (daylight and night, classified as above) and phase (incubation and chick rearing) as factors, and setting the individual as a random factor. We started with a full model (random effects and interaction between day and phase), and then compared it with increasingly simpler models, using ANOVA-like test for random and fixed effects. These tests were carried out using lmerTest (Kuznetsova et al. 2017) and lme4 packages (Bates et al. 2015) for R (R Core Team 2021). We estimated the bearing of the position of each individual in relation to the colony at a distance of 10 km, and tested the uniformity in the direction of departure from the colony, using Rayleigh uniformity test in circular package (Agostinelli and Lund 2017).
Results
GPS retrieval
Overall, 66 out the 77 deployed GPS were recovered. All individuals tracked during the chick rearing period were recaptured at their nest. During incubation, nine birds could not be recaptured and eventually deserted their nest. Two birds lost the GPS attached. Four GPS did not deliver data due to device failure. From the 62 remaining GPS with data, we were only able to extract 55 trips for trip metrics calculation. There were no significant differences in trip duration between the recaptured birds with GPS and the control group [means (± SD): 6.5 ± 1.4 days (n = 109) vs 6.2 ± 1.7 days (n = 53), respectively, GLM χ21 = 0.59, P = 0.44].
Foraging trips
Total distance travelled, maximum distance from the colony, and trip duration were not significantly different between years during the incubation period (ANOVA, F1,25 = 0.78, P = 0.39, F1,26 = 1.16, P = 0.29 and F1,21 = 3.79, P = 0.06), so data from the 2 years were pooled.
Trip characteristics during incubation and chick rearing are summarized in Table 1 and are based on 30 complete trips and 19 nearly complete trips recorded, for both breeding phases. No significant differences in total distance, maximum distance, and trip duration were found between breeding periods (all P > 0.05; Table 1). However, there was a tendency for more distant foraging trips during incubation [18 birds (67%) undertook foraging trips over 500 km and 10 individuals (37%) travelled over 1000 km] compared to the chick rearing period [seven birds (49%) undertook foraging trips over 500 km, and only three birds (17%) travelled over 1000 km] (see Online Resource 2).
Throughout the breeding period, birds were mostly associated with offshore pelagic areas (average depth > 2000 m; Table 1 and Fig. 2). Most trips targeted deep waters around the colony. During the chick rearing period, four birds foraged in the shelf edge/slope, two near the African coast and the others on the west side of Fuerteventura Island (Fig. 2b). Birds set off in all directions from the colony during the incubation period (Rayleigh uniformity test: test statistic = 0.29, P = 0.06, Fig. 2a), but tended to leave the island to the east during chick rearing (83° from the geographical north, Rayleigh uniformity test: test statistic = 0.53, P = 0.002). White-faced Storm Petrel did not seem to concentrate in any well-defined foraging hotspots (Fig. 2).
Birds travelled slightly but significantly faster during the night (effect of period: day = 6.6 ± 2.2 km/h, night = 8.4 ± 2.3 km/h, LMM, t = 3.0, P = 0.004, Fig. 3) and also faster during chick rearing (effect of breeding phase: incubation = 7.1 ± 2.3 km/h, chick rearing = 8.6 ± 2.0 km/h, LMM t = 2.7, P = 0.01), with no significant interaction (t = 1.8, P = 0.08). The 50% UD of all individuals tended to contain slightly more diurnal than nocturnal fixes (day:night ratios, incubation = 1.20 (n = 1512 fixes); chick rearing = 1.49 (n = 558).
Diet
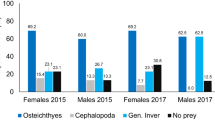
Prey sequences were detected in 17 samples (out of 20) and they all contained fish. European pilchard Sardina pilchardus (Clupeidae) predominated in samples (FO = 71%), followed by Warming’s lantern fish Ceratoscopelus warmingii (Myctophidae, FO = 29%) and Longspine snipefish Macroramphosus scolopax (FO = 24%) (Table 2). Myctophids were represented by 8 species, from 6 genus. Mesopelagic prey were present in 94% of the samples.
Cephalopods occurred in 24% of the faecal samples, with Mastigoteuthis magna being the most detected species. The regurgitate of the adult presented a high number of taxa (9 different prey), including some crustaceans.
Mercury
The mean mercury concentration in chick back body feathers was 3.45 ± 1.44 mg kg−1 dry weight (n = 10), with concentrations ranging from 1.68 to 6.01 mg kg−1 dw. Following the equation published by Ackerman et al. (2016), the average mercury concentration of chick feathers would correspond approximately to a blood mercury concentration of 0.43 ± 0.24 mg kg−1 wet weight (range 0.27–0.63 mg kg−1).
Discussion
In this study, we present novel data on foraging movements of the White-faced Storm Petrel during the incubation and chick rearing periods from the largest colony of this species in the North Atlantic. The White-faced Storm Petrel departed from the colony without a strong preference in directionality and used mostly deep oceanic waters. They seem to have a marked nocturnal activity during the chick rearing period, feeding mostly on mesopelagic prey, which is corroborated by the relatively high concentrations of mercury accumulated in feathers.
Individual foraging trips of White-faced Storm Petrel covered an average distance of more than 700 km while foraging during the incubation and more than 500 km during the chick rearing periods. Some individuals travelled more than 1000 km in one foraging trip during incubation, and further than 400 km off Selvagem Grande. Although remarkable that a small petrel such as the White-faced Storm Petrel is able to travel so far during the breeding season, this is consistent with recent tracking results for other small species of Procellariiformes. For instance, the Leach’s Storm Petrel Hydrobates leucorhous and Fork-tailed Storm Petrel Hydrobates furcatus, both weighing ca. 50 g birds, are able to travel distances up to 1600 km from the colony during the breeding season (Pollet et al. 2014; Halpin et al. 2018; Hedd et al. 2018; Bolton 2021; Collins et al. 2022). Rotger et al. (2020) also reported that Mediterranean Storm Petrels Hydrobates pelagicus melitensis in the Mediterranean Sea ranged up to 350 km from breeding colonies during incubation. As commonly observed in Procellariiformes (e.g., Guilford et al. 2008; Cecere et al. 2013), White-faced Storm Petrels made shorter foraging trips during chick rearing compared to incubation to ensure regular feed to their chicks (the lack of statistical significance is probably due to the small sample size and to the large variability in the tracking data).
Petrels are highly efficient flyers, using updrafts, slope, and dynamic soaring to exploit wind energy (Warham, 1990). Some small seabirds (e.g., Bulwer’s petrel Bulweria bulwerii; Gadfly petrels Pterodroma spp.) often choose to fly with favorable side winds that enable them to travel at high ground speeds and low energetic cost (Dias et al. 2016; Ventura et al. 2020). Storm petrels, mostly the northern storm petrels (Family Hydrobatidae), use dynamic soaring to travel over the ocean surface (Pennycuick 1982; Warham 1990) but this method does not seem to be used by White-faced Storm Petrel to any large extent (Pennycuick 1982; Erickson 1955; Cramp and Simmons 1997). In fact, White-faced Storm Petrel most frequently exhibit a distinctive flight pattern when feeding as they hop using both feet along the surface of the water facing the wind with extended wings (Marchant and Higgins 1990). This behavior is used to a varying degree among species, and it seems to be related to the general morphological differences between species (del Hoyo et al. 1992; Sausner et al. 2016). Some species, such as the White-faced Storm Petrel, use pattering almost exclusively, whereas other species, such as Leach’s Storm Petrel (Hydrobates leucorhoa), rarely use it (del Hoyo et al. 1992). Pattering is also prominent in the Oceanitidae family (southern storm petrels). Moreover, Sausner et al. (2016) suggested that species that pattering the most have low wing loading (mass (g)/total wing area (cm2)), low foot loading (relative foot size − mass (g)/foot area (cm2)), and a long tarsus in contrast to species that were classified as intermediate or least pattering (e.g., Hydrobates leucorhous). This might can explain differences in flight performance, as species increase flight speed with increasing wing loading. This unique flight behavior and morphology (wide wings and very long legs and feet) may be limiting White-faced Storm Petrel capability of undertaking exceptionally long foraging trips as the Hydrobates species mentioned above do (e.g., Pollet et al. 2014; Hedd et al. 2018; Collins et al. 2022).
Many seabird species breeding in oceanic tropical environments are known to forage in multiple directions owing to the spatial unpredictability of prey (Hennicke and Weimerskirch 2014; Oppel et al. 2015; Mott et al. 2016). A recent study by Oppel et al. (2018) showed that some families of seabirds, such as storm petrels, disperse widely at sea and exhibited large foraging ranges. Our data indicate that during the incubation period, White-faced Storm Petrels also seem to travel without directionality, not showing a clear choice for any well-defined foraging hotspots (the east directionality shown in chick rearing is probably due to the small sample size). This movement pattern suggests that while prey may be typically unpredictable in this area, they are widely distributed.
The oceanic areas of the subtropical eastern North Atlantic are characterised by warm sea surface temperatures and low productivity, differing from the nutrient-rich waters of the coastal upwelling of West Africa associated with the Canary Current (Cropper et al. 2014; Paiva et al. 2010). White-faced Storm Petrel does not seem to be associated with seamounts or core upwelling areas in the African coast, in contrast with other Procellariiform species from this and adjacent colonies. For example, Cory’s shearwater Calonectris borealis from Selvagem Grande forage the oceanic domain around the islands, the African continental shelf (from Morocco to Mauritania), and the nearest seamounts (e.g., Alonso et al. 2012; Ramos et al. 2013; Romero et al. 2021). On the other hand, the Bulwer’s petrel explores areas around the colony and waters close to the Azorean archipelago (mid-Atlantic) (Dias et al. 2016), but birds from the Canary Islands also use the shelf-break to forage during the breeding season (Rodríguez et al. 2013). Deserta and Madeira’s petrel (Pterodroma deserta and P. madeira) perform very large clockwise foraging trips assisted by favourable winds, and use a large pelagic region around the archipelagos of Madeira and Azores (Ramos et al. 2016; Ventura et al. 2020). The distribution of White-faced Storm Petrels far from the continental shelf confirms the highly pelagic behavior of this species. Notwithstanding, they also forage in the continental shelf edge and near the Canary Islands. Due to the influence of the Canary Current, the African shelf and shelf-break represent productive areas in the north-east Atlantic (Barton et al. 1998), which leads to enhanced productivity of the shelf edge areas (Hunt et al. 1999; Weimerskirch 2007).
The diet of White-faced Storm Petrels, as assessed through DNA metabarcoding of chick faeces during the chick rearing period, was dominated by fishes and a few cephalopods species. The main fish family found was Myctophidae (FO = 71%), and is in accordance with the study by Spear et al. (2007) in the Pacific and by Waap (2015) in the North Atlantic. The presence of mesopelagic prey in the diet of surface seizing seabirds is striking. They are probably available to White-faced Storm Petrel during the night, when they ascend to more superficial waters to feed on zooplankton. Alternatively, they can be forced to the surface by underwater predators, such as whales, dolphins, and tuna, which are abundant in the region. Still, the presence of such prey is also frequent in the diet of other small seabirds that nest in the North Atlantic, such as Bulwer’s Petrel, Madeiran Storm Petrel Hydrobates castro, and Leach’s Storm Petrel (Zonfrillo 1985; Monteiro et al. 1996; Hedd and Montevecchi 2006; Hedd et al. 2009; Waap 2015; Waap et al. 2017; Carreiro et al. 2020). Cephalopods were the second most abundant group in the diet of White-faced Storm Petrel (FO = 24%), with Mastigoteuthis magna being the most detected species. The cephalopod species present occur mainly in mesopelagic or even bathypelagic environments, although some species are epipelagic when in larval or juvenile stages (Clarke 1986).
The European pilchard, an epipelagic species, also occurred frequently (FO = 71%) in the diet of White-faced Storm Petrel. Sardines are abundant in coastal waters being one of the most abundant pelagic species off the NW African Coast (Machu et al. 2009). In the coastal (neritic) waters of the Madeira Archipelago, especially off the south coast of Madeira Island, there is a year-round fishery for small pelagic fishes, including the European pilchard. Fish larvae, e.g., of Sardina pichardus, from the spawning area of northwest Africa are known to be transported to the waters of the Canary Islands. This can explain the foraging movements of some tracked White-faced Storm Petrel near the African continental shelf edge and near the Canary Islands. Storm petrels in general are not usually observed foraging inshore, although the European Storm petrel (Hydrobates pelagicus) frequently does so (D’Elbee and Hemery 1998; Poot 2008).
Although crustaceans are known to be an important group in White-faced Storm Petrel diet (Croxall et al. 1997; Spear et al. 2007; Waap 2015), we only recorded them in the adult regurgitate sample. None were recorded in the chick faecal samples. The efficiency of the crustacean DNA amplification in the faecal samples might have been reduced because of the lower yield of DNA extracted. Another hypothesis is that crustaceans were not detected, owing to a high degradation of their DNA which prevented PCR amplification. Due to longer gut retention, high assimilation, and digestion efficiency, samples derived from chick faeces contain more degraded DNA sequences, and hence, less identifiable DNA sequences than those obtained from adult regurgitates (e.g., Wilson et al. 1989; Hilton et al. 2000). The same PCR protocol applied to the regurgitate sample returned several crustacean species, which is consistent with the macroscopic observation of regurgitated tissues, containing about a dozen of small sized Euphausiidae (< 1 cm) partially digested.
The absence of crustaceans in the chick faeces may also result from parents selecting higher quality food for their offspring (Wanless et al. 2005). In Newfoundland and Nova Scotia, Leach’s storm petrels rely heavily on mesopelagic fish while raising chicks (Hedd and Montevecchi 2006; Hedd et al. 2018), as these are energy-rich fish (Lea et al. 2002; Hedd and Montevecchi 2006) but also smaller proportions of euphausiid and hyperiid crustaceans of lower energy content (Hedd and Montevecchi 2006). Conversely, in winter, their diet likely consisted of a significant proportion of crustaceans (Hedd and Montevecchi 2006). Wilson’s Storm Petrel Oceanites oceanicus also adjust their diet for more energetic prey during the chick rearing period, increasing the amount of myctophid fish and decreasing of krill (Quillfeldt 2002; Gladbach et al. 2007).
Seabirds that are more specialized in mesopelagic prey, such as several species of Oceanodroma, Fregetta, Pterodroma, and Bulweria, tend to forage more frequently in offshore/oceanic waters and are markedly nocturnal (Warham 1990; Brooke and Prince 1991; Spear et al. 2007). Mesopelagic fish (e.g., Myctophidae, Photichthyidae, and Sternoptychidae) and cephalopods display diel vertical migrations that make them available to shallow divers at night (Gjøsaeter and Kawaguchi 1980; Watanabe et al. 1999). These groups were frequent in the diet of White-faced Storm Petrel, which fits well with the observed high activity during the night. Our results showed that White-faced Storm Petrels seem to behave differently during the incubation period than during the chick rearing period. Due to a higher travel speed during the night in the incubation period, we hypothesized that White-faced Storm Petrel may be pattering less, and therefore, it may not be feeding as much at night as during the chick rearing period. It is possible that during the chick rearing as this is a more energetically demanding time, birds tend to search for prey both day and night, presenting similar travel speed during day and night at this breeding period. This fits also with the finding that the birds consumed both mesopelagic and epipelagic prey during the chick rearing period. This is also in line with their oceanic distribution while foraging, since mesopelagic fish are scarce or absent in the continental shelf and other shallower areas (Gjøsaeter and Kawaguchi 1980; Nybakken 2001; Pusch et al. 2004).
The chicks of White-faced Storm Petrel showed no significant differences in mercury concentration in body feathers compared to the chicks of Bulwer’s Petrel, a specialist predator of mesopelagic prey (Waap et al. 2017) from the Deserta Grande, Madeira (275 km north of our study site), that showed a mercury concentration in body feathers of 4.38 ± 1.69 mg kg−1 dw in 2018 (mean ± SE, Furtado et al. 2021) (Welch’s t test, t = − 1.491, df = 0.28, P = 0.148). Seabirds feeding on mesopelagic prey present higher mercury concentrations in feathers than those feeding predominantly on epipelagic prey (Monteiro and Furness 1995; Kim et al. 1996; Monteiro et al. 1996; Bond and Diamond 2009; Carravieri et al. 2018; Furtado et al. 2019, 2021). Hence, mercury measurements in feathers support the idea that White-faced Storm Petrels raise their chicks mostly on mesopelagic prey. In addition, deep water pelagic fishes are known to accumulate higher mercury concentrations than nearshore species (Monteiro et al. 1996; Burger and Gochfeld 2000).
Currently, there are insufficient data to evaluate the cumulative effects of mercury for many seabird populations. A recent study by Pollet et al. (2017) found that levels of mercury in blood of adult Leach’s storm petrels (0.78 ± 0.43 mg kg−1 wet weight) were relatively high (compared to other species of seabirds from the same region), and yet did not appear to adversely affect their offspring development or the return rates of adults from previous years. It is also expected that the mercury concentration in feathers found in our study (corresponding to 0.43 ± 0.24 mg kg−1 wet weight in blood, following Ackerman et al. 2016) will not cause negative effects in chicks.
Smaller seabird species, such as White-faced Storm Petrels, can reflect changes that occur at lower trophic levels and thus be potential bioindicators of marine conditions and therefore sentinels to environmental changes which respond at a faster speed compared to larger seabirds (Grémillet and Charmantier 2010). This study presents the first baseline information on the foraging ecology of this species, and one of the still few studies that document the foraging strategies of storm petrels, which broadens our view on the range of behaviors displayed by pelagic seabirds.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the authors on reasonable request.
References
Ackerman J, Eagles-Smith C, Herzog M, Hartman A, Peterson S, Evers D, Jackson A, Elliott John S, Bryan C (2016) Avian mercury exposure and toxicological risk across western North America: a synthesis. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2016.03.071
Agostinelli C, Lund U (2017) R package ‘circular’: circular statistics (version 0.4-93). https://r-forge.r-project.org/projects/circular/. Accessed 10 May 2022
Alonso H, Granadeiro JP, Paiva VH, Dias AS, Ramos JA, Catry P (2012) Parent-offspring dietary segregation of Cory’s shearwaters breeding in contrasting environments. Mar Biol 159:1197–1207. https://doi.org/10.1007/s00227-012-1900-2
Barton ED, Arístegui J, Tett P, Cantón M, García-Braun J, Hernández-León S, Nykjaer L, Almeida C, Almunia J, Ballesteros S, Basterretxea G, Escánez J, García-Weil L, Hernández-Guerra A, López-Laatzen F, Molina R, Montero MF, Navarro-Pérez E, Rodríguez JM, van Lenning K, Vélez H, Wild K (1998) The transition zone of the Canary Current upwelling region. Prog Oceanogr 41:455–504
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
BirdLife (2020) Birdlife International seabird tracking database | Tracking Ocean Wanderers. http://www.seabirdtracking.org. Accessed 20 Nov 2021
Bivand R, Lewin-Koh N (2020) maptools: Tools for handling spatial objects. R package version 1.0-2. https://CRAN.R-project.org/package=maptools. Accessed 15 Jan 2022
Bokulich NA, Kaehler BD, Rideout JR, Dillon M, Bolyen E, Knight R, Huttley GA, Gregory Caporaso J (2018) Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 6:90. https://doi.org/10.1186/s40168-018-0470-z
Bolton M (2021) GPS tracking reveals highly consistent use of restricted foraging areas by European Storm-petrels Hydrobates pelagicus breeding at the largest UK colony: implications for conservation management. Bird Conserv Int 31:35–52. https://doi.org/10.1017/S0959270920000374
Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodríguez AM, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang L, Kaehler BD, Kang KB, Keefe CR, Keim P, Kelley ST, Knights D, Koester I, Kosciolek T, Kreps J, Langille MGI, Lee J, Ley R, Liu YX, Loftfield E, Lozupone C, Maher M, Marotz C, Martin BD, McDonald D, McIver LJ, Melnik AV, Metcalf JL, Morgan SC, Morton JT, Naimey AT, Navas-Molina JA, Nothias LF, Orchanian SB, Pearson T, Peoples SL, Petras D, Preuss ML, Pruesse E, Rasmussen LB, Rivers A, Robeson MS 2nd, Rosenthal P, Segata N, Shaffer M, Shiffer A, Sinha R, Song SJ, Spear JR, Swafford AD, Thompson LR, Torres PJ, Trinh P, Tripathi A, Turnbaugh PJ, Ul-Hasan S, van der Hooft JJJ, Vargas F, Vázquez-Baeza Y, Vogtmann E, von Hippel M, Walters W, Wan Y, Wang M, Warren J, Weber KC, Williamson CHD, Willis AD, Xu ZZ, Zaneveld JR, Zhang Y, Zhu Q, Knight R, Caporaso JG (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857. https://doi.org/10.1038/s41587-019-0209-9
Bond AL, Diamond AW (2009) Mercury concentrations in seabird tissues from Machias Seal Island, New Brunswick, Canada. Sci Total Environ 407(14):4340–4347. https://doi.org/10.1016/j.scitotenv.2009.04.018
Brooke ML, Prince PA (1991) Nocturnality in seabirds. In: Bell BD, Cossee RO, Flux JEC, Heather BD, Hitchmough RA, Robertson CJR, Williams MJ (eds) Acta XX Congressus Internationalis Ornithologici. New Zealand Ornithological Congress, Wellington, pp 1113−1121
Brooke ML (2004a) Albatrosses and petrels across the world. Oxford University Press, New York
Brooke ML (2004b) The food consumption of the world’s seabirds. Proc R Soc Lond 271(Suppl):246–248
Buglione M, Maselli V, Rippa D, de Filippo G, Trapanese M, Fulgione D (2018) A pilot study on the application of DNA metabarcoding for non-invasive diet analysis in the Italian hare. Mamm Biol 88:31–42. https://doi.org/10.1016/j.mambio.2017.10.010
Burger J, Gochfeld M (2000) Metal levels in feathers of 12 species of seabirds from Midway Atoll in the northern Pacific Ocean. Sci Total Environ 257(1):37–52. https://doi.org/10.1016/S0048-9697(00)00496-4
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP (2016) Dada2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13(7):581. https://doi.org/10.1038/nmeth.3869
Calenge C (2006) The package adehabitat for the R software: a tool for the analysis of space and habitat use by animals. Ecol Model 197:516–525. https://doi.org/10.1016/j.ecolmodel.2006.03.017
Camphuysen KCJ, Shamoun-Baranes J, Bouten W, Garthe S (2012) Identifying ecologically important marine areas for seabirds using behavioural information in combination with distribution patterns. Biol Conserv 156:22–29. https://doi.org/10.1016/j.biocon.2011.12.024
Campos AR, Granadeiro JP (1999) Breeding biology of the White-faced Storm-Petrel on Selvagem Grande Island, north-east Atlantic. Waterbirds Int J Waterbird Biol 22:199–206
Carravieri A, Fort J, Tarroux A, Cherel Y, Love OP, Prieur S, Brault-Favrou M, Bustamante P, Descamps S (2018) Mercury exposure and short-term consequences on physiology and reproduction in Antarctic petrels. Environ Pollut. https://doi.org/10.1016/j.envpol.2017.11.004
Carreiro AR, Paiva VH, Medeiros R, Franklin KA, Oliveira N, Fagundes AI, Ramos JA (2020) Metabarcoding, stables isotopes, and tracking: unraveling the trophic ecology of a winter-breeding storm petrel (Hydrobates castro) with a multimethod approach. Mar Biol 167:1–13
Cecere JG, Catoni C, Maggini I, Imperio S, Gaibani G (2013) Movement patterns and habitat use during incubation and chick-rearing of Cory’s shearwaters (Calonectris diomedea diomedea) (Aves: Vertebrata) from Central Mediterranean: influence of seascape and breeding stage. Ital J Zool 80:82–89. https://doi.org/10.1080/11250003.2012.710654
Choy C, Popp B, Kaneko J, Drazen J (2009) The influence of depth of mercury levels in pelagic fishes and their prey. Proc Natl Acad Sci 106:13865–13869
Clarke MR (1986) A handbook for the identification of cephalopod beaks. Clarendon Press, Oxford
Collins S, Hedd A, Fifield D, Wilson D, Wi M (2022) Foraging paths of breeding Leach’s Storm-Petrels in relation to offshore oil platforms, breeding stage, and year. Front Mar Sci. https://doi.org/10.3389/fmars.2022.816659
Costley CT, Mossop KF, Dean JR, Garden LM, Marshall J, Carroll J (2000) Determination of mercury in environmental and biological samples using pyrolysis atomic absorption spectrometry with gold amalgamation. Anal Chim Acta 405:179–183. https://doi.org/10.1016/S0003-2670(99)00742-4
Coulson JC (2002) Colonial breeding in seabirds. In: Schreiber EA, Burger J (ed) Biology of Marine Birds CRS Press, Boca Raton, FL, pp 87–113
Cramp S, Simmons KEL (1997) The birds of the western Palearctic, vol I. Oxford University Press, Oxford
Cropper TE, Hanna E, Bigg GR (2014) Spatial and temporal seasonal trends in coastal upwelling off Northwest Africa 1981–2012. Deep-Sea Res I: Oceanogr Res Pap 86:94–111. https://doi.org/10.1016/j.dsr.2014.01.007
Croxall JP, Prince PA, Reid K (1997) Dietary segregation of krill-eating South Georgia seabirds. J Zool (Lond) 242:531–555
De Barba M, Miquel C, Boyer F, Mercier C, Rioux D, Coissac E, Taberlet P (2014) DNA metabarcoding multiplexing and validation of data accuracy for diet assessment: application to omnivorous diet. Mol Ecol Resour 14:306–323. https://doi.org/10.1111/1755-0998.12188
Del Hoyo J, Elliott A, Sargatal J (1992) Handbook of the birds of the world. Vol. 1. Ostrich to ducks, vol 1. Lynx Edicions, Barcelona
D’Elbee J, Hemery G (1998) Diet and foraging behaviour of the British Storm Petrel Hydrobates pelagicus in the Bay of Biscay during summer. Ardea 86:1–10
Depot KM, Scopel LC, Kress SW, Shannon P, Diamond AW, Elliott KH (2020) Atlantic puffin diet reflects haddock and redfish abundance in the Gulf of Maine. Mar Ecol Prog Ser. https://doi.org/10.3354/meps13537
Dias MP, Romero J, Granadeiro JP, Catry T, Pollet IL, Catry P (2016) Distribution and at-sea activity of a nocturnal seabird, the Bulwer’s petrel Bulweria bulwerii, during the incubation period. Deep Sea Res I Oceanogr Res Pap 113:49–56. https://doi.org/10.1016/j.dsr.2016.03.006
Erickson J (1955) Flight behavior of the Procellariiformes. Auk 72:415–420. https://doi.org/10.2307/4081455
Fauchald P (2009) Spatial interaction between seabirds and prey: review and synthesis. Mar Ecol Prog Ser 391:139–151
Fauchald P, Erikstad KE, Skarsfjord H (2000) Scale-dependent predator-prey interactions: the hierarchical spatial distribution of seabirds and prey. Ecology 81:773–783. https://doi.org/10.1890/0012-9658(2000)081[0773:SDPPIT]2.0.CO;2
Furness RW (1978) Energy requirements of seabird communities: a bio-energetic model. J Anim Ecol 47:39–53
Furtado R, Menezes D, Santos CJ, Catry P (2016) White-faced storm-petrels Pelagodroma marina predated by gulls as biological monitors of plastic pollution in the pelagic subtropical Northeast Atlantic. Mar Pollut Bull 112:117–122. https://doi.org/10.1016/j.marpolbul.2016.08.031
Furtado R, Pereira ME, Granadeiro JP, Catry P (2019) Body feather mercury and arsenic concentrations in five species of seabirds from the Falkland Islands. Mar Pollut Bull. https://doi.org/10.1016/j.marpolbul.2019.110574
Furtado R, Granadeiro JP, Gatt MC, Rounds R, Horikoshi K, Paiva VH, Menezes D, Pereira E, Catry P (2021) Monitoring of mercury in the mesopelagic domain of the Pacific and Atlantic oceans using body feathers of Bulwer’s petrel as a bioindicator. Sci Total Environt 775:145796. https://doi.org/10.1016/j.scitotenv.2021.1457
Gjøsaeter J, Kawaguchi K (1980) A review of the world resources of mesopelagic fish. FAO Fisheries Technical Paper No. 193, Rome, Food and Agriculture Organization of the United Nations
Gladbach A, McGill RAR, Quillfeldt P (2007) Foraging areas of Wilson’s storm-petrel Oceanites oceanicus in the breeding and inter-breeding period determined by stable isotope analysis. Polar Biol 30:1005–1012. https://doi.org/10.1007/s00300-007-0258-2
Grémillet D, Charmantier A (2010) Shifts in phenotypic plasticity constrain the value of seabirds as ecological indicators of marine ecosystems. Ecol Appl 20(6):1498–1503. https://doi.org/10.1890/09-1586.1
Guilford TC, Meade J, Freeman R, Biro D, Evans T, Bonadonna F, Boyle D, Roberts S, Perrins CM (2008) GPS tracking of the foraging movements of Manx Shearwaters Puffinus puffinus breeding on Skomer Island, Wales. Ibis 150:462–473. https://doi.org/10.1111/j.1474-919X.2008.00805.x
Halpin LR, Pollet IL, Lee C, Morgan KH, Carter HR (2018) Year-round movements of sympatric Fork-tailed (Oceanodroma furcata) and Leach’s (O. leucorhoa) storm-petrels. J Field Ornithol 89:207–220. https://doi.org/10.1111/jofo.12255)
Hedd A, Montevecchi WA (2006) Diet and trophic position of Leach’s storm-petrel Oceanodroma leucorhoa during breeding and moult, inferred from stable isotope analysis of feathers. Mar Ecol Prog Ser 322:291–301. https://doi.org/10.3354/meps322291
Hedd A, Montevecchi WA, Davoren GK, Fifield DA (2009) Diets and distributions of Leach’s storm-petrel (Oceanodroma leucorhoa) before and after an ecosystem shift in the Northwest Atlantic. Can J Zool 87:787–801. https://doi.org/10.1139/Z09-060
Hedd A, Pollet IL, Mauck RA, Burke CM, Mallory ML, McFarlane Tranquilla LA, Montevecchi WA, Robertson GJ, Ronconi RA, Shutler D, Wilhelm SI, Burgess NM (2018) Foraging areas, offshore habitat use, and colony overlap by incubating leach’s storm-petrels Oceanodroma leucorhoa in the northwest Atlantic. PLoS One 13:1–18. https://doi.org/10.1371/journal.pone.0194389
Hennicke JC, Weimerskirch H (2014) Coping with variable and oligotrophic tropical waters: foraging behaviour and flexibility of the Abbott’s booby Papasula abbotti. Mar Ecol Prog Ser 499:259–273. https://doi.org/10.3354/meps10664
Hilton GM, Ruxton GD, Furness RW, Houston DC (2000) Optimal digestion strategies in seabirds: a modelling approach. Evol Ecol Res 2:207–230
Hunt GL Jr, Schneider DC (1987) Scale-dependent processes in the physical and biological environment of marine birds. In: Croxall JP (ed) Seabirds: feeding biology and role in marine ecosystems. Cambridge University Press, Cambridge, pp 7–41
Hunt GL, Mehlum F, Russell RW, Irons D, Decker MB, Becker PH (1999) Physical processes, prey abundance, and the foraging ecology of seabirds. Proc Int Ornithol Congr 22:2040–2056
Imber MJ (1984) Migration of White-faced Storm-Petrels (Pelagodroma marina) in the South Pacific and the status of the Kermadec subspecies. Emu 84:32–35
Kim EY, Murakami T, Saeki K, Tatsukawa R (1996) Mercury levels and its chemical form in tissues and organs of seabirds. Arch Environ Contam Toxicol 30:259–266. https://doi.org/10.1007/s002449900035
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: tests in linear mixed effects models. J Stat Softw 82:1–26. https://doi.org/10.18637/jss.v082.i13
Lea MA, Nichols PD, Wilson G (2002) Fatty acid composition of lipid-rich myctophids and mackerel ice fish (Champsocephalus gunnari)—Southern Ocean food-web implications. Polar Biol 25:843–854
Machida RJ, Leray M, Ho SL, Knowlton N (2017) Metazoan mitochondrial gene sequence reference datasets for taxonomic assignment of environmental samples. Sci Data 4:170027. https://doi.org/10.1038/sdata.2017.27
Machu E, Ettahiri O, Kifani S, Benazzouz A, Makaoui A, Demarcq H (2009) Environmental control of the recruitment of sardines (Sardina pilchardus) over the western Saharan shelf between 1995 and 2002: a coupled physical-biogeochemical modelling experiment. Fish Oceanogr 18:287–300. https://doi.org/10.1111/j.1365-2419.2009.00511.x
Marchant S, Higgins PJ (1990) Handbook of Australian, New Zealand, and Antarctic birds. Oxford University Press, Melbourne
Menkhorst PW, Pescott TW, Gaynor GF (1984) Results of banding White-faced storm-petrels, Pelagodroma marina at Mud Islands, Victoria. Corella 8:53–56
Monteiro LR, Furness RW (1995) Seabirds as monitors of mercury in the marine environment. Water Air Soil Pollut 80:851–870
Monteiro LR, Furness RW (2001) Kinetics, dose-response, and excretion of methylmercury in free-living adult Cory’s shearwaters. Environ Sci Technol 35:739–746. https://doi.org/10.1021/es000114a
Monteiro LR, Ramos JA, Furness RW, Del Nevo AJ (1996) Movements, morphology, breeding, molt, diet and feeding of seabirds in the Azores. Waterbirds 19:82–97. https://doi.org/10.2307/1521810
Mott R, Herrod A, Clarke RH (2016) Resource partitioning between species and sexes in Great Frigatebirds and Lesser Frigatebirds. Auk 134:153–167
Nybakken JW (2001) Marine biology: an ecological approach, 5th edn. Benjamin Cummings, San Francisco
Oppel S, Beard A, Fox D, Mackley E, Leat E, Henry L, Clingham E, Fowler N, Sim J, Sommerfeld J, Weber N, Weber S, Bolton M (2015) Foraging distribution of a tropical seabird supports Ashmole’s hypothesis of population regulation. Behav Ecol Sociobiol 69:915–926
Oppel S, Bolton M, Carneiro APB, Dias MP, Green JA, Masello JF, Phillips RA, Owen E, Quillfeldt P, Beard A, Bertrand S, Blackburn J, Boersma PD, Borges A, Broderick AC, Catry P, Cleasby I, Clingham E, Creuwels J, Crofts S, Cuthbert RJ, Dallmeijer H, Davies D, Davies R, Dilley BJ, Dinis HA, Dossa J, Dunn MJ, Efe MA, Fayet AL, Figueiredo L, Frederico AP, Gjerdrum C, Godley BJ, Granadeiro JP, Guilford T, Hamer KC, Hazin C, Hedd A, Henry L, Hernández-Montero M, Hinke J, Kokubun N, Leat E, Tranquilla LM, Metzger B, Militão T, Montrond G, Mullié W, Padget O, Pearmain EJ, Pollet IL, Pütz K, Quintana F, Ratcliffe N, Ronconi RA, Ryan PG, Saldanha S, Shoji A, Sim J, Small C, Soanes L, Takahashi A, Trathan P, Trivelpiece W, Veen J, Wakefield E, Weber N, Weber S, Zango L, Daunt F, Ito M, Harris MP, Newell MA, Wanless S, González-Solís J, Croxall J (2018) Spatial scales of marine conservation management for breeding seabirds. Mar Policy 98:37–46. https://doi.org/10.1016/j.marpol.2018.08.024
Oro D (2014) Seabirds and climate: knowledge, pitfalls and opportunities. Front Ecol Evol 2:79. https://doi.org/10.3389/fevo.2014.00079
Paiva VH, Geraldes P, Ramírez I, Meirinho A, Garthe S, Ramos JA (2010) Oceanographic characteristics of areas used by Cory’s shearwaters during short and long foraging trips in the North Atlantic. Mar Biol 157:1385–1399
Pennycuick CJ (1982) The flight of petrels and albatrosses (Procellariiformes), observed in South Georgia and its vicinity. Philos Trans R Soc Lond B 300:75–106. https://doi.org/10.1098/rstb.1982.0158
Phillips RA, Xavier JC, Croxall J (2003) Effects of satellite transmitters on albatrosses and petrels. Auk 120(4):1082–1090. https://doi.org/10.1093/auk/120.4.1082
Poot M (2008) Nocturnal and diurnal foraging of European Storm petrels Hydrobates sp. along the Lisbon coast, Portugal. Airo 18:13–21
Pollet IL, Ronconi RA, Jonsen ID, Leonard ML, Taylor PD, Shutler D (2014) Foraging movements of Leach’s storm-petrels Oceanodroma leucorhoa during incubation. J Avian Biol 45:305–314. https://doi.org/10.1111/jav.00361
Pollet IL, Leonard ML, O’Driscoll NJ, Burgess NM, Shutler D (2017) Relationships between blood mercury levels, reproduction, and return rate in a small seabird. Ecotoxicology 26:97–103. https://doi.org/10.1007/s10646-016-1745-4
Pusch C, Beckmann A, Porteiro FM, Westernhagen H (2004) The influence of seamounts on mesopelagic fish communities. Arch Fish Mar Res 51:165–186
Quillfeldt P (2002) Seasonal and annual variation in the diet of breeding and non-breeding Wilson’s storm-petrels on King George Island, South Shetland Islands. Polar Biol 25:216–221
Ramos R, Granadeiro JP, Rodríguez B, Navarro J, Paiva VH, Bécares J, Reyes-González JM, Fagundes I, Ruiz A, Arcos P, González-Solís J, Catry P (2013) Meta-population feeding grounds of Cory’s shearwater in the subtropical Atlantic Ocean: implications for the definition of Marine Protected Areas based on tracking studies. Divers Distrib 19:1284–1288. https://doi.org/10.1111/ddi.12088
Ramos R, Ramírez I, Paiva VH, Militão T, Biscoito M, Menezes D, Phillips RA, Zino F, González-Solís J (2016) Global spatial ecology of three closely-related gadfly petrels. Sci Rep 6:1–11
Rankin MN, Duffey EA (1948) A study of the bird life of the North Atlantic. Br Birds 41:1–42
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 13 Jan 2022
Richdale LE (1943–1944) The White-faced Storm-petrel or Takahi-kare-moana (Pelagodroma marina maoriana, Mathews). Trans Proc R Soc N Z 73:97–115, 217–232, 335–350
Rodríguez B, De León L, Martín A, Alonso J, Nogales M (2003) Status and distribution of breeding seabirds in the northern islets of Lanzarote (Canary Islands). Atl Seab 5:41–56
Rodríguez B, Bécares J, Martínez JM, Rodríguez A, Ruiz A, Arcos JM (2013) Satellite tracking of Bulwer’s Petrels Bulweria bulwerii in the Canary Islands. Bird Study 60:270–274. https://doi.org/10.1080/00063657.2013.778226
Rodríguez A, Arcos JM, Bretagnolle V, Dias MP, Holmes ND, Louzao M, Provencher J, Raine AF, Ramírez F, Rodríguez B, Ronconi RA, Taylor RS, Bonnaud E, Borrelle SB, Cortés V, Descamps S, Friesen VL, Genovart M, Hedd A, Hodum P, Humphries GRW, Le Corre M, Lebarbenchon C, Martin R, Melvin EF, Montevecchi WA, Pinet P, Pollet IL, Ramos R, Russell JC, Ryan PG, Sanz-Aguilar A, Spatz DR, Travers M, Votier SC, Wanless RM, Woehler E, Chiaradia A (2019) Future directions in conservation research on petrels and shearwaters. Front Mar Sci. https://doi.org/10.3389/fmars.2019.00094
Rognes T, Flouri T, Nichols B, Quince C, Mahé F (2016) Vsearch: a versatile open-source tool for metagenomics. PeerJ 4:2584. https://doi.org/10.7717/peerj.2584
Romero J, Catry P, Alonso H, Granadeiro JP (2021) Seabird diet analysis suggests sudden shift in the pelagic communities of the subtropical Northeast Atlantic. Mar Environ Res 165:105232. https://doi.org/10.1016/j.marenvres.2020.105232
Rotger A, Sola A, Tavecchia G, SanzAguilar A (2020) Foraging far from home: Gps-tracking of Mediterranean Storm-Petrels Hydrobates pelagicus melitensis reveals long-distance foraging movements. Ardeola 68:3–16. https://doi.org/10.13157/arla.68.1.2021.ra1
Sausner J, Torres-Mura J, Robertson J, Hertel F (2016) Ecomorphological differences in foraging and pattering behavior among storm-petrels in the eastern Pacific Ocean. Auk 133:397–414. https://doi.org/10.1642/AUK-15-158.1
Silva MC, Matias R, Wanless RM, Ryan PG, Stephenson BM, Bolton M, Ferrand N, Coelho MM (2015) Understanding the mechanisms of antitropical divergence in the seabird White-faced Storm-petrel (Procellariiformes: Pelagodroma marina) using a multilocus approach. Mol Ecol 24:3122–3137. https://doi.org/10.1111/mec.13212
Sousa L, Silva S, Xavier R (2019) DNA metabarcoding in diet studies: unveiling ecological aspects in aquatic and terrestrial ecosystems. Environ DNA 1(3):199–214. https://doi.org/10.1002/edn3.27
Spear LB, Ainley DG, Walker WA (2007) Foraging dynamics of seabirds in the eastern tropical Pacific Ocean. Stud Avian Biol 35:1–99
Stewart FM, Phillips RA, Catry P, Furness RW (1997) Influence of species, age and diet on mercury concentrations in Shetland seabirds. Mar Ecol Prog Ser 151:237–244
Symondson WOC (2002) Molecular identification of prey in predator diets. Mol Ecol 11:627–641
Taberlet P, Coissac E, Hajibabaei M, Rieseberg LH (2012) Environmental DNA. Mol Ecol 21:1789–1793
Underwood M, Bunce A (2004) The breeding biology of the White-faced Storm Petrel (Pelagodroma marina) on Mud Islands, Port Phillip Bay, Victoria. Emu Austral Ornithol 104:213–220
Valentini A, Miquel C, Nawaz MA, Bellemain E, Coissac E, Pompanon F, Gielly L, Cruaud C, Nascetti G, Wincker SJE, Taberlet P (2009) New perspectives in diet analysis based on DNA barcoding and parallel pyrosequencing: the trnL approach. Mol Ecol Res 9:51–60
Ventura F, Granadeiro JP, Padget O, Catry P (2020) Gadfly petrels use knowledge of the windscape, not memorized foraging patches, to optimize foraging trips on ocean-wide scales. Proc R Soc B 287:20191775. https://doi.org/10.1098/rspb.2019.1775
Waap S (2015) Trophic relationships among pelagic predators of the deep seas of the Madeira islands. Doctoral Dissertation. Cardiff University
Waap S, Symondson WOC, Granadeiro JP, Alonso H, Serra-Gonçalves C, Dias MP, Catry P (2017) The diet of a nocturnal pelagic predator, the Bulwer’s petrel, across the lunar cycle. Sci Rep. https://doi.org/10.1038/s41598-017-01312-3
Wanless S, Harris MP, Redman P, Speakman JR (2005) Low energy values of fish as a probable cause of a major seabird bird breeding failure in the North Sea. Mar Ecol Prog Ser 294:1–8
Warham J (1990) The petrels: their ecology and breeding systems. Academic Press, London, p 440
Watanabe H, Moku M, Kawaguchi K, Ishimaru K, Ohno A (1999) Diel vertical migration of myctophid fishes (family Myctophidae) in the transitional waters of the western North Pacific. Fish Oceanogr 8:115–127
Watson GE, Lee DS, Backus ES (1986) Status and subspecific identity of White-faced Storm-petrels in the western North Atlantic Ocean. Anon Am Birds 40(3):401–408
Weimerskirch H (2007) Are seabirds foraging for unpredictable resources? Deep Sea Res II Top Stud Oceanogr 54:211–223
Wilson RP, Ryan PG, Wilson MP (1989) Sharing food in the stomachs of seabirds between adults and chicks: a case for delayed gastric emptying. Comp Biochem Physiol 94(3):461–466. https://doi.org/10.1016/0300-9629(89)90121-7
Yoda K (2019) Advances in bio-logging techniques and their application to study navigation in wild seabirds. Adv Robot 33:108–117. https://doi.org/10.1080/01691864.2018.1553686
Zonfrillo B (1985) Diet of Bulwer’s Petrel Bulweria bulwerii in the Madeiran Archipelago. Ibis 128(4):570–572. https://doi.org/10.1111/j.1474-919X.1986.tb02708.x
Acknowledgements
This study was financial supported by Foundation for Science and Technology (FCT; Portugal) through projects granted to CESAM (UIDP/50017/2020 + UIDB/50017/2020 + LA/P/0094/2020), MARE (UIDB/04292/2020 and UIDP/04292/2020), cE3c (UIDB/BIA/00329/2020), by the project PTDC/BIA-EVL/28565/2017 and to a doctoral grant SFRH/BD/133561/2017 awarded to MA. MCS is funded by FCT through a contract foreseen in DL 57/2016, changed by Law 57/2017. We would like to acknowledge Instituto das Florestas e Conservação da Natureza, especially Dília Menezes, Carolina Santos, and Paulo Oliveira, for providing permissions to carry out the work in Selvagens Nature Reserve and for logistical support. We would like to thank Filipe Moniz, Daniel Lopes, Maria Dias, Edna Correia, Marie Claire, and Francesco Ventura for helping during field work and to park-wardens of the Nature Reserve during our stays in the island. We are also grateful to Ricardo Furtado for his contribution in the laboratory analysis of mercury in the feathers of White-faced Storm Petrel chicks and to Eduarda Pereira (Department of Chemistry and CESAM/REQUIMTE, University of Aveiro) for providing the laboratory and equipment necessary for the analysis.
Funding
This work was financial supported by Foundation for Science and Technology (FCT; Portugal) through projects granted to CESAM (UIDP/50017/2020 + UIDB/50017/2020 + LA/P/0094/2020), MARE (UIDB/04292/2020 and UIDP/04292/2020), cE3c (UIDB/BIA/00329/2020), by the project PTDC/BIA-EVL/28565/2017 and to a doctoral grant SFRH/ BD/133561/2017 awarded to MA. MCS is funded by FCT through a contract foreseen in DL 57/2016, changed by Law 57/2017.
Author information
Authors and Affiliations
Contributions
All authors contributed to study conception and design. Fieldwork was performed by MA. DNA metabarcoding analysis performed by MCS and VLN. JPG and MA analysed spatial data. MA wrote the initial manuscript. All the authors commented on previous versions of the manuscript, and they read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interests.
Ethics approval
The work reported in this paper comply with the standards and procedures laid down by national legislation.
Additional information
Responsible Editor: T. A. Clay.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alho, M., Catry, P., Silva, M.C. et al. Revealing the foraging movements and diet of the White-faced Storm Petrel Pelagodroma marina in the NE Atlantic. Mar Biol 169, 91 (2022). https://doi.org/10.1007/s00227-022-04078-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-022-04078-z