Abstract
Comparisons between shark nursery populations are limited, however recent work has shown different populations exhibit distinct space use patterns. This study examined the residency and space use of young of the year (YOY) and juvenile Australian blacktip sharks, Carcharhinus tilstoni in a northern Australian nursery using acoustic telemetry. Presence and space use patterns exhibited by C. tilstoni were highly variable among individuals. In contrast to other shark nursery populations, the majority of YOY individuals left the nursery area within three months of release, while most juveniles exhibited long-term residency (6 months - 1 year). In addition, YOY individuals used smaller amounts of space than juveniles. Variable activity space size and location indicated individuals used different areas and often moved into new areas. High individual variation in juvenile populations is atypical for carcharhinid sharks, and contrasts with other nursery species, including the common blacktip shark C. limbatus, which are known to exhibit residency and consistent space use patterns in nursery areas. The unique patterns observed among C. tilstoni may be due to a number of factors, including differences in nursery habitat and population structure, or strategies to improve survival. This study highlights the importance of investigating nursery behaviour across different habitats and populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nearshore areas are highly productive environments exploited by a wide array of species including birds, teleosts, and crustaceans (Nixon et al. 1986; Beck et al. 2001; Spalding et al. 2007). Nearshore areas also provide valuable habitats for a range of shark populations across different life stages (Heupel et al. 2007; Knip et al. 2010). Some shark species, such as the Australian sharpnose shark Rhizoprionodon taylori, use nearshore habitats throughout life (Knip et al. 2010; Munroe et al. 2014); while large-bodied species, such as the sandbar shark Carcharhinus plumbeus, often use nearshore habitats as nursery areas (Springer 1967; Rechisky and Wetherbee 2003; Grubbs 2010). Nearshore nurseries can be highly beneficial to juvenile sharks by improving feeding opportunities and providing protection from potential predators (Heupel et al. 2007). As a result, juvenile sharks typically exhibit high residency to nursery areas, potentially for several years after birth, before ultimately leaving to utilize new habitats and resources (Heithaus 2007; Hussey et al. 2009; Murchie et al. 2010).
Study of shark nursery areas has taken a number of forms, ranging from identification of single species nurseries (Gruber et al. 1988), to recognition of communal or multi-species nurseries (Castro 1993; Simpfendorfer and Milward 1993). Recent research has expanded beyond the scope of looking to identify single species nurseries to more complex studies of nursery area definition (Heupel et al. 2007), and contributions individual nurseries could make to the adult population (Yates et al. 2012). Comparison among and across nursery habitats and populations is difficult and uncommon. There are, however, several recent exceptions. Froeschke et al. (2010) examined juvenile bull shark Carcharhinus leucas presence in eight coastal bays to determine whether they functioned as nursery areas. Comparisons among bays revealed spatial and temporal differences in the abundance of individuals indicating diverse use of habitat within a species. Yates et al. (2015) examined shark community structure in five bays and found the composition of shark populations varied among bays, indicating different locations may meet different biological needs of individual species. Comparisons of shark movement and tagging studies have also shown that different nursery populations of the same species exhibit distinct residency and space use patterns, potentially due to differences in habitat structure (Legare et al. 2015). Although use of nearshore habitats varies between species and locations, previous studies suggest movement is often consistent within locations and populations (e.g. Heupel and Hueter 2002; Grubbs 2010).
The Australian blacktip shark, Carcharhinus tilstoni is a coastal species endemic to northern Australian waters (Last and Stevens 2009), where it can be found from close inshore to depths of 150 m. Carcharhinus tilstoni have a size at birth of approximately 600 mm total length (TL), mature at approximately 1200 mm TL, and grow to 1600–1800 mm TL (Davenport and Stevens 1988; Harry et al. 2012). Despite being one of the most commonly captured species in northern Australian commercial inshore shark fisheries (Harry et al. 2011; Bradshaw et al. 2013), little is known about its movements and habitat use. In contrast, the closely related common blacktip shark, C. limbatus, which is widely distributed in tropical and warm temperate regions around the world, has been comparatively well researched in the US (Heupel and Hueter 2002; Heupel et al. 2012), the Virgin Islands (DeAngelis et al. 2008; Legare et al. 2015) and Galapagos (Hirschfeld 2013). These studies have consistently shown that C. limbatus use nearshore habitats as nursery areas, and revealed distinct patterns in juvenile space use and offshore recruitment with age. The morphological and biological similarities between C. tilstoni and C. limbatus in northern Australia have been well documented (Harry et al. 2012; Morgan et al. 2012), and reported hybridisation provides further evidence of how closely related these two species are (Ovenden et al. 2010). Although the biological similarities between C. tilstoni and C. limbatus might suggest similar nursery use and juvenile behaviour, it is unclear if C. tilstoni shares any ecological similarities with other blacktip or nursery populations. Therefore, the aims of this study were to: 1) describe and compare presence and space use by young of the year (YOY) and juvenile C. tilstoni in a coastal bay, 2) examine changes in activity space and presence in relation to season and time since release, and 3) compare the space use patterns of C. tilstoni relative to other documented nursery populations.
Materials and methods
Study site
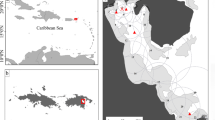
This study was conducted in Cleveland Bay, Queensland, Australia (Fig. 1). Cleveland Bay covers an area of approximately 225 km2 with the majority of the bay having depths of less than 10 m. The most common bottom types are soft mud and sandy substrate, although areas of seagrass and coastal patch reefs are also present. An array of sixty-three VR2W acoustic receivers (Vemco Ltd., Canada) was deployed in Cleveland Bay to monitor C. tilstoni movements. Data were downloaded from receivers approximately every three months.
Field methods
Sharks were collected using baited rod and reel, bottom-set 400 m long-lines, and 200 m long 11.45 cm mesh gillnets. Rod and reel and long-lines were baited with blue threadfin (Eleutheronema tetradactylum), mullet (Mugil cephalus), butterfly bream (Nemipterus spp.), or squid (Loligo sp.) Long-lines were made of 6-mm nylon mainline and were anchored at both ends. Gangions were made of 1 m of 4 mm nylon cord and 1 m of 1.5 mm wire leader. Fifty to 70 size 14/0 Mustad tuna circle hooks were used per line. Gillnets were set for 15 to 20 min and long-lines were set for 45 to 60 min.
Acoustic transmitters (Vemco Ltd., Canada; V16) were surgically implanted into the body cavity of individuals to ensure long-term retention. An incision was made, the transmitter inserted into the body cavity, and the incision closed with absorbable sutures. Individuals were tagged with an individually numbered Rototag in the first dorsal fin, stretch total length (STL) measured to the nearest millimeter, sexed, and released. Transmitters emitted a unique code at 69 kHz to allow for the identification of individuals. Acoustic codes were emitted at random 45 to 90 s intervals to minimise signal overlap. Transmitters had a battery life of 24 months and a maximum detection range of 900 m based on 5 % probability of detection (Kessel et al. 2013). Umbilical scar condition, clasper calcification, and published length-at-age data (Harry et al. 2012) were used to classify individuals as either young of the year (YOY) or juveniles (>1 year).
Statistical methods
Residency
Presence was evaluated each day with individuals considered present if they were detected two or more times in the array in a given day. Residency was expressed as a residency index that calculated the number of days an individual was present in the array as a proportion of the total days monitored. The index ranged from 1 to 0, indicating high to low residency, respectively. An ANOVA was used to test for differences in residency between sexes and age classes (YOY and juveniles).
Space use
Individual positions were estimated using a mean position algorithm to determine individual centre of activity (COA) locations (Simpfendorfer et al. 2002). The COA represented a weighted mean position for each 30-min interval an individual was detected in the array. COA locations were used to calculate individual monthly activity space as 50 % and 95 % kernel utilisation distributions (KUDs) using the adehabitatHR package in R version 3.0 (Calenge 2006). Individuals that were present in the bay for less than 14 days were excluded from space use analysis. To prevent overestimation of KUD size, KUD calculations incorporated an impassable boundary that represented the Cleveland Bay coastline. All KUD calculations used a smoothing parameter of 0.008 and were calculated in km2.
Changes in activity space size and position were evaluated using multiple methods. Monthly KUDs for individuals present more than 14 days in a given month were examined with a linear mixed effects model to determine if 50 % and 95 % KUD size was affected by age class, month since release or diel period. Individual identity was incorporated as a random factor in the resultant models to account for repeated measures in the data. Models were computed using the nlme package in R (Pinheiro et al. 2013). Models were compared using Akaike information criterion with a small sample size bias correction (AICc). Models with the lowest AICc were considered the most significant drivers of KUD size. Akaike weights were calculated to aid model assessment.
Cumulative monthly 50 % and 95 % KUDs were calculated for individuals that were present more than three consecutive months. Cumulative KUDs were calculated by adding the next month’s COA locations of an individual to all the past month’s COA locations and recalculating activity space (Heupel et al. 2004). Cumulative fortnightly 50 % and 95 % KUDs were also calculated for YOY individuals that were present in the bay less than three consecutive months.
Within-individual monthly KUD overlap was also calculated for each consecutive month individuals were detected. This metric determined how much of the previous month’s activity space an individual re-used the following month. Between-individual monthly KUD overlap was also calculated for each possible pair of individuals. Between-individual KUD overlap determined the amount of space two individuals shared in a given month. Space use overlap was only calculated for individuals that were present more than three consecutive months. All overlap values were measured as a percent using the adehabitatHR package in R (Calenge 2006).
Results
Twenty-three juvenile C. tilstoni (10 male, 13 female) were caught and released with acoustic transmitters between September and December 2012. Size ranged from 668 to 1150 mm STL (mean ± SE = 829 ± 34). Based on length-at-age data and umbilical scar condition 13 sharks were YOY and 10 were juvenile (one to four years old). Two YOY individuals (one male, one female) died following release and were excluded from analyses. The female was recaptured four weeks after release in Cleveland Bay and the male died approximately six weeks after release, potentially from predation. The remaining 21 individuals were monitored in Cleveland Bay from December 2012 to November 2013.
Presence
Presence of individuals in Cleveland Bay ranged from 7 to 372 days (mean ± SE = 138 ± 30) (Fig. 2) and residency ranged from 0.02 to 0.87 (mean ± SE = 0.32± 0.07). Residency data was non-normal and was arcsine transformed. A type III sum of squares correction for the ANOVA was used to properly evaluate the unbalanced data. There was no significant difference in residency between sexes (ANOVA, F (1,18) = 0.095, P > 0.05). However, juveniles had significantly higher residency than YOY individuals (ANOVA, F (1,18) = 18.26, P < 0.05).
With the exception of one YOY female, all YOY individuals spent less than three consecutive months within the array. YOY individuals that exited the array left over the same four-week period (January to February 2013). Two YOY individuals were last detected on the north-western boundary of the array, suggesting they departed the bay via the passage between Magnetic Island and the mainland. In contrast, the majority of juvenile individuals were present for the duration of the monitoring period.
Space use
Individual monthly activity space ranged from 2.6 to 19.8 km2 (mean ± SE =10.6 km2 ± 0.3) for 50 % KUDs and 9.1 to 81.9 km2 (mean ± SE = 47.9 km2 ± 1.0) for 95 % KUDs. The best possible model to explain both 50 % and 95 % KUD size included age class, month since release, and diel period as factors (Table 1). For both 50 % and 95 % KUDs, juvenile C. tilstoni had larger activity spaces than YOY individuals (Fig. 3a, c), and day-time KUDs were larger than night-time KUDs (Fig. 3b, d). The effect of month since release was most pronounced in May 2013 where KUD size was largest, and August 2013, where KUD size was smallest (Fig. 4).
Eight individuals (7 juvenile, 1 YOY) were present in the array for more than three consecutive months following release. Cumulative home range analysis showed that during the first four to six months after release, juvenile cumulative KUD size increased, but the rate of increase was low (Fig. 5). By May 2013, all juvenile cumulative activity space curves for both 50 % and 95 % KUDs reached an asymptote, indicating that individuals were no longer moving into new areas. Most juveniles reached a maximum cumulative KUD size between 10 to 20 km2 for 50 % KUDs and 60 to 80 km2 for 95 % KUDs. The exception to this was one juvenile female (#46,990) that showed rapid expansion into new areas in January 2013, a decrease in space use in February 2013, followed by a second rapid increase in space use in March and April 2013. Increases in cumulative KUD size corresponded with use of unique sections of the bay compared to other individuals (Fig. 6b). As a result, this individual had the largest cumulative KUDs, ranging from 20 to 25 km2 for 50 % KUDs and 100 to 120 km2 for 95 % KUDs.
The single YOY individual present for more than three consecutive months (#47002) used consistent and small amounts of space for the first four months it was monitored in the array (December 2012 - March 2013). This individual used a small area concentrated in the far northeast corner of the bay (Fig. 6a). In April 2013, #47002 exhibited rapid space use expansion and began to use new areas of the bay. The cumulative KUD trends for #47002 began to asymptote, most notably for the 95 % KUDs, at the end of the monitoring period (November 2013), suggesting that after approximately 1 year this individual was no longer moving into new areas. This individual reached a maximum cumulative KUD size of 23.0 km2 for 50 % KUDs and 89.4 km2 for 95 % KUDs.
Eight YOY individuals were present less than three months following release. Trends in 50 % and 95 % fortnightly cumulative KUD size varied between individuals. Some individuals exhibited a relatively rapid and/or consistent increase in cumulative KUD size with time, indicating the use of new areas and activity space expansion prior to exiting the array. However, two individuals showed no sign of space use expansion and consistently used small areas close to shore. The activity space positions of resident individuals fluctuated according to the same monthly pattern as KUD size. From December 2012 to July 2013, most individual KUD areas were widely spread throughout the bay (Fig. 6). However, in August 2013, individual KUDs were concentrated near the southeastern creek mouths of the bay (Fig. 7a, b). Most individuals remained in this area until October 2013. Only one individual did not move toward the southeastern creek mouths at this time (Fig. 7c).
Individual monthly juvenile KUD overlap was highly variable. Several individuals reused areas while others regularly moved into new areas. Individual juvenile overlap patterns also varied over time (Fig. 8a). The single resident YOY individual exhibited high space use overlap during the first four months after its release and consistently used the northeast portion of Cleveland Bay. This was followed by a low degree of overlap for the remainder of the monitoring period. This change in space use overlap corresponded with expansion into new areas of the bay. In May 2013, the majority of individuals exhibited high KUD overlap. This was likely because KUD size was largest at this time, which would increase the likelihood of overlap between months. Between July and August 2013, most individuals exhibited a large decrease in KUD overlap as a result of the sudden shift in position to the southeastern shore of the bay.
Between-individual overlap for both 50 % and 95 % KUDs was relatively low from December 2012 to March 2013 (Fig. 8b). However 95 % between-individual overlap increased in May 2013 when KUD size began to increase. The 50 % KUD between-individual overlap was greatest in August and September 2013, when most individuals moved to the southeast corner of Cleveland Bay.
Discussion
The presence and space use patterns of juvenile and YOY C. tilstoni were highly variable among individuals and strongly contrast with the more consistent, long-term residency observed in other juvenile populations, such as lemon Negaprion brevirostris (Morrissey and Gruber 1993), pigeye C. amboinensis (Knip et al. 2011), and common blacktip C. limbatus sharks (Heupel et al. 2004; DeAngelis et al. 2008; Legare et al. 2015). Low residency and variation in movement was unexpected, as juveniles of large-bodied, slow-growing sharks often exhibit high site fidelity for periods of months (Wetherbee et al. 2007; Conrath and Musick 2010) to years, to increase survival (Heithaus 2007; Chapman et al. 2009; Knip et al. 2011). Juvenile shark populations also often continuously use small, specific portions of the nursery area (Heupel et al. 2004; Knip et al. 2011). The highly variable movement and space use of C. tilstoni indicated use of different areas of the bay and regular movement into new areas and habitats. These unique movement and residency patterns suggest that C. tilstoni use nursery areas differently to other shark populations.
The notable differences in movement strategies between C. tilstoni and other nursery populations are difficult to explain, however differences in habitat structure and resources could be a primary cause (Legare et al. 2015). For example, while C. limbatus in Florida, USA and the Virgin Islands used less space than juvenile C. tilstoni, the former were tracked in relatively small bays with narrow openings. Cleveland Bay has a comparatively expansive and open structure. This more open structure may allow individuals to move over greater distances. Roaming movement patterns may also be highly beneficial to individuals. Juvenile bonnethead sharks Sphyrna tiburo and adult Australian sharpnose sharks Rhizoprionodon taylori are also known to use relatively consistent amounts of space but roam between different locations over time (Heupel et al. 2006; Munroe et al. 2014). It was proposed that this movement strategy may increase access to prey items, especially if resources in a previously used area have declined (Munroe et al. 2014).
The majority of YOYs that left the study site exhibited rapid activity space use expansion and used new areas prior to their last detections. These behaviours suggest many YOY left the region. Ontogenetic increases in space use and activity (Morrissey and Gruber 1993; Dicken et al. 2006; Knip et al. 2011), followed by decreased nursery residency (Hussey et al. 2009; Andrews et al. 2010; Conrath and Musick 2010) and migration have been observed in numerous shark nursery populations, including C. limbatus (Heupel et al. 2004). Expansions in space use have been presumed to be related to individuals learning to successfully forage, avoid predators, and prepare for migration (Heupel et al. 2004). Ontogeny had a clear influence on C. tilstoni activity space size, with YOY using less space than juveniles, but progressively using more space with time. After approximately one year the single resident YOY used similar amounts of space to older juveniles. Convergence in activity space size indicates that most juveniles ultimately use similar amounts of space within the bay over time. Although ontogenetic changes in space use as identified in this individual and other species seems a plausible explanation for some C. tilstoni movement patterns, residence of older juveniles and the rapid departure of most YOYs suggests ontogenetic shifts in space use are not exclusively driving individual residency and movement.
Given that most YOY departed over the same four week period, it is possible that a social or seasonal environmental stimulus triggered movement out of the bay (Schlaff et al. 2014). Environmental cues for movement among sharks are common (Heupel et al. 2004; Grubbs et al. 2007; Legare et al. 2015). However, environmental parameters in February 2013 were consistent with those of the preceding months, suggesting environmental conditions did not prompt movement from the area. The individuals that left Cleveland Bay were part of the same cohort, suggesting a biological parameter could have caused this movement. However, not all YOY left the bay, and a large juvenile population was consistently present, suggesting that whatever driver caused individuals to leave was not persistent enough or relevant to all individuals from other size and age classes. This suggests movement from the area was based on the individual, or represents a varied life history strategy with individuals adopting different patterns. Individual variability in YOY residency has also been observed among C. limbatus at San Cristobal Island, Galapagos where some individuals were resident while others departed (Hirschfeld 2013). Acoustic tracking of juvenile C. leucas showed that some individuals were highly risk adverse and typically used less productive but sheltered areas within an estuarine nursery, while others used more exposed, productive areas (Matich and Heithaus 2015). These examples indicate that juvenile movement to different habitats occurs and may be the result of internal drivers.
High residency to nursery areas is thought to benefit populations if nearshore areas are highly productive and provide shelter from predation (Branstetter 1990; Beck et al. 2001). Previous work has indicated that Cleveland Bay is a highly productive environment capable of supporting numerous elasmobranch populations (Simpfendorfer and Milward 1993; Knip et al. 2011; Kinney et al. 2011; Knip et al. 2012a). Therefore, residency in Cleveland Bay is a potentially beneficial strategy for C. tilstoni and likely explains why at least a portion of the population was highly resident. However, transitory strategies can also be highly beneficial depending on environmental and individual conditions. Movement into new areas can increase access to novel prey resources, increase foraging success (Collins et al. 2007), and help reduce competition for resources (McMahon and Tash 1988; Taylor et al. 2013). Matich and Heithaus (2015) found that juvenile C. leucas were more likely to move into new areas that were exposed to predation, but also more productive, if they had relatively poor body condition. In contrast, C. leucas with relatively healthy body condition remained in less productive but safer areas of the nursery, potentially because they were able to outcompete other juveniles in the area. Thus, for some C. leucas, the risk of exposure to predation appeared to be outweighed by access to better resources. Although Cleveland Bay is considered highly productive, it is also a known communal nursery with historically high numbers of juvenile sharks with potentially high competition for some resources (Simpfendorfer and Milward 1993). Therefore the potential benefits of moving to a new area may have prompted some C. tilstoni to leave Cleveland Bay in search of better resource conditions and/or to reduce competition, despite the potential increased exposure to predators outside the nursery (Näslund et al. 1993).
The movement of some individuals to seek out new, and possibly better habitat, while part of the population remains in a viable habitat may be an evolutionarily effective strategy. Both strategies have levels of risk and reward relative to survival and employing both may be an effective way of ensuring populations continue to thrive. Movement by youngest individuals may occur because they are least efficient at foraging and require more abundant resources than more experienced individuals (Bowler and Benton 2005). Mortality rates of YOY and juvenile sharks can be high (Heupel and Simpfendorfer 2002: Knip et al. 2012b), so taking risks at this life stage may be worthwhile. Employing both resident and transitory behaviours may be a life history strategy to increase survival rates of young C. tilstoni and maintain populations.
The results of this study demonstrate that juvenile and YOY C. tilstoni residency and movement are driven by a complex set of factors including age class, time of year, and individual variation. Overall, the patterns observed in C. tilstoni do not reflect those observed for most other large coastal shark species, most notably based on dispersal of YOY individuals while juveniles remained in the area. This suggests that C. tilstoni may have different habitat use patterns or life history strategies, that the region offers an array of suitable habitats individuals can exploit, or some other factor influences presence and movement of a portion of the population. Further study of C. tilstoni populations in this and other regions will help elucidate differences in habitat use to that of the widely accepted norm for sharks in coastal nursery areas.
References
Andrews KS, Williams GD, Levin PS (2010) Seasonal and ontogenetic changes in movement patterns of sixgill sharks. PLoS One 5:e12549. doi:10.1371/journal.pone.0012549
Beck MW et al. (2001) The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience 51:633–641. doi:10.1641/0006-3568(2001)051[0633:ticamo]2.0.co;2
Bowler DE, Benton TG (2005) Cause and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol Rev 80:205–225. doi:10.1017/S1464793104006645
Bradshaw CJA, Field IC, McMahon CR, Johnson GJ, Meekan MG, Buckworth RC (2013) More analytical bite in estimating targets for shark harvest. Mar Ecol Prog Ser 488:221–232
Branstetter S (1990) Early life-history implications of selected carcharhinoid and lamnoid sharks of the Northwest Atlantic. NOAA Tech Rep NMFS 90:17–28
Calenge C (2006) The package adehabitat for the R software: a tool for the analysis of space and habitat use by animals. Ecol Model 197:516–519
Castro J (1993) The shark nursery of Bulls Bay, South Carolina, with a review of the shark nurseries of the southeastern coast of the United States. In: LS D, JP W (eds) The reproduction and development of sharks, skates, rays and ratfishes. Developments in environmental biology of fishes, vol 14. Springer, Netherlands, pp. 37–48
Chapman DD et al. (2009) Long-term natal site-fidelity by immature lemon sharks (Negaprion brevirostris) at a subtropical island. Mol Ecol 18:3500–3507. doi:10.1111/j.1365-294X.2009.04289.x
Collins AB, Heupel MR, Motta PJ (2007) Residence and movement patterns of cownose rays Rhinoptera bonasus within a south-West Florida estuary. J Fish Biol 71:1159–1178. doi:10.1111/j.1095-8649.2007.01590.x
Conrath CL, Musick JA (2010) Residency, space use and movement patterns of juvenile sandbar sharks (Carcharhinus plumbeus) within a Virginia summer nursery area. Mar Freshw Res 61:223–235. doi:10.1071/MF09078
Davenport S, Stevens J (1988) Age and growth of two commercially imported sharks (Carcharhinus tilstoni and C. sorrah) from Northern Australia. Mar Freshw Res 39:417–433. doi:10.1071/MF9880417
DeAngelis BM, McCandless CT, Kohler NE, Recksiek CW, Skomal GB (2008) First characterization of shark nursery habitat in the United States Virgin Islands: evidence of habitat partitioning by two shark species. Mar Ecol Prog Ser 358:257–271
Dicken M, Smale M, Booth A (2006) Spatial and seasonal distribution patterns of the ragged-tooth shark Carcharias taurus along the coast of South Africa. Afr J Mar Sci 28:603–616
Froeschke JT, Stunz GW, Sterba-Boatwright B, Wildhaber ML (2010) An empirical test of the ‘shark nursery area concept’in Texas bays using a long-term fisheries-independent data set. Aquat Biol 11:65–76
Grubbs RD (2010) Ontogenetic shifts in movements and habitat use. In: Carrier JC, Musick JA, Heithaus MR (eds) Sharks and their relatives II: biodiversity, adaptive physiology, and conservation. CRC Press, Boca Raton, pp. 319–342
Grubbs RD, Musick JA, Conrath CL, Romine JG (2007) Long-term movements, migration, and temporal delineation of a summer nursery for juvenile sandbar sharks in the Chesapeake Bay region. In: American Fisheries Society symposium, vol 50. American Fisheries Society, p 87
Gruber SH, Nelson DR, Morrissey JF (1988) Patterns of activity and space utilization of lemon sharks, Negaprion brevirostris, in a shallow bahamian lagoon. Bull Mar Sci 43:61–76
Harry AV et al. (2011) Evaluating catch and mitigating risk in a multispecies, tropical, inshore shark fishery within the great barrier reef world heritage area. Mar Freshw Res 62:710–721. doi:10.1071/MF10155
Harry AV, Morgan JAT, Ovenden JR, Tobin AJ, Welch DJ, Simpfendorfer CA (2012) Comparison of the reproductive ecology of two sympatric blacktip sharks (Carcharhinus limbatus and Carcharhinus tilstoni) off North-Eastern Australia with species identification inferred from vertebral counts. J Fish Biol 81:1225–1233. doi:10.1111/j.1095-8649.2012.03400.x
Heithaus MR (2007) Nursery areas as essential shark habitats: a theoretical perspective. In: American Fisheries Society symposium, vol 50. American Fisheries Society, p 3
Heupel MR, Hueter RE (2002) Importance of prey density in relation to the movement patterns of juvenile blacktip sharks (Carcharhinus limbatus) within a coastal nursery area. Mar Freshw Res 53:543–550. doi:10.1071/MF01132
Heupel MR, Simpfendorfer CA (2002) Estimation of mortality of juvenile blacktip sharks, Carcharhinus limbatus, within a nursery area based on telemetry data. Can J Fish Aquat Sci 59:624–632
Heupel MR, Simpfendorfer CA, Hueter RE (2004) Estimation of shark home ranges using passive monitoring techniques. Environ Biol Fish 71:135–142. doi:10.1023/B:EBFI.0000045710.18997.f7
Heupel MR, Simpfendorfer CA, Collins AB, Tyminski JP (2006) Residency and movement patterns of bonnethead sharks, Sphyrna tiburo, in a large Florida estuary. Environ Biol Fish 76:47–67. doi:10.1007/s10641-006-9007-6
Heupel MR, Carlson JK, Simpfendorfer CA (2007) Shark nursery areas: concepts, definition, characterization and assumptions. Mar Ecol Prog Ser 337:287–297. doi:10.3354/meps337287
Heupel MR, Simpfendorfer CA, Olsen EM, Moland E (2012) Consistent movement traits indicative of innate behavior in neonate sharks. J Exp Mar Biol Ecol 432–433:131–137. doi:10.1016/j.jembe.2012.07.013
Hirschfeld M (2013) Habitat use and movement patterns of juvenile and neonate blacktip sharks, Carcharhinus limbatus in nursery areas on San Cristobal Island, Galápagos. Universidad San Francisco De Quito, Quito.
Hussey NE, McCarthy ID, Dudley SFJ, Mann BQ (2009) Nursery grounds, movement patterns and growth rates of dusky sharks, Carcharhinus obscurus: a long-term tag and release study in south African waters. Mar Freshw Res 60:571–583. doi:10.1071/MF08280
Kessel ST et al. (2013) A review of detection range testing in aquatic passive acoustic telemetry studies. Rev Fish Biol Fish:1–20. doi:10.1007/s11160-013-9328-4
Kinney M, Hussey N, Fisk A, Tobin A, Simpfendorfer C (2011) Communal or competitive? stable isotope analysis provides evidence of resource partitioning within a communal shark nursery. Mar Ecol Prog Ser 439:263–276. doi:10.3354/meps09327
Knip DM, Heupel MR, Simpfendorfer CA (2010) Sharks in nearshore environments: models, importance, and consequences. Mar Ecol Prog Ser 402:1–11. doi:10.3354/meps08498
Knip DM, Heupel MR, Simpfendorfer CA, Tobin AJ, Moloney J (2011) Ontogenetic shifts in movement and habitat use of juvenile pigeye sharks Carcharhinus amboinensis in a tropical nearshore region. Mar Ecol Prog Ser 425:233–246. doi:10.3354/meps09006
Knip DM, Heupel MR, Simpfendorfer CA (2012a) Habitat use and spatial segregation of adult spottail sharks Carcharhinus sorrah in tropical nearshore waters. J Fish Biol 80:767–784. doi:10.1111/j.1095-8649.2012.03223.x
Knip DM, Heupel MR, Simpfendorfer CA (2012b) Mortality rates for two shark species occupying a shared coastal environment. Fish Res 125-126:184–189
Last PR, Stevens JD (2009) Sharks and rays of Australia 2nd edn. CSIRO Publishing Collingwood, Victoria
Legare B, Kneebone J, DeAngelis B, Skomal G (2015) The spatiotemporal dynamics of habitat use by blacktip (Carcharhinus limbatus) and lemon (Negaprion brevirostris) sharks in nurseries of St. John, United States Virgin Islands. Mar Biol 162:699–716
Matich P, Heithaus MR (2015) Individual variation in ontogenetic niche shifts in habitat use and movement patterns of a large estuarine predator (Carcharhinus leucas). Oecologia 178:347–359. doi:10.1007/s00442-015-3253-2
McMahon TE, Tash JC (1988) Experimental analysis of the role of emigration in population regulation of desert pupfish. Ecology 69:1871–1883
Morgan JAT et al. (2012) Detection of interspecies hybridisation in chondrichthyes: hybrids and hybrid offspring between Australian (Carcharhinus tilstoni) and common (C. limbatus) blacktip shark found in an Australian fishery. Conserv Genet 13:455–463. doi:10.1007/s10592-011-0298-6
Morrissey JF, Gruber SH (1993) Habitat selection by juvenile lemon sharks, Negaprion brevirostris. Environ Biol Fish 38:311–319. doi:10.1007/bf00007524
Munroe SEM, Simpfendorfer CA, Heupel MR (2014) Habitat and space use of an abundant nearshore shark, Rhizoprionodon taylori. Mar Freshw Res 65:959–968. doi:10.1071/MF13272
Murchie KJ et al. (2010) Spatial ecology of juvenile lemon sharks (Negaprion brevirostris) in tidal creeks and coastal waters of Eleuthera, the Bahamas. Environ Biol Fish 89:95–104. doi:10.1007/s10641-010-9693-y
Näslund I, Milbrink G, Eriksson L, Holmgren S (1993) Importance of habitat productivity differences, competition and predation for the migratory behaviour of Arctic charr. Oikos 66:538–546
Nixon SW, Oviatt CA, Frithsen J, Sullivan B (1986) Nutrients and the productivity of estuarine and coastal marine ecosystems. J Limnol Soc South Africa 12:43–71. doi:10.1080/03779688.1986.9639398
Ovenden JR, Morgan JAT, Kashiwagi T, Broderick D, Salini J (2010) Towards better management of Australia’s shark fishery: genetic analyses reveal unexpected ratios of cryptic blacktip species Carcharhinus tilstoni and C. limbatus. Mar Freshw Res 61:253–262. doi:10.1071/MF09151
Pinheiro J, Bates D, DebRoy S, Sarkar D (2013) nlme: Linear and nonlinear mixed effects models. vol R package version 3.1
Rechisky E, Wetherbee B (2003) Short-term movements of juvenile and neonate sandbar sharks, Carcharhinus plumbeus, on their nursery grounds in Delaware Bay. Environ Biol Fish 68:113–128
Schlaff AM, Heupel MR, Simpfendorfer CA (2014) Influence of environmental factors on shark and ray movement, behaviour and habitat use: a review. Rev Fish Biol Fish:1–15. doi:10.1007/s11160-014-9364-8
Simpfendorfer C, Milward N (1993) Utilisation of a tropical bay as a nursery area by sharks of the families carcharhinidae and sphyrnidae. Environ Biol Fish 37:337–345. doi:10.1007/BF00005200
Simpfendorfer CA, Heupel MR, Hueter RE (2002) Estimation of short-term centers of activity from an array of omnidirectional hydrophones and its use in studying animal movements. Can J Fish Aquat Sci 59:23–32. doi:10.1139/f01-191
Spalding MD et al. (2007) Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience 57:573–583. doi:10.1641/b570707
Springer S (1967) Social organization of shark populations. In: Gilbert PW, Mathewson RW, Rall DP (eds) Sharks, skates and rays. John Hopkins Press, Baltimore, pp. 149–174
Taylor MD, Fairfax AV, Suthers IM (2013) The race for space: using acoustic telemetry to understand density-dependent emigration and habitat selection in a released predatory fish. Rev Fish Sci 21:276–285
Wetherbee BM, Gruber SH, Rosa RS (2007) Movement patterns of juvenile lemon sharks Negaprion brevirostris within atol das rocas, Brazil: a nursery characterized by tidal extremes. Mar Ecol Prog Ser 343:283–293
Yates P, Heupel M, Tobin A, Simpfendorfer C (2012) Diversity in young shark habitats provides the potential for portfolio effects. Mar Ecol Prog Ser 458:269–281. doi:10.3354/meps09759
Yates PM, Heupel MR, Tobin AJ, Moore SK, Simpfendorfer CA (2015) Diversity in immature-shark communities along a tropical coastline. Mar Freshw Res 66:399–410
Acknowledgments
We thank all the staff and students at the Centre for Sustainable Tropical Fisheries and Aquaculture including Merritt Adkins, Fernanda de Faria, Mario Espinoza, Madeline Green, Lauren Meyer, Jonathan Smart, and countless volunteers for their help and support of this project. We would also like to thank Vinay Udaywer for field and statistical assistance. Primary financial support came from the National Environmental Research Program awarded to MRH and CAS. All research was conducted in accordance with James Cook University (JCU) animal ethics permit A1566 and Great Barrier Reef (G11/346181.1) and QDAF (144482) permits for animal collection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Munroe, S.E.M., Simpfendorfer, C.A. & Heupel, M.R. Variation in blacktip shark movement patterns in a tropical coastal bay. Environ Biol Fish 99, 377–389 (2016). https://doi.org/10.1007/s10641-016-0480-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-016-0480-2