Abstract
Sharks are a highly diverse predatory taxon and are regularly found in large, potentially competitive, assemblages. However, the mechanisms that enable long-term coexistence and factors that drive complementary movement are poorly understood. As interspecific interactions can have a large influence on survival and trophic linkages, research on shark assemblages could substantially increase our understanding of marine community dynamics. In this study, we used passive acoustic telemetry to compare the activity space size, spatial overlap and habitat use patterns of six co-occurring shark species from the same family in a tropical nearshore embayment. Our results indicated that all sizes of Rhizoprionodon taylori (a small-bodied, highly productive species) used significantly larger amounts of space (e.g., mean 95% KUD = 85.9 km2) than juveniles of large-bodied, less productive species (e.g., Carcharhinus amboinensis; 62.3 km2) that use nearshore areas as nursery areas. Most large, less productive species appeared risk averse by using less space, while the smaller more productive species took greater risk by roaming broadly. These movement strategies are likely a means of avoiding predation or gaining access to new or additional resources. Spatial overlap patterns varied substantially between species with overlap in core use areas ranging from 1.2 to 27.6%, but were consistent over time. Most species exhibited low spatial overlap, suggesting spatial partitioning to reduce interspecific competition. While a few species exhibited a high degree of spatial overlap (up to 60% of activity space extent), dietary diversity may reduce competition to support co-occurrence. These data suggest that complex interactions occur in communal nurseries in nearshore waters where species are in direct competition for resources at vulnerable life stages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coastal habitats are both dynamic and highly productive (Allen et al. 2006; Tobin et al. 2014). These habitats are used by a wide array of marine and estuarine species and serve a variety of ecosystem functions (Barbier et al. 2011). For example, coastal habitats serve as nursery areas for a number of species and critical habitat for many others (Beck et al. 2001; Munsch et al. 2016; Heupel et al. 2018a). The reliance of a large number of species on these habitats creates communities that must not only coexist, but survive and thrive in these shared habitats. Although the coexistence of coastal species is widely known, our understanding of niche partitioning and resource use in facilitating coexistence is limited.
Resource partitioning may be a critical component of survival. Limited resources or high levels of competition can have direct impact on the survival of individuals and populations and may require species to employ differing behaviours to reduce niche overlap (White and Potter 2004; Matley et al. 2016a,b; Matich et al. 2017; Heupel et al. 2018b). For example, recent studies have revealed sympatric species adopt differing diets or movement patterns to reduce competition (Lédée et al. 2016; Matley et al. 2016a, b). Interestingly, different strategies were applied among the species observed. For example, two coral trout species that co-occur on offshore reefs (Plectropomus leopardus, P. laevis) showed overlapping space use but different diets (Matley et al. 2016a). In contrast, two coral trout species that co-occur on inshore reefs (P. leopardus, P. maculatus) feed on the same prey items, but segregate spatially in the water column (Matley et al. 2016b). Although employing different strategies, the behaviours of these species work to reduce competition, facilitate coexistence and presumably improve survival. Similar patterns of spatial separation have also been observed in skate (Humphries et al. 2016) and reef shark (Heupel et al. 2018b) populations with species with similar diets segregating by depth.
Animal movement and behaviour patterns are also driven by aspects of their environment (e.g., Schlaff et al. 2014). Species are constrained by physiological limits governed by salinity, temperature or habitat type (e.g., seagrass beds, coral reef). Therefore, the use of habitats and interactions among species are further complicated by the biological limitations and requirements of a given species. As in the examples above for coral trout and reef sharks, species that depend on specific habitat types may need to employ behaviour patterns to reduce competition or interactions, since dispersal to new or different habitats is not feasible or optimal. The intersection of environmental and behavioural factors is a key component of the composition and functioning of coastal ecosystems.
Elasmobranchs (sharks and rays) are one of the planet’s most morphologically diverse and mobile predatory taxon (Carrier et al. 2012; Ebert et al. 2013). In the past, research on elasmobranch movement and behaviour has been primarily focused on the influence of factors such as foraging and changes in environmental conditions (Heupel and Hueter 2002; Abascal et al. 2011; Nakamura et al. 2011; Schlaff et al. 2014). However, juvenile elasmobranchs commonly co-occur in coastal habitats for long periods (Castro 1993; Simpfendorfer and Milward 1993; Dale et al. 2011; Bethea et al. 2015; Yates et al. 2015). Since interspecific competition is observed more often than not in most communities (Connell 1983; Schoener 1986), it can be assumed that co-occurring elasmobranchs potentially experience high levels of interspecific interaction and competition. However, the long-term coexistence of sharks in coastal habitats indicates that species have developed strategies to limit competition for resources (White and Potter 2004; Papastamatiou et al. 2006; Speed et al. 2011; Heithaus et al. 2013).
Coastal shark communities typically include individuals exhibiting one of two life history patterns (Knip et al. 2010): juveniles of large-bodied, slow-growing, late maturing species that use nearshore environments as nursery areas (e.g., Springer 1967; Heupel et al. 2007; Froeschke et al. 2010), and small-bodied, highly productive, fast-growing sharks, which use nearshore habitats throughout their lives (e.g., Carlson et al. 2008; Munroe et al. 2014). Thus, coastal habitats are used for distinct purposes by different shark species with contrasting life histories. These differences have been hypothesized to have a substantial effect on how sharks use space (Knip et al. 2010), but directed comparative movement studies are limited (Heupel et al. 2018b).
Methods for investigating and comparing how coastal species use space have traditionally used measures of activity space, such as kernel utilisation distributions or minimum convex polygons (Heupel et al. 2004). These types of metrics can be compared between and within species to examine spatial and temporal separation (Munroe et al. 2016), but do not provide information on how individuals move within their activity spaces. Recently, use of network analysis to examine animal movement and space use, especially those tracked using acoustic monitoring, has proved to be a useful analytical tool to resolve additional information about spatial ecology (Jacoby et al. 2012; Lédée et al. 2015; Mourier et al. 2018). Network analysis examines the movement (edges) of animals between locations (acoustic receiver stations, nodes) and provides a series of metrics to understand habitat use and partitioning, and movement pathways.
The aim of this study was to examine the space use, movement, and habitat use of sharks in a coastal bay to determine whether niche separation was present and identifiable based on behaviour patterns. Passive acoustic telemetry and network analysis were used to determine the spatial overlap, relative positioning, and space use of six coastal shark populations over time. Our hypothesis was that coexisting species use different areas, exhibit limited spatial overlap and/or use different movement pathways, and that spatial partitioning provides a mechanism to limit competition for resources. Differences in space use and movement strategies were examined relative to modern coastal shark habitat use theories (Heupel et al. 2007, Knip et al. 2010).
Methods
Study site
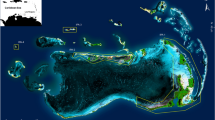
Cleveland Bay is a shallow embayment on the northeast coast of Queensland, Australia. The bay is 27 km wide and covers an area of approximately 225 km2 (Fig. 1). Most of the bay is less than 10 m deep, and has a maximum tidal range of 4.2 m. The bay contains a diverse range of habitat types and substrates. The western side of the bay contains rocky substrate composed of coral rubble and sand with patches of coral reef on the southern coast of Magnetic Island. The eastern side of the bay is predominantly intertidal mudflat, and in deeper waters the bottom is composed of soft mud and seagrass. Mangroves line the southeastern shore. This area is a well-known communal elasmobranch nursery that is home to approximately 25 shark species (Simpfendorfer and Milward 1993).
The acoustic telemetry array in Cleveland Bay, Queensland, Australia. Points indicate locations of acoustic receivers deployed in 2008 (circles), 2009 (squares), and 2011 (triangles). Reef habitat is marked with dashed lines. Colours represent habitat type: green = deep habitat, violet = mudflat, blue = reef flats, pink = sand and orange = seagrass beds
An array of up to 61 VR2 and VR2W acoustic receivers (Vemco Ltd., Canada) was installed in Cleveland Bay to monitor shark species movements. Receivers were deployed over a period of several years; 47 receivers were deployed in 2008, an additional 9 in 2009, and 5 more in 2011. Receivers were installed in primary habitats within the bay, specifically intertidal mudflats, areas with > 5 m depth, sandy inshore substrate, reefs, and seagrass. Data were downloaded from receivers approximately every 3 months.
Study species
Long-term acoustic tracking data were collected for six shark species that consistently co-occur in Cleveland Bay. Data collection occurred over a period of 7 years and not all species were tracked simultaneously (Table 1). Previous analysis of individual species included in this study showed that species tracked over multiple years exhibited similar presence and movement patterns between years (e.g., Knip et al. 2011, 2012). This inter-annual consistency indicated data from different species collected in different years were comparable and suitable for community-level overlap and space use analysis.
Australian sharpnose (Rhizoprionodon taylori) and spottail (Carcharhinus sorrah) sharks are fast-growing, highly productive species that spend their entire lives in shallow coastal habitats (Davenport and Stevens 1988; Simpfendorfer 1992, 1993). The creek whaler Carcharhinus fitzroyensis is a medium-bodied coastal shark that is also relatively productive and inhabits shallow coastal areas its entire life (Last and Stevens 2009). However, unlike C. sorrah and R. taylori, C. fitzroyensis primarily uses turbid, muddy, and seagrass habitat (Munroe et al. 2015). Pigeye (Carcharhinus amboinensis) and Australian blacktip (C. tilstoni) sharks are relatively slow-growing, large-bodied, late maturing species that primarily use nearshore areas as nursery habitat (Knip et al. 2011; Tillett et al. 2011; Harry et al. 2012). Finally, the blacktip reef shark C. melanopterus, is a specialized species that primarily uses reef habitats (Chin et al. 2013a, b). In addition to tracking data, diet and life history information is available for most of these species, much of which was gathered from Cleveland Bay (e.g., Simpfendorfer 1993; Simpfendorfer and Milward 1993; Simpfendorfer 1998; Harry et al. 2011; Kinney et al. 2011; Harry et al. 2012, 2013).
Field methods
Sharks were captured using bottom-set 400 m longlines, 11.45 cm mesh gillnets, and/or baited rod and reel. Longlines were made of 6 mm nylon mainline that was anchored at both ends. Gangions were composed of 1 m of 4 mm nylon cord and 1 m of 1.5 mm wire leader. Capture procedures and bait were the same for all the species. Specific capture procedures have been described in previous publications (Knip et al. 2011, 2012; Munroe et al. 2014, 2015). Rhizoprionodon taylori were fitted with V13 (13 mm × 36 mm), while all other species were fitted with V16 (16 mm × 65 mm) acoustic transmitters (Vemco Ltd., Canada). Transmitters were implanted into the body cavity to ensure long-term retention. An incision was made on the body midline anterior to the cloaca and then the transmitter was inserted into the body cavity. The incision was closed with absorbable sutures. Individuals were measured to the nearest millimetre for their stretch total length (STL), sexed, tagged with an individually numbered Rototag in the first dorsal fin and released. To reduce the overlap of acoustic signals, each transmitter emitted a unique code at 69 kHz on a random repeat interval of 45–75 s. Range testing indicated V13 and V16 transmitters had a maximum detection range of 525 m and 900 m, respectively, based on 5% probability of detection (Kessel et al. 2013). Range testing was conducted in each habitat type and results showed that the maximum detection range was consistent throughout the bay.
Space use and overlap analysis
Space use comparisons between resident and transitory sharks were considered invalid, because the movement and space use patterns of transitory individuals may not be indicative of local habitat selection and space use strategies. Therefore, space use analysis was restricted to resident individuals that were present for more than 2 consecutive months following release with acoustic transmitters. Individuals were considered present in the bay in a given month if they were detected for 3 or more days in the array. Sharks were considered present on a given day if they were detected at least twice on that day. If a shark was not detected for > 30 days, it was presumed that the shark had left the bay. Based on these criteria, 75 of 157 tagged sharks were excluded from analyses. Majority of the excluded individuals were detected in the bay for less than 6 weeks after release and did not return to Cleveland Bay during the monitoring period. Resident individuals were present in Cleveland Bay for 4–18 months. The only exception was a single R. taylori, which was present in Cleveland Bay for 3 months.
Individual positions were estimated for resident individuals using a mean position algorithm to determine centre of activity (COA) locations. COA locations were weighted mean positions for each 30-min interval a shark was detected in the array (Simpfendorfer et al. 2002). COAs were used to calculate individual activity space as 50% (core use) and 95% (extent) kernel utilisation distributions (KUDs) using the adehabitatHR package (Calenge 2006) in the R statistical environment (R Core Development Team 2017) version 3.0. To prevent overestimation of KUD size, calculations used an impassable boundary that approximated the Cleveland Bay coastline. This boundary did not extend around the Magnetic Island, as the coastline of this area was too complex to include in the calculations. All KUD calculations used a smoothing parameter of 0.008. Since the total amount of time each individual spent in the bay was variable and individuals were monitored across different years, individual KUDs were calculated for the total amount of time each individual spent in the bay (hereafter referred to as total KUD). To enable more direct comparisons in activity space size between individuals, KUDs were also calculated for each calendar month an individual was present (hereafter referred to as monthly KUD). Total 50% and 95% KUDs were overlaid to establish core and extent activity spaces for each species (Speed et al. 2011). A one-way ANCOVA was used to determine if total 50% and 95% KUD sizes were affected by species or STL (p < 0.05). Given the imbalanced nature of the data, all ANCOVAs were run with type III sum of squares. A linear mixed effects model was used to test the effect of species and STL on monthly 50% and 95% KUD size. Individual tag ID was included as a random factor to account for the repeated measures in these data. Monthly and total residency values (number of days detected/monitoring period) were variable between species. For example, R. taylori had lower residency values than C. amboinensis (Knip et al. 2011; Munroe et al. 2014). These residency trends have been identified previously and may be linked to differences in life history traits between species (Heupel et al. 2007; Knip et al. 2010; Munroe et al. 2014). Since previous work has shown that residency values were consistent within species (e.g., Knip et al. 2010, 2012; Munroe et al. 2014, 2015), we did not include residency as a factor in analyses, because it is correlated with species. Models were computed using the nlme package in R (Pinheiro et al. 2012). Models were compared using Akaike’s information criterion with a small sample size bias correction (AICc). Likelihood ratio tests were used to compare final models to respective null models. Data were assessed for normality prior to analysis and log10 transformed if necessary. Total and monthly KUD overlaps were calculated using the adehabitatHR package in R (Calenge 2006). Overlap was calculated between all the individuals of all the species and was measured as a percent (%). An ANOVA was used to determine if total 50% and 95% KUD overlap varied between species pairs. A linear mixed effects model was used to determine if monthly 50% and 95% KUD overlap was influenced by species pair and month. As overlap data were measured as a proportion, it was arcsine transformed prior to analysis.
Network analysis
Network analysis was used to assess species habitat use and partitioning, and movement pathways between different habitats types within Cleveland Bay. Networks were analysed using sna (Butts 2013), igraph (Csardi and Nepusz 2006) and tnet (Opsahl 2009) packages in R. Detection data for each individual were used to create non-square matrices that counted the frequency of use of habitat type for each individual; frequency was measured by dividing the total detections in each habitat by the number of receivers deployed in that same habitat. The non-square matrices were used to create two-mode habitat networks that represented the habitat use of a species for the entire study period. In this case, because non-square matrices were based on two sets of entities (i.e., habitat and individual), also referred to as nodes, the network created was bipartite, with one node representing habitats and another individuals. Species habitat networks were visualised and canonical correspondence analysis (CCA) was used to examine habitat partitioning within Cleveland Bay.
To examine the movement of individuals between habitat types, acoustic receivers deployed within Cleveland Bay were grouped according to the habitat type (Fig. 1). Receivers deployed in deeper habitat of the bay were separated into two groups: eastern deep (e_deep) and western deep (w_deep) for movement pathway analyses. Detection data for each individual were used to create square matrices that counted presence at, and relative movements between, habitat types. A 5 min interval was used to filter individuals’ detections at the same habitat to filter out possible false detections. Relative movement was defined as the number of times an individual moved between two habitats divided by the total number of movements made by the individual (i.e., total number of edges in the network (Jacoby et al. 2012). Directed and weighted habitat movement networks representing individual habitat use in the study area were created from the square matrices. Each network was tested for non-random patterns using link re-arrangement (i.e., permutation) via a bootstrap approach (n = 10,000; Croft et al. 2011). Observed movements were randomly shuffled between habitat types and new networks were generated using the same degree distribution as the original network. Transitivity was calculated for each random network to compare to values from the observed network using coefficient of variation and likelihood ratio tests (χ2, p < 0.05). Pathway number and frequency were calculated for each individual habitat movement network. Pathway number refers to the number of routes between two habitats used by an individual and pathway frequency (or relative movement) measures the number of times an individual moved between two habitats divided by the overall number of movements in the network.
Linear mixed effect models using an information theoretic approach were used to investigate the influence of species, STL and their interaction with pathway count and frequency. Linear models were implemented using the lme function from the nlme package (Pinheiro et al. 2012). Stretched total length was centred to simplify interpretation (Schielzeth 2010). Individual tag ID was included as a random factor to account for the repeated-measures nature of the data (Bolker et al. 2009). Data normality was tested prior to statistical analysis and data were transformed to normal when required. Collinearity between factors was assessed using the variance inflation factors function from the car package (Fox and Weisberg 2011). Goodness of fit was evaluated using diagnostics plots (i.e., residuals plot and auto-correlation function plot; Burnham and Anderson 2002; Zuur et al. 2010). Models were fitted with different weights and correlation functions to account for heteroscedasticity and temporal auto-correlation when required; models with the lowest AICc values were selected for analyses. Likelihood ratio tests were used to compare final models to respective null models. Analysis of deviance and post hoc multiple comparisons (Tukey’s HSD, α = 0.05) were used to test the effects of species, STL and their interaction with the pathway count and frequency metrics.
Results
Eighty-two sharks were tracked in Cleveland Bay between 2008 and 2014. The number of individuals tracked per calendar month was high and consistent for all species except R. taylori (Supplementary Table 1). The number of resident R. taylori varied from 1 to 5 individuals. Activity space size varied substantially among species (Fig. 2). Species had a significant effect on total 50% (ANCOVA, F(5,81) = 6.19, p < 0.05) and 95% (ANCOVA, F(5,81) = 4.05, p < 0.05) KUD size. Carcharhinus amboinensis and C. melanopterus had the smallest total KUDs, with C. melanopterus using approximately half the space of other species. Rhizoprionodon taylori and C. tilstoni had the largest total KUDs (Fig. 2). Stretch total length (STL) also had a significant effect on total 50% (ANCOVA, F(1,31) = 13.55, p < 0.05) and 95% (ANCOVA, F(1,31) = 7.94, P < 0.05) KUD size (Supplementary Figure 1). A significant negative trend between shark STL and total KUD size suggested as individuals increased in size, space use decreased. In contrast to total KUD results, the model that best explained monthly 50% and 95% KUD size only included species as a factor (Table 2; Fig. 2). Monthly KUDs revealed the same pattern where C. amboinensis and C. melanopterus had the smallest and R. taylori and C. tilstioni had the largest KUDs compared to other species. However, there was no relationship between shark STL and monthly KUD size.
Distribution patterns also differed substantially between species. Overlaying total 50% and 95% KUDs indicated that some species used large portions of the bay while others repeatedly used small, consistent areas (Fig. 3). Collectively, R. taylori and C. sorrah used more of the bay than other species and used core areas on both sides of the bay. The unique individual distributions of R. taylori and C. sorrah suggest high intraspecific variability in movement and habitat use. Carcharhinus amboinensis, C. fitzroyensis, and C. tilstoni consistently used the southeastern part of the bay with core areas centralised in shallow regions near river mouths. Carcharhinus melanopterus were almost exclusively detected on the western side of Cleveland Bay over reef habitat and adjacent reef-associated rocky/sandy substrate.
Total kernel utilisation distributions (KUDs) for a C. amboinensis, b C. fitzroyensis, c R. taylori, d C. melonpterous, e C. tilstoni and f C. sorrah. The 95% KUD contour (dashed line) are the 95% KUDs (extent of space use) for all individuals combined. The 50% KUDs (core use areas; grey fill) are shown for each individual. Increasingly dark fill indicates more individuals in that area
Analysis of total 95% KUD overlap showed that all species had some degree of spatial overlap with other species (Fig. 4), however, there was a significant difference in the degree of spatial overlap between species (ANOVA, F(14, 32261) = 504.48, p < 0.05). Carcharhinus amboinensis, C. fitzroyensis, and C. tilstoni exhibited the highest degree of overlap. This was the result of high residency of these species in the southeastern section of the bay. Rhizoprionodon taylori exhibited consistent spatial overlap with C. amboinensis, C. fitzroyensis, and C. tilstoni, with mean overlap ranging from 57.5 to 60.1%. Overlap between C. melanopterus and all other species was low (< 15%) and driven by limited use of reef and reef-associated habitats by other species. Carcharhinus sorrah overlap with all other species (except C. melanopterus) was consistent with mean overlap values between 24.7 and 35.1%.
Total 50% KUD overlaps were lower than 95% KUD overlap values and showed greater spatial partitioning between species (Fig. 4). There was a significant difference in 50% KUD overlap between species pairs (ANOVA, F(14, 32261) = 583.26, p < 0.05). Overlap of 50% KUDs was highest between C. amboinensis, C. fitzroyensis, and C. tilstoni. Rhizoprionodon taylori overlap was variable between species pairs, ranging from 1.2 to 27.6%. In contrast, C. melanopterus and C. sorrah exhibited little or no 50% KUD overlap with other species.
Monthly 50% and 95% KUD overlap values varied significantly between species pairs and months, and there was a significant interaction between these factors (Table 3). Overlap patterns for monthly 50% and 95% KUDS were consistent with total KUD overlap (Supplementary Tables 2, 3). However, close evaluation of monthly overlap values indicated most species pairs exhibited < 20% variation in overlap between months. In general, species pairs that exhibited low or high total overlap exhibited similar overlap values regardless of time of year. The notable exception to this was R. taylori, which exhibited more variable overlap patterns over time. KUD overlap between R. talyori and other species varied as much as 50% between consecutive months. This was likely due to the high individual variability in movement patterns and the inconsistent and low number of R. taylori monitored each month. Therefore, temporal changes in KUD overlap between R. taylori and other species should be interpreted with caution and R. taylori were likely driving the observed importance of month in the model output.
Species habitat networks and full CCA (on all six shark species) showed complex habitat partitioning within Cleveland Bay (Fig. 5, 6a and Supplementary Table 4) with C. melanopterus using reef and reef-associated habitats more than other species. The other five species followed a depth distribution within the bay with C. amboinensis and C. fitzroyensis using mudflats followed by C. tilstoni and R. taylori using seagrass beds and finally C. sorrah using deeper habitats (Fig. 6a, b). To confirm this depth distribution pattern, a partial CCA was performed on only five shark species, not including C. melanopterus. Carcharhinus amboinensis, C. fitzroyensis, C. sorrah, C. tilstoni and R. taylori distribution within Cleveland Bay was confirmed by the partial CCA (Fig. 6b). Furthermore, C. amboinensis, C. fitzroyensis and C. tilstoni had a more defined habitat use (smaller ellipses) compared with C. sorrah and R. taylori in which individuals used various habitats/sides of the bay (western vs. eastern) (Fig. 6b).
Species habitat networks for Carcharhinus amboinensis, C. fitzroyensis, C. melanopterus, C. tilstoni, C. sorrah and Rhizoprionodon taylori within Cleveland Bay, Australia. Node colours represent the different individuals in grey and the different habitat types in green for deeper habitat within the bay, violet for mudflat, blue for reef flats, pink for sand, orange for seagrass beds. Node size represents the detection count for the individual while in the bay and the frequency of habitat use by the individuals
a Full canonical correspondence analysis (CCA) showing the position of the six-shark species according to their habitat use. b Partial CCA showing the position of five-shark species (not including C. melanopterus) according to their habitat use: from more to least complex habitats within the bay on the x-axis and from deeper to shallower habitats within the bay on the y-axis
Testing networks (Fig. 7) for random characteristics revealed 4% of C. amboinensis, 0% of C. fitzroyensis, C. melanopterus and C. sorrah, 18% of C. tilstoni and 29% of R. taylori showed evidence of random movement (χ2, p < 0.05), and these networks were excluded from subsequent analyses. Pathway numbers varied between species (Table 4) with C. melanopterus having a significantly lower pathway count than C. amboinensis (~ 90%) and C. sorrah (~ 80%—Table 5a, Supplementary Figure 2). This difference may indicate that C. melanopterus moved more selectively between habitat types than C. amboinensis and C. sorrah. Pathway frequency (Table 5a, b and Supplementary Figure 3) did not vary significantly between species, although C. melanopterus pathway frequency was on average ≥ 63% higher than any other species. However, the lower pathway frequency median and wider range of C. melanopterus suggest that they heavily used a small number of pathways. This result is likely based on the limited amount of reef habitats available and subsequently the low number of receivers in this habitat type. The other five species had similar pathway counts and frequencies within their networks, indicating each moved similarly within their habitat. There was no significant effect of STL or species and STL interaction on tested metrics (Supplementary Table 5).
Example of individual habitat movement network for Carcharhinus amboinensis, C. fitzroyensis, C. melanopterus, C. tilstoni, C. sorrah and Rhizoprionodon taylori within Cleveland Bay, Australia. Node colours represent the different habitat types in green for deeper habitat within the bay, violet for mudflat, blue for reef flats, pink for sand, orange for seagrass beds. Node size represents the detection count at the habitat used by the individuals
Mean pathway frequency was 0.21 with a median at 0.05 which suggests that a small number of pathways was heavily used compared to others. At the pathway level, frequencies differed between types with pathways within the same habitat type more frequently (> 0.05) used (ranging from 0.75 to 1.64) than pathways between different habitat types (ranging from 0.004 to 0.50; Supplementary Figure 3). Furthermore, within habitat pathways were more frequently (> 0.05) used by most species. For example, pathways between seagrass beds were frequently used by C. amboinensis, C. fitzroyensis, C. tilstoni, C. sorrah and R. taylori, while pathways between mudflats were highly used by C. amboinensis, C. fitzroyensis, C. tilstoni and R. taylori. This suggests that species moved frequently within their main habitat types (Fig. 5). Pathway frequencies also differed with the region of the bay or with pathway frequency within the same region (east or west) ~ 92% higher than pathways crossing from one side to the other (Supplementary Figure 3). This may suggest that species mostly used one region within the bay and supports the consistent, relatively small 50% KUD estimates derived.
Discussion
Coastal habitats are widely recognised as productive areas that support an array of ecosystem services and species. Use of coastal habitats by elasmobranch species has been documented for decades (Springer 1967; Castro 1993), but little attention has been paid to how coexisting species interact. Research by Yates et al. (2015) and others (e.g., Simpfendorfer and Milward 1993; Bethea et al. 2004, 2015) has revealed overlapping distributions of a number of shark species in coastal systems, but few have considered whether coastal habitats are simply productive enough to support these shark species assemblages, or whether species partition habitats, space or prey to reduce or avoid competition. Competition for resources can be detrimental to species, resulting in decreased growth and survivorship (McMahon and Tash 1988; Webster 2004; Benkwitt 2013). Therefore, species will often go to great lengths to reduce competition, including using less favourable habitats, reducing the number of resources they utilise, or moving to new areas (Fausch and White 1981; Taylor et al. 2013). Here, we have demonstrated that six coexisting elasmobranch species from the same family use different habitat types, movement strategies, and amounts of space within a productive coastal bay, which may be indicative of niche separation to reduce competition for resources.
Differences in space use observed in the monitored species are likely due to two primary factors. First, the range of habitat types present within Cleveland Bay, from coral reef to mudflat and deeper water habitats, could explain species distinct distributions, possibly due to their unique and strong habitat preferences. For example, C. melanopterus used the smallest amount of space of any tracked species, had relatively consistent KUD locations, and had highly specialized habitat networks that almost exclusively included reef and reef-associated habitat receivers. This pattern is likely the result of the high dependence of this species on reef and associated habitats and the small and localised amount of these habitats available within Cleveland Bay. Our findings are consistent with the previous analysis by Chin et al. (2013a) who concluded that Cleveland Bay provides a critical habitat for juvenile and adult female C. melanopterus. In contrast, R. taylori, which had the largest KUDs and most diverse habitat use network, has been shown to be more flexible in its habitat use (Munroe et al. 2014). This flexibility might allow R. taylori to move more broadly than species with higher habitat dependence or more specific preferences. The movement patterns, space use, and habitat preferences shown by the six species thus provide for some degree of spatial separation that should reduce the competition for prey. This is supported in part by observations of overlap in diet among species (Simpfendorfer and Milward 1993; Kinney et al. 2011) which would suggest spatial separation is required to reduce competition for resources.
The second factor likely driving species-level variation in space use was that the species tracked had differing life history strategies and were monitored at different stages of maturity. Several species in this study are slow growing and large bodied with low productivity, while others are small to medium bodied, fast growing and highly productive. For example, C. amboinensis and C. tilstoni only use Cleveland Bay as juveniles with individuals recruiting offshore as they mature. Carcharhinus melanopterus has a somewhat similar strategy with juveniles and adult females using the bay, with large males rarely being present (Chin et al. 2013a). Tracking of C. amboinensis and C. melanopterus has revealed ontogenetic changes in space use by year classes of juvenile individuals with increasing size and age (Knip et al. 2011; Chin et al. 2016). The remaining species (C. fitzroyensis, C. sorrah, R. taylori) use nearshore coastal embayments throughout their life, and adults and juveniles often have overlapping home ranges (Knip et al. 2012; Munroe et al. 2014, 2015). Juveniles of slow-growing, large-bodied species typically use restricted home ranges which increase as they grow (e.g., Duncan and Holland 2006; Conrath and Musick 2010). This space use strategy is believed to decrease the risk of predation and help ensure survival until reproductive maturity (Heupel et al. 2007). This is likely why juvenile C. amboinensis used relatively small amounts of space and a consistent area of the bay. In contrast, small-bodied C. sorrah and R. taylori may be less risk averse, because their growth rate means they reach maturity more rapidly than species with lower productivity and the greater proportion of individuals reaching maturity can offset the predation risk that results from moving broadly to obtain resources (Knip et al. 2010; Munroe et al. 2014). Thus, differences in age classes tracked and species life history may help explain the contrasting distribution and habitat use patterns of elasmobranchs in Cleveland Bay.
Individual size also appeared to affect activity space size at the community level. Species with the largest activity spaces included the smallest individuals tracked (R. taylori) and juveniles of one of the large-bodied species (C. tilstoni), while species with the smallest KUDs included larger individuals of C. melanopterus and C. amboinensis. Collectively, these results indicated that as size increased, home range size decreased. This result appears counterintuitive, because previous work with sharks, as well as a wide range of other species, has shown that activity space size generally increases with body size (Knip et al. 2011; Tamburello et al. 2015). However, the negative trend observed in the present study may be explained by the fact that most species tracked were small-bodied adults or juveniles of large-bodied species. As previously discussed, the life history of the large-bodied species dictates that they will move offshore as they increase in size. This strategy limited our ability to collect movement data for larger C. amboinensis and C. tilstoni. Adult female C. melanopterus were the largest individuals in the data set. Their highly restricted home range, even at large sizes, was therefore the likely driver for this result.
Examination of spatial overlap indicated differences in overlap between species, but also between spatial scales. Overlap ranged from 9 to 70% for 95% KUDs which suggests some species may share large amounts of space. However, the temporal resolution of this analysis is insufficient to determine whether individuals were in the same areas at the same time. In contrast, 50% KUD overlap was greatly reduced (0–47%), indicating that core areas where individuals spent the majority of their time had limited overlap. This result suggests the species in this study used distinct core areas and adopted different space use and movement patterns. Network analyses and the CCA analysis support this finding through the presence of high numbers of pathways within the same habitat, and clear differences in the habitats used by the species. This indicates consistently high use of key habitat types. It is possible that these species partition core areas within selected habitats as a means of niche separation. These results are consistent with previous studies of spatial and dietary overlap of aquatic predator communities. Matich et al. (2017) found that co-occurring bull sharks, alligators, and dolphins within the resource-limited Shark River estuary (Florida, USA) partitioned both habitat and dietary resources, suggesting these predators used a combination of strategies to reduce competition. Similarly, White and Potter (2004) found evidence of habitat partitioning in a diverse coastal shark community where shark species composition changed markedly between different habitat types. The diverse habitat structure of Cleveland Bay may ultimately play an important role in supporting local elasmobranch habitat partitioning and coexistence. Highly complex ecosystems can facilitate and sustain coexistence by providing species with a variety of distinct and non-overlapping habitats and resources (Almany 2004a, b). Given the similarity in size of most species and commonalities in diet (Simpfendorfer and Milward 1993), it is likely they are in direct competition for prey resources. Thus, the combination of habitat selection and amount of space used might provide a mechanism to reduce interactions and increase survival.
The highest amount of overlap occurred in the southeast corner of the bay where C. amboinensis, C. fitzroyensis and C. tilstoni co-occur. These three species showed 45–47% overlap in their 50% KUD areas, the highest of all species combinations. Species habitat networks based on CCA revealed smaller ellipses for these species and consistent movement within their habitat type (mudflat and seagrass). Consistent use of this area combined with high overlap suggests this region is critical to these three species. It is unclear what benefit is derived from this location. It is possible that this area, which is located near primary river outflows, is highly productive and provides adequate prey resources for these species to coexist. Analysis of the stable isotopes of sharks in Cleveland Bay has shown evidence of some partitioning of prey resources (Kinney et al. 2011), but only amongst some groups of species. They demonstrated that C. amboinensis and C. fitzroyensis consume different prey and feed at different trophic levels, while C. fitzroyensis and C. tilstoni have similar diets (Kinney et al. 2011). This suggests that both spatial and dietary partitioning are occurring in Cleveland Bay, and this likely reduced the competition for resources. Dietary partitioning has previously been observed in elasmobranch assemblages (White et al. 2004; Heithaus et al. 2013; O’Shea et al. 2013) and is considered an important way by which elasmobranchs may limit competition in shared areas (Marshall et al. 2008; Sommerville et al. 2011; Yick et al. 2011). The shallow nature of this habitat may also provide protection from predation. Since C. amboinensis and C. tilstoni are both long-lived species with low productivity, survival of juveniles is critical for population persistence (Kinney and Simpfendorfer 2009). Therefore, predator avoidance may be a key strategy for these species at these early life stages. The shared use of small but protected shallow nurseries may in fact be a calculated ecological trade-off between the benefits of increased predator avoidance and the negative effects of increased competition for limited resources (Heithaus 2007; Matich and Heithaus 2015). It may be that a combination of these factors is ultimately what drives or enables the use of this area despite the large amount of distributional overlap and potential for competition.
While there are potential detriments to communal habitat use by sharks (e.g., competition for resources), this strategy would not occur if there were no benefits to the species. One potential benefit is increased protection from predation, because adults of large-bodied species are often spatially segregated from their young. This ontogenetic segregation may reduce predation of all species that coexist with young of these species (Simpfendorfer and Milward 1993). This allows individuals of all species to be less vigilant against predation and devote more energy to other important tasks, such as foraging (Beauchamp 2003; Sridhar et al. 2009). In addition, if social behaviours are present, co-occurring individuals may work together to capture prey (Ebert 1991) or learn from one another. For example, Guttridge et al. (2013) have shown that juvenile lemon sharks can learn behaviours from trained conspecifics. Therefore, shared space use and periodic overlap may be beneficial for populations.
Long-term tracking of six shark species revealed distinct differences in their space use that may increase their survival by reducing competition and exposure to predators. Subtle differences in the amount of space used and location of those spaces within coastal bays may provide adequate separation to allow a suite of similar species to coexist. Habitat selection appears to play a key role in the distribution of these species and their ultimate use of the bay. This indicates the importance for maintaining intact ecosystems with a variety of habitat types (Almany 2004a, b). Because it is unclear which ecosystem services are most crucial to these species (prey abundance, preferred environmental conditions, predator avoidance), we must maintain the health of coastal bays and estuaries that serve as critical habitat for multiple life stages of elasmobranch and other resident populations.
References
Abascal FJ, Quintans M, Ramos-Cartelle A, Mejuto J (2011) Movements and environmental preferences of the shortfin mako, Isurus oxyrinchus, in the southeastern Pacific Ocean. Mar Biol 158:1175–1184. https://doi.org/10.1007/s00227-011-1639-1
Allen LG, Yoklavich MM, Cailliet GM, Horn MH (2006) Bays and estuaries. In: Allen LG, Pondella DJ, Horn MH (eds) The ecology of marine fishes: California and adjacent waters. University of California Press, Los Angeles, pp 119–148
Almany GR (2004a) Differential effects of habitat complexity, predators and competitors on abundance of juvenile and adult coral reef fishes. Oecologia 141:105–113. https://doi.org/10.1007/s00442-004-1617-0
Almany GR (2004b) Does increased habitat complexity reduce predation and competition in coral reef fish assemblages? Oikos 106:275–284. https://doi.org/10.1111/j.0030-1299.2004.13193.x
Barbier EB, Hacker SD, Kennedy C, Koch EW, Stier AC, Silliman BR (2011) The value of estuarine and coastal ecosystem services. Ecol Monogr 81:169–193. https://doi.org/10.1890/10-1510.1
Beauchamp G (2003) Group-size effects on vigilance: a search for mechanisms. Behav Process 63:111–121. https://doi.org/10.1016/S0376-6357(03)00002-0
Beck MW, Heck KL, Able KW, Childers DL, Eggleston DB, Gillanders BM, Halpern B, Hays CG, Hoshino K, Minello TJ, Orth RJ, Sheridan PF, Weinstein MP (2001) The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience 51:633–641. https://doi.org/10.1641/0006-3568(2001)051%5b0633:ticamo%5d2.0.co;2
Benkwitt CE (2013) Density-dependent growth in invasive lionfish (Pterois volitans). PLoS One 8:e66995. https://doi.org/10.1371/journal.pone.0066995
Bethea DM, Buckel JA, Carlson JK (2004) Foraging ecology of the early life stages of four sympatric shark species. Mar Ecol Prog Ser 268:245–264. https://doi.org/10.3354/meps268245
Bethea DM, Ajemian MJ, Carlson JK, Hoffmayer ER, Imhoff JL, Grubbs RD, Peterson CT, Burgess GH (2015) Distribution and community structure of coastal sharks in the northeastern Gulf of Mexico. Environ Biol Fishes 98:1233–1254. https://doi.org/10.1007/s10641-014-0355-3
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MH, White JS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135. https://doi.org/10.1016/j.tree.2008.10.008
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer Science & Business Media, New York
Butts C (2013) sna: tools for social network analysis.–R package ver. 2.3-1
Calenge C (2006) The package adehabitat for the R software: a tool for the analysis of space and habitat use by animals. Ecol Model 197:516–519. https://doi.org/10.1016/j.ecolmodel.2006.03.017
Carlson J, Heupel M, Bethea D, Hollensead L (2008) Coastal habitat use and residency of juvenile Atlantic sharpnose sharks (Rhizoprionodon terraenovae). Estuaries Coast 31:931–940. https://doi.org/10.1007/s12237-008-9075-2
Carrier JC, Musick JA, Heithaus MR (2012) Biology of sharks and their relatives. CRC Press, Boca Raton
Castro JI (1993) The shark nursery of Bulls Bay, South Carolina, with a review of the shark nurseries of the southeastern coast of the United States. Environ Biol Fishes 38:37–48. https://doi.org/10.1007/bf00842902
Chin A, Heupel MR, Simpfendorfer CA, Tobin AJ (2013a) Ontogenetic movements of juvenile blacktip reef sharks: evidence of dispersal and connectivity between coastal habitats and coral reefs. Aquat Conserv 23:468–474. https://doi.org/10.1002/aqc.2349
Chin A, Tobin AJ, Heupel MR, Simpfendorfer CA (2013b) Population structure and residency patterns of the blacktip reef shark Carcharhinus melanopterus in turbid coastal environments. J Fish Biol 82:1192–1210. https://doi.org/10.1111/jfb.12057
Chin A, Heupel MR, Simpfendorfer CA, Tobin AJ (2016) Population organisation in reef sharks: new variations in coastal habitat use by mobile marine predators. Mar Ecol Prog Ser 544:197–211
Connell JH (1983) On the prevalence and relative importance of interspecific competition: evidence from field experiments. Am Nat 122:661–696. https://doi.org/10.2307/2460847
Conrath CL, Musick JA (2010) Residency, space use and movement patterns of juvenile sandbar sharks (Carcharhinus plumbeus) within a Virginia summer nursery area. Mar Freshw Res 61:223–235. https://doi.org/10.1071/MF09078
Core Development Team R (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Croft DP, Madden JR, Franks DW, James R (2011) Hypothesis testing in animal social networks. Trends Ecol Evol 26:502–507. https://doi.org/10.1016/j.tree.2011.05.012
Csardi G, Nepusz T (2006) The igraph software package for complex network research. Complex Syst 1695:1–9
Dale JJ, Stankus AM, Burns MS, Meyer CG (2011) The shark assemblage at French Frigate Shoals Atoll, Hawaii: species composition, abundance and habitat use. PLoS One 6:e16962. https://doi.org/10.1371/journal.pone.0016962
Davenport S, Stevens J (1988) Age and growth of two commercially imported sharks (Carcharhinus tilstoni and C. sorrah) from Northern Australia. Mar Fresh Res 39:417–433. https://doi.org/10.1071/MF9880417
Duncan KM, Holland KN (2006) Habitat use, growth rates and dispersal patterns of juvenile scalloped hammerhead sharks Sphyrna lewini in a nursery habitat. Mar Ecol Prog Ser 312:211–221. https://doi.org/10.3354/meps312211
Ebert DA (1991) Observations on the predatory behaviour of the sevengill shark Notorynchus cepedianus. S Afr J Mar Sci 11:455–465. https://doi.org/10.2989/025776191784287637
Ebert D, Fowler S, Compagno L (2013) Sharks of the world. Princeton University Press, Princeton
Fausch KD, White RJ (1981) Competition between brook trout (Salvelinus fontinalis) and brown trout (Salmo trutta) for positions in a Michigan stream. Can J Fish Aquat Sci 38:1220–1227. https://doi.org/10.1139/f81-164
Fox J, Weisberg S (2011) An R companion to applied regression. Sage, Thousand Oaks
Froeschke JT, Stunz GW, Sterba-Boatwright B, Wildhaber ML (2010) An empirical test of the ‘shark nursery area concept’ in Texas bays using a long-term fisheries-independent data set. Aquat Biol 11:65–76. https://doi.org/10.3354/ab00290
Guttridge TL, van Dijk S, Stamhuis EJ, Krause J, Gruber SH, Brown C (2013) Social learning in juvenile lemon sharks, Negaprion brevirostris. Anim Cogn 16:55–64. https://doi.org/10.1007/s10071-012-0550-6
Harry AV, Macbeth WG, Gutteridge AN, Simpfendorfer CA (2011) The life histories of endangered hammerhead sharks (Carcharhiniformes, Sphyrnidae) from the east coast of Australia. J Fish Biol 78:2026–2051
Harry AV, Morgan JAT, Ovenden JR, Tobin AJ, Welch DJ, Simpfendorfer CA (2012) Comparison of the reproductive ecology of two sympatric blacktip sharks (Carcharhinus limbatus and Carcharhinus tilstoni) off north-eastern Australia with species identification inferred from vertebral counts. J Fish Biol 81:1225–1233. https://doi.org/10.1111/j.1095-8649.2012.03400.x
Harry AV, Tobin AJ, Simpfendorfer CA (2013) Age, growth and reproductive biology of the spot-tail shark, Carcharhinus sorrah, and the Australian blacktip shark, C. tilstoni, from the Great Barrier Reef World Heritage Area, north-eastern Australia. Mar Freshw Res 64:277–293
Heithaus MR (2007) Nursery areas as essential shark habitats: a theoretical perspective. Am Fish Soc Symp 50:3–13
Heithaus MR, Vaudo JJ, Kreicker S, Layman CA, Krützen M, Burkholder DA, Gastrich K, Bessey C, Sarabia R, Cameron K (2013) Apparent resource partitioning and trophic structure of large-bodied marine predators in a relatively pristine seagrass ecosystem. Mar Ecol Prog Ser 481:225–237. https://doi.org/10.3354/meps10235
Heupel MR, Hueter RE (2002) Importance of prey density in relation to the movement patterns of juvenile blacktip sharks (Carcharhinus limbatus) within a coastal nursery area. Mar Freshw Res 53:543–550. https://doi.org/10.1071/MF01132
Heupel MR, Simpfendorfer CA, Hueter RE (2004) Estimation of shark home ranges using passive monitoring techniques. Environ Biol Fishes 71:135–142
Heupel MR, Carlson JK, Simpfendorfer CA (2007) Shark nursery areas: concepts, definition, characterization and assumptions. Mar Ecol Prog Ser 337:287–297. https://doi.org/10.3354/meps337287
Heupel MR, Kanno S, Martins AP, Simpfendorfer CA (2018a) Advances in understanding the roles and benefits of nursery areas for elasmobranch populations. Mar Fresh Res. https://doi.org/10.1071/mf18081
Heupel MR, Lédée EJ, Simpfendorfer CA (2018b) Telemetry reveals spatial separation of co-occurring reef sharks. Mar Ecol Prog Ser 589:179–192. https://doi.org/10.3354/meps12423
Humphries NE, Simpson SJ, Wearmouth VJ, Sims DW (2016) Two’s company, three’s a crowd: fine-scale habitat partitioning by depth among sympatric species of marine mesopredator. Mar Ecol Prog Ser 561:173–187. https://doi.org/10.3354/meps11937
Jacoby DMP, Brooks EJ, Croft DP, Sims DW (2012) Developing a deeper understanding of animal movements and spatial dynamics through novel application of network analyses. Methods Ecol Evol 3:574–583. https://doi.org/10.1111/j.2041-210X.2012.00187.x
Kessel ST, Cooke SJ, Heupel MR, Hussey NE, Simpfendorfer CA, Vagle S, Fisk AT (2013) A review of detection range testing in aquatic passive acoustic telemetry studies. Rev Fish Biol Fish 24:199–218. https://doi.org/10.1007/s11160-013-9328-4
Kinney MJ, Simpfendorfer CA (2009) Reassessing the value of nursery areas to shark conservation and management. Conserv Lett 2:53–60. https://doi.org/10.1111/j.1755-263X.2008.00046.x
Kinney M, Hussey N, Fisk A, Tobin A, Simpfendorfer C (2011) Communal or competitive? Stable isotope analysis provides evidence of resource partitioning within a communal shark nursery. Mar Ecol Prog Ser 439:263–276. https://doi.org/10.3354/meps09327
Knip DM, Heupel MR, Simpfendorfer CA (2010) Sharks in nearshore environments: models, importance, and consequences. Mar Ecol Prog Ser 402:1–11. https://doi.org/10.3354/meps08498
Knip DM, Heupel MR, Simpfendorfer CA, Tobin AJ, Moloney J (2011) Ontogenetic shifts in movement and habitat use of juvenile pigeye sharks Carcharhinus amboinensis in a tropical nearshore region. Mar Ecol Prog Ser 425:233–246. https://doi.org/10.3354/meps09006
Knip DM, Heupel MR, Simpfendorfer CA (2012) Habitat use and spatial segregation of adult spottail sharks Carcharhinus sorrah in tropical nearshore waters. J Fish Biol 80:767–784. https://doi.org/10.1111/j.1095-8649.2012.03223.x
Last PR, Stevens JD (2009) Sharks and rays of Australia, 2nd edn. CSIRO Publishing Collingwood, Victoria
Lédée EJI, Heupel MR, Tobin AJ, Simpfendorfer CA (2015) Movements and space use of giant trevally in coral reef habitats and the importance of environmental drivers. Anim Biotelem 3:6. https://doi.org/10.1186/s40317-015-0024-0
Lédée EJ, Heupel MR, Tobin AJ, Mapleston A, Simpfendorfer CA (2016) Movement patterns of two carangid species in inshore habitats characterised using network analysis. Mar Ecol Prog Ser 553:219–232. https://doi.org/10.3354/meps11777
Marshall AD, Kyne PM, Bennett MB (2008) Comparing the diet of two sympatric urolophid elasmobranchs (Trygonoptera testacea Müller & Henle and Urolophus kapalensis Yearsley & Last): evidence of ontogenetic shifts and possible resource partitioning. J Fish Biol 72:883–898. https://doi.org/10.1111/j.1095-8649.2007.01762.x
Matich P, Heithaus MR (2015) Individual variation in ontogenetic niche shifts in habitat use and movement patterns of a large estuarine predator (Carcharhinus leucas). Oecologia 178:347–359. https://doi.org/10.1007/s00442-015-3253-2
Matich P, Ault JS, Boucek RE, Bryan DR, Gastrich KR, Harvey CL, Heithaus MR, Kiszka JJ, Paz V, Rehage JS (2017) Ecological niche partitioning within a large predator guild in a nutrient-limited estuary. Limnol Oceanogr 62:934–953. https://doi.org/10.1002/lno.10477
Matley J, Tobin A, Lédée E, Heupel M, Simpfendorfer C (2016a) Contrasting patterns of vertical and horizontal space use of two exploited and sympatric coral reef fish. Mar Biol 163:253. https://doi.org/10.1007/s00227-016-3023-7
Matley JK, Heupel MR, Fisk AT, Simpfendorfer CA, Tobin AJ (2016b) Measuring niche overlap between co-occurring Plectropomus spp. using acoustic telemetry and stable isotopes. Mar Fresh Res 68:1468–1478. https://doi.org/10.1071/MF16120
McMahon TE, Tash JC (1988) Experimental analysis of the role of emigration in population regulation of desert pupfish. Ecology 69:1871–1883
Mourier J, Jacoby DMP, Guttridge TL (2018) Network analysis and theory in shark ecology-methods and applications. In: Carrier JC, Heithaus MR, Simpfendorfer CA (eds) Shark research: emerging technologies and applications for the field and laboratory. CRC Press, Boca Roton, pp 337–356
Munroe SEM, Simpfendorfer CA, Heupel MR (2014) Habitat and space use of an abundant nearshore shark, Rhizoprionodon taylori. Mar Freshw Res 65:959–968. https://doi.org/10.1071/MF13272
Munroe SEM, Simpfendorfer CA, Moloney J, Heupel MR (2015) Nearshore movement ecology of a medium-bodied shark, the creek whaler Carcharhinus fitzroyensis. Anim Biotelem 3:10. https://doi.org/10.1186/s40317-015-0026-y
Munroe SEM, Simpfendorfer CA, Heupel MR (2016) Variation in blacktip shark movement patterns in a tropical coastal bay. Environ Biol Fishes 99:377–389. https://doi.org/10.1007/s10641-016-0480-2
Munsch SH, Cordell JR, Toft JD (2016) Fine-scale habitat use and behavior of a nearshore fish community: nursery functions, predation avoidance, and spatiotemporal habitat partitioning. Mar Ecol Prog Ser 557:1–15. https://doi.org/10.3354/meps11862
Nakamura I, Watanabe YY, Papastamatiou YP, Sato K, Meyer CG (2011) Yo-Yo vertical movements suggest a foraging strategy for tiger sharks Galeocerdo cuvier. Mar Ecol Prog Ser 424:237–246. https://doi.org/10.3354/meps08980
Opsahl T (2009) Structure and evolution of weighted networks. University of London, London
O’Shea O, Thums M, Van Keulen M, Kempster R, Meekan M (2013) Dietary partitioning by five sympatric species of stingray (Dasyatidae) on coral reefs. J Fish Biol 82:1805–1820. https://doi.org/10.1111/jfb.12104
Papastamatiou YP, Wetherbee BM, Lowe CG, Crow GL (2006) Distribution and diet of four species of carcharhinid shark in the Hawaiian Islands: evidence for resource partitioning and competitive exclusion. Mar Ecol Prog Ser 320:239–251. https://doi.org/10.3354/meps320239
Pinheiro J, Bates D, DebRoy S, Sarkar D (2012) R Development Core Team. nlme: linear and nonlinear mixed effects models, R package version:3.1-103. http://CRAN.R-project.org/package=nlme
Schielzeth H (2010) Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol 1:103–113. https://doi.org/10.1111/j.2041-210X.2010.00012.x
Schlaff AM, Heupel MR, Simpfendorfer CA (2014) Influence of environmental factors on shark and ray movement, behaviour and habitat use: a review. Rev Fish Biol Fisheries 24:1089–1103. https://doi.org/10.1007/s11160-014-9364-8
Schoener TW (1986) Resource partitioning. In: Kilkkawa J, Anderson DJ (eds) Community ecology: pattern and process. Blackwell Scientific Publications, Boston, pp 91–126
Simpfendorfer C (1992) Reproductive strategy of the Australian sharpnose shark, Rhizoprionodon taylori (Elasmobranchii: Carcharhinidae), from Cleveland Bay, Northern Queensland. Mar Freshw Res 43:67–75. https://doi.org/10.1071/MF9920067
Simpfendorfer CA (1993) Age and growth of the Australian sharpnose shark, Rhizoprionodon taylori, from north Queensland, Australia. Environ Biol Fishes 36:233–241. https://doi.org/10.1007/bf00001718
Simpfendorfer C (1998) Diet of the Australian sharpnose shark, Rhizoprionodon taylori, from northern Queensland. Mar Freshw Res 49:757–761
Simpfendorfer CA, Milward NE (1993) Utilisation of a tropical bay as a nursery area by sharks of the families Carcharhinidae and Sphyrnidae. Environ Biol Fishes 37:337–345. https://doi.org/10.1007/BF00005200
Simpfendorfer CA, Heupel MR, Hueter RE (2002) Estimation of short-term centers of activity from an array of omnidirectional hydrophones and its use in studying animal movements. Can J Fish Aquat Sci 59:23–32. https://doi.org/10.1139/f01-191
Sommerville E, Platell ME, White WT, Jones AA, Potter IC (2011) Partitioning of food resources by four abundant, co-occurring elasmobranch species: relationships between diet and both body size and season. Mar Fresh Res 62:54–65. https://doi.org/10.1071/MF10164
Speed CW, Meekan MG, Field IC, McMahon CR, Stevens JD, McGregor F, Huveneers C, Berger Y, Bradshaw CJA (2011) Spatial and temporal movement patterns of a multi-species coastal reef shark aggregation. Mar Ecol Prog Ser 429:261–618. https://doi.org/10.3354/meps09080
Springer S (1967) Social organization of shark populations. In: Gilbert PW, Mathewson RF, Rall DP (eds) Sharks, skates and rays. John Hopkins Press, Baltimore, pp 149–174
Sridhar H, Beauchamp G, Shanker K (2009) Why do birds participate in mixed-species foraging flocks? A large-scale synthesis. Anim Behav 78:337–347. https://doi.org/10.1016/j.anbehav.2009.05.008
Tamburello N, Côté IM, Dulvy NK (2015) Energy and the scaling of animal space use. Am Nat 186:196–211. https://doi.org/10.1086/682070
Taylor MD, Fairfax AV, Suthers IM (2013) The race for space: using acoustic telemetry to understand density-dependent emigration and habitat selection in a released predatory fish. Rev Fish Sci 21:276–285. https://doi.org/10.1080/10641262.2013.796813
Tillett BJ, Meekan MG, Field IC, Hua Q, Bradshaw CJA (2011) Similar life history traits in bull (Carcharhinus leucas) and pigeye (C. amboinensis) sharks. Mar Fresh Res 62:850–860. https://doi.org/10.1071/MF10271
Tobin AJ, Mapleston A, Harry AV, Espinoza M (2014) Big fish in shallow water; use of an intertidal surf-zone habitat by large-bodied teleosts and elasmobranchs in tropical northern Australia. Environ Biol Fishes 97:821–838. https://doi.org/10.1007/s10641-013-0182-y
Webster MS (2004) Density dependence via intercohort competition in a coral-reef fish. Ecology 85:986–994. https://doi.org/10.1890/02-0576
White W, Potter I (2004) Habitat partitioning among four elasmobranch species in nearshore, shallow waters of a subtropical embayment in Western Australia. Mar Biol 145:1023–1032. https://doi.org/10.1007/s00227-004-1386-7
White WT, Platell ME, Potter IC (2004) Comparison between the diets of four abundant species of elasmobranch in a subtropical embayment: implications for resource partitioning. Mar Biol 144:439–448. https://doi.org/10.1007/s00227-003-1218-1
Yates PM, Heupel MR, Tobin AJ, Moore SK, Simpfendorfer CA (2015) Diversity in immature-shark communities along a tropical coastline. Mar Fresh Res 66:399–410. https://doi.org/10.1071/MF14033
Yick JL, Tracey SR, White RWG (2011) Niche overlap and trophic resource partitioning of two sympatric batoids co-inhabiting an estuarine system in southeast Australia. J Appl Ichthyol 27:1272–1277. https://doi.org/10.1111/j.1439-0426.2011.01819.x
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14. https://doi.org/10.1111/j.2041-210X.2009.00001.x
Acknowledgements
We thank all the staff and students at the Centre for Sustainable Tropical Fisheries and Aquaculture and the countless volunteers for their assistance, especially D Knip.
Funding
Funding for this research was provided by the Australian Research Council and Great Barrier Reef Marine Park Authority (GBRMPA) awarded to MRH and CAS; additional support was provided by the National Environmental Research Program awarded to CAS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All research was conducted in accordance with James Cook University (JCU) animal ethics permit A1566 and Great Barrier Reef (G11/346181.1) and QDAF (144482) permits for animal collection.
Additional information
Responsible Editor: J.K. Carlson.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by D. Jacoby and an undisclosed expert.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Heupel, M.R., Munroe, S.E.M., Lédée, E.J.I. et al. Interspecific interactions, movement patterns and habitat use in a diverse coastal shark assemblage. Mar Biol 166, 68 (2019). https://doi.org/10.1007/s00227-019-3511-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-019-3511-7