Abstract
The Arctic is facing major environmental changes impacting marine biodiversity and ecosystem functioning. One way of assessing the responses of an ecosystem to these changes is to quantitatively study food web dynamics. Here, we used stable isotope (δ15N and δ13C) analyses of 39 Arctic marine species to investigate trophic relationships and isotopic niches of the West Greenland food web in 2000–2004. The lowest δ15N values were found for suspension feeding blue mussel (Mytilus edulis; 6.1 ‰) and the highest for polar bear (Ursus maritimus; 20.2 ‰). For δ13C, copepods (Calanus spp.) had the lowest values (−20.4 ‰) and snow crab (Chionoecetes opilio) the highest values (−15.8 ‰). Our results show that the three trophic enrichment factor (TEF) approaches used to quantify species trophic positions (fixed TEF of 3.8 and 3.4 ‰ or scaled TEF) did not generally affect trophic modelling and provided similar conclusions. Overall, the findings in this study are in good agreement with previous investigations of other Arctic marine ecosystems. Interestingly, we found little overlap of core isotopic niches used by the four investigated functional groups (mammals, seabirds, fish and invertebrates), except for seabirds and fish where an overlap of 24 % was found. These results provide new insights into species and functional group interactions, as well as into the food web structure and ecosystem functioning of an important Arctic region that can be used as a template to guide future modelling of carbon, energy and contaminant flow in the region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Marine ecosystems are being affected by the combined effects of climate change (Beaugrand et al. 2002) and anthropogenic influences including fisheries (Halpern et al. 2008), non-native species invasions and release of contaminants (Wiese and Robertson 2004; Hooper et al. 2005). To get a thorough understanding of how an ecosystem reacts to these changes, information is needed on its ecology, functioning and mechanisms of trophic transfer (Thompson et al. 2012). One way of combining these approaches is to study the food web dynamics within a system (Thompson et al. 2012). However, there are major obstacles in deriving long-term information on food web dynamics in marine food webs using conventional approaches such as stomach content analysis (Votier et al. 2003; Polito et al. 2011). A now well-established alternative method to study the trophic structure and dynamics of a community is the application of stable isotope analysis, as stable carbon (δ13C) and nitrogen (δ15N) isotope ratios in tissues of animals provide information on their source of feeding and their relative trophic position, respectively (e.g. Hobson and Welch 1992; Boecklen et al. 2011; Middelburg 2014).
The marine ecosystem off the coast of West Greenland is one of the most biologically productive areas across the entire Arctic (Smidt 1979; Juul-Pedersen et al. 2015) and supports large populations of fish, seabirds and marine mammals (Born et al. 2003; Boertmann et al. 2004) as well as important spawning and nursery grounds, especially for northern shrimp (Pandalus borealis) and Greenlandic halibut (Reinhardtius hippoglossoides) (Buch et al. 2004; Simonsen et al. 2006). However, this region is undergoing major environmental changes. For instance, in West Greenland, as well as other parts of the Arctic, global warming has resulted in a reduction in sea ice thickness, distribution and seasonal duration (e.g. Comiso et al. 2008; Kwok and Rothrock 2009; Swart et al. 2015). Locally, the reduction in sea ice, as well as changes in the nutrient balance, has already changed the length and initiation of the growth season for primary producers and the distribution of fish, thereby fundamentally influencing the structure and functioning of the food web (Arrigo et al. 2008; Grebmeier et al. 2006; Wassmann et al. 2011).
Compared to other marine systems in the Arctic, the pelagic food web off West Greenland has been relatively well studied (e.g. Nielsen and Hansen 1995; Munk et al. 2003; Arendt et al. 2010; Kjellerup et al. 2015). However, substantial knowledge gaps remain, in particular on the trophic coupling to the higher trophic levels. The spring phytoplankton bloom develops immediately after the breakup of sea ice (Dünweber et al. 2010). Sea ice conditions are therefore important for the initiation of primary production in spring which, in turn, needs to be matched by the arrival of overwintering copepods of the genus Calanus, to get an efficient transfer of energy to higher trophic levels (Swalethorp et al. 2011; Møller et al. 2006, 2016). Current knowledge of marine shellfish and fish in West Greenland is based primarily on single species information (e.g. Møller et al. 2010; Hedeholm et al. 2012) and on research related to fisheries and environmental impact assessments related to hydrocarbon extraction and other anthropogenic activities (e.g. Bergström and Vilhjalmarsson 2007; ABA 2013). Seabird and marine mammal distribution and abundance have similarly been studied in relation to harvest management and planning of oil activities (e.g. Boertmann et al. 2013). However, in general, dietary and trophic information is currently fragmentary in West Greenland, complicating prediction on how species will respond to future environmental changes.
Since climate and anthropogenic changes can readily modify the ecosystem, it is of key importance to acquire baseline knowledge about the trophic structure of this marine ecosystem in order to provide a solid base for detection of the impacts of current and pending environmental change. Furthermore, understanding the ecological niche space occupied by species within an ecosystem, and the extent to which they overlap with other species or functional groups, is key for assessing their role in food web structure and energy transfer (McMeans et al. 2013a; Quillien et al. 2016). Here, we analysed isotopic data from key components of the West Greenland marine food web collected in 2000–2004 to provide a baseline against which current and future isotopic studies can be contrasted. Using these data, we describe the trophic relationships and isotopic niche space from invertebrate to marine mammals and compare our data with that available from other high-latitude marine food webs.
Materials and methods
Sample collections
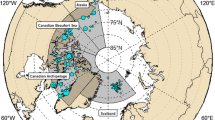
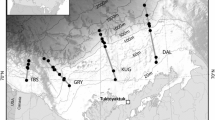
Thirty-nine species were analysed (Table 1). Invertebrates, fish and seabirds were sampled between 62°00′ and 69°30′N, whereas marine mammals were sampled up to 71°30′N (Fig. 1). Twenty-six of the 39 species were collected during July–October 2003 (northern summer/fall, Table 1). Deviations from this main sampling period by the following species were unavoidable due to seasonal migrations. Copepods (Calanus spp.), little auks (Alle alle), ringed seals (Phoca hispida), hooded seals (Cystophora cristata) and polar bear (Ursus maritimus) were sampled from March to June (northern spring/summer) in 2003 (polar bear in 2001), while the remaining three species of seabirds [king eider (Somateria spectabilis), common eider (Somateria molissima) and Brünnich’s guillemot (Uria lomvia)] were sampled during November–January (northern winter) 2003/2004, and three species of marine mammals [walrus (Odobenus rosmarus), beluga (Delphinapterus leucas) and narwhal (Monodon monoceros)] were sampled during November–January (northern winter) in 2000/2002.
Copepods were sampled from the research vessel R.V. Porsild (University of Copenhagen) at a permanent station located 1 nautical mile off Qeqertarsuaq (69°15′N, 53°33′W) (Hansen et al. 2012b) and collected in the upper 50 m of the water column using a WP-2 net (mesh size 200 µm). Samples were diluted in surface water in a 100-L thermo box and brought to the laboratory where the dominating Calanus species (Calanus hyperboreus, C. glacialis and C. finmarchicus) were carefully sorted and rinsed in filtered surface water before being transferred to a test tube and deep frozen (−28 °C).
Samples of snow crab were collected during a routine pot survey using squid as bait (Carl and Burmeister 2006). Samples of northern shrimp, northern krill (Meganyctiphanes norvegica), Boreoatlantic armhook squid (Gonatus fabricii), capelin (Mallotus villosus), polar cod (Boreogadus saida), sandeel (Ammodytes sp.), deepwater redfish (Sebastes mentella), golden redfish (Sebastes marinus), Greenland halibut, great silver smelt (Argentina silus), American plaice (Hippoglossoides platessoides), haddock (Melanogrammus aeglefinus), Atlantic cod, daubed shanny (Leptoclinus maculatus), Atlantic wolffish (Anarhichas lupus), spotted wolffish (Anarhichas minor) and shorthorn sculpin (Myoxocephalus scorpius) were collected by a 3000/20-mesh Skjervøy bottom trawl (Kanneworff and Wieland 2003). All samples taken were deep frozen (−50 °C) on board immediately after collection.
Samples of additional invertebrates [blue mussel (Mytilus edulis), Iceland scallop (Chlamys islandica)], fish [Atlantic salmon (Salmo salar)], seabirds [Brünnich’s guillemot, black-legged kittiwake (Rissa tridactyla), common eider, king eider, black guillemot (Cepphus grylle)] and marine mammals [harp seal (Pagophilus groenlandicus) and minke whale (Balaenoptera acutorostrata)] along with supplementary samples of spotted wolffish and Atlantic wolffish were collected from coastal Inuit subsistence catches. For marine mammals, tissue samples were deep frozen (−28 °C/− 50 °C) 6–24 h postmortem.
All necessary permits to collect samples from the different species of animals were obtained from the Ministry of Fisheries, Hunting and Agriculture (APNN) in Greenland.
Stable isotope analysis
Muscle samples from all species [except blue mussels, copepods and northern krill where the whole animal (minus shell) was used] were prepared for δ13C and δ15N analyses by the isotope laboratory at the Department of Soil Science, University of Saskatchewan, Saskatoon, Canada. Samples were washed in distilled water, freeze-dried, powdered and treated with a 2:1 chloroform–methanol solution to remove lipids. Our approach was to normalize sample measurements in this way in order to compare our data with other major isotopic food web studies conducted in the region (e.g. the North Water Polynya and the Canadian Arctic, Hobson et al. 1995, 2002a). We recognize that such lipid extractions while controlling for differential lipid content in order to make δ13C measurements more easily interpreted, can have small (0 ‰) to moderate (0.5 ‰) effects on tissue δ15N values (Søreide et al. 2006). However, we favoured our approach over the use of calibrations based on the elemental C:N ratios which have not been developed specifically for our food web of interest. Our approach thus provides key information on protein pathways and not necessarily on lipid pathways and should be interpreted as such. Samples were then dried under a fume hood. Additionally, zooplankton were soaked in 0.1 N HCl to remove carbonates and then dried without rinsing. Homogenized samples of 1 mg were loaded into tin cups and combusted at 1200 °C in a Robo-Prep elemental analyser. Resultant CO2 and N2 gases were then analysed using an interfaced Europa 20:20 continuous-flow isotope ratio mass spectrometer (CFIRMS), with every five samples separated by two laboratory standards (Bowhead whale baleen and egg albumen). Stable isotope abundances were expressed in the δ-notation as the deviation from standards in parts per thousand (‰) according to the following equation:
where X is 13C or 15N and R is the corresponding ratio 13C/12C or 15N/14N. The R standard values were based on the PeeDee Belemnite for 13C and atmospheric N2 for 15N. Replicate measurements of internal laboratory standards of similar matrix (Bowhead whale baleen and egg albumin) indicate within-run (n = 6) measurement errors of ±0.1 ‰ and ±0.3 ‰ for δ13C and δ15N values, respectively.
Estimations of trophic positions
Stable isotope analysis has, over the past 20 years, increasingly been used to study animal diets and trophic positions (see review by Boecklen et al. 2011). One very important aspect of using stable isotopes to describe the food web structure of a community is determining the diet–tissue discrimination factor (Δ15N) or trophic enrichment factor (TEF), namely the isotopic offset between a consumer tissues and its diet (Hobson and Clark 1992; Post 2002). It remains a challenge to choose the most appropriate TEF value for any given system, especially those involving a wide variety of trophic positions and taxa. A meta-analysis conducted by Post (2002) suggests that a TEF of 3.4 ‰ is a reasonable estimate (see also Minagawa and Wada 1984). However, for Arctic marine systems, modelling based on extensive food web isotopic assays suggests that a TEF of 3.8 ‰ is more appropriate (Hobson and Welch 1992; Hobson et al. 1994, 2002a, 2002b; Hobson and Bond 2012). More recently, the idea of varying TEF values (Olive et al. 2003; Olin et al. 2013) has resulted in the suggestion of using a scaled approach (Hussey et al. 2014a). Our primary motivation was to produce a dataset that could be readily compared with previous work (i.e. by using a TEF of 3.8 ‰). However, we also modelled our trophic positions based on the generalized TEF value of 3.4 ‰ and the scaled approach suggested by Hussey et al. (2014a).
Trophic positions (TP) using the generalized estimated TEF values were calculated according to the formula:
We assigned the copepod Calanus finmarchicus (average δ15N value 7.92 ‰ in our study area) to the second trophic level (i.e. TPprimary consumer = 2.0).
To apply the scaled trophic estimates we used the formula (see Hussey et al. 2014a, b for details):
where
Values of β 0 (5.92) and β 1 (−0.27) were taken from Hussey et al. (2014a, p. 243). Again we assigned copepod Calanus finmarchicus (average δ15N value 7.92 ‰ in our study area) to the second trophic level (i.e. TP = 2.0).
Niche width and overlap
The position of a species in the δ-space is mainly driven by resource use and foraging habitats. The isotopic niche width (‰2) was measured for each species by calculating the standard ellipse area SEA (40 %, SIAR default), corrected for small sample sizes (SEAC) following Jackson et al. (2011). The individual isotope niche width (SEAC) was calculated for all species except northern krill, great silver smelt, Greenland shark, walrus and polar bear due to too small sample sizes (Table 1). In addition, niche widths and (semi-quantitative) niche overlaps were calculated for the four functional groups (invertebrates, fish, seabirds and marine mammals). Niche overlap is given as the proportion of the overlapping area between the niches of groups A and B out of the summed niche area of A and B. All analyses were done using the package SIAR in R (R Development Core Team 2011).
Results
Stable isotopes
The lowest δ15N values sampled in our study were for the suspension feeding blue mussel (6.1 ± 0.5 ‰ SD), and the highest were for polar bear (20.2 ‰) (Table 1, Electronic Supplementary Material Figure S1). Groups of invertebrates, fish, seabirds and marine mammals had mean δ15N values ranging from 6.1 to 13.1 ‰, 10.0 to 17.0 ‰, 11.3 to 13.8 ‰ and 12.0 to 20.2 ‰, respectively (Table 1; Figs. 2, 3, 4, 5). Species having the lowest δ15N values generally also had the lowest δ13C values, except for marine mammals, where the opposite was the case. The lowest δ13C values were observed in Calanus finmarchicus (−20.4 ± 0.3 ‰ SD) and the highest in the snow crab (−15.8 ± 0.3 ‰ SD). Groups of invertebrates, fish, seabirds and marine mammals had mean δ13C values ranging from −20.4 to −15.8 ‰, −19.7 to −16.4 ‰, −19.5 to −17.3 ‰ and −19.5 to −16.8 ‰, respectively (Table 1; Electronic Supplementary Material Figure S1).
Stable isotope biplot illustrating the isotopic niche of the invertebrate community in West Greenland. Ellipses represent the isotopic niche width of 40 % (SIAR default) corrected for small sample size (Jackson et al. 2011)
Stable isotope biplot illustrating the isotopic niche of the fish community in West Greenland. Ellipses represent the isotopic niche width of 40 % (SIAR default) corrected for small sample size (Jackson et al. 2011)
Stable isotope biplot illustrating the isotopic niche of the seabird community in West Greenland. Ellipses represent the isotopic niche width of 40 % (SIAR default) corrected for small sample size (Jackson et al. 2011)
Stable isotope biplot illustrating the isotopic niche of the marine mammal (including shark) community in West Greenland. Ellipses represent the isotopic niche width of 40 % (SIAR default) corrected for small sample size (Jackson et al. 2011)
Food web length
With a TEF of 3.8 ‰ the mean TPs of all organisms ranged from 1.5 to 5.2 indicating a food web in West Greenland spanning 5 trophic levels (Table 1; Fig. 6). A TEF of 3.4 ‰ showed similar results with mean TPs of organisms ranging from 1.5 to 5.6, also indicating a food web of 5 trophic levels (Table 1). Using a scaled TEF mean TPs ranged from 1.6 to 8.6 indicating a food web with 8 trophic levels (Table 1).
Isotopic niche width and overlap
The isotopic niche width of all individual invertebrate species ranged from 0.3 ‰2 (Calanus glacialis and snow crab) to 2.5 ‰2 (Boreoatlantic armhook squid), with an overall width for the group of 11.4 ‰2. The niche width of the individual fish species ranged from 0.2 ‰2 (Greenland halibut – small) to 3.2 ‰2 (Atlantic wolffish) with a group niche width of 4.4 ‰2. The individual seabird species ranged in niche width from 0.4 ‰2 (king eider) to 1.3 ‰2 (common eider) and an overall group width of 3.4 ‰2. The individual marine mammal species ranged in isotopic niche width from 0.3 ‰2 (ringed seal and narwhal) to 2.0 ‰2 (minke whale) and had a group size of 3.3 ‰2.
We found the core isotopic niche areas used by seabirds and fish to have an overlap of 24.3 % (Table 2; Fig. 7). Seabird and invertebrate niches had an overlap of 9.9 % and fish and invertebrate niches an overlap of 3.0 % (Table 2; Fig. 7). The isotopic niche area of marine mammals and fish had an overlap of 1.2 % (Table 2; Fig. 7), whereas no overlap was found between the core niche areas of marine mammals and invertebrates. However, looking exclusively at the Boreoatlantic armhook squid, where the wide niche width is driven by high δ15N values, we found the total niche area of squid and marine mammals to have an overlap of 10.3 %.
Stable isotope biplot illustrating the isotopic niche of the four functional groups (invertebrates, fish, seabirds and marine mammals) in West Greenland. Ellipses represent the isotopic niche width of 40 % (SIAR default) corrected for small sample size (SEAc; Jackson et al. 2011)
Discussion
Our isotopic analysis of the marine food web of West Greenland has provided a trophic model describing relationships among diverse organisms from herbivorous zooplankton to polar bears. Our work based on a TEF of 3.8 ‰ contributes to the circumpolar understanding by allowing direct comparisons with models developed for other Arctic regions such as the North Water Polynya (Hobson et al. 2002a), North East Water Polynya (Hobson et al. 1995), Lancaster Sound (Hobson and Welch 1992) and the sub-Arctic Gulf of Alaska (Hobson et al. 1997). We acknowledge that our choice of TEF can clearly influence our interpretations of food web structure and so also applied two other approaches. However, we found that a trophic model using a TEF of 3.4 ‰ and the scaling approach advocated by Hussey et al. (2014a) did not result in substantial differences in our trophic estimates with the exception of marine mammals. Polar bear, especially, where the scaling approach estimated this species at a TP of 8.6, while this value was 5.2 and 5.6 with TEF 3.8 ‰ and 3.4 ‰, respectively, seems to be clearly overestimated. However, considering we only had one polar bear sample, this needs further investigation. To a lesser degree, we also found the scaling approach provided higher TP estimates for ringed seal (range between TEF 3.8 ‰ and scaling method: 4.0–4.4), beluga (4.1–4.7), narwhal (4.2–4.8) and hooded seal (4.8–5.3). With these exceptions noted, our study confirmed an expected 5–5.5 trophic-level system with considerable overlap between isotopic niches of species at each level, as illustrated from other Arctic areas, such as the North East Water Polynya (Hobson et al. 1995) and Lancaster Sound (Hobson and Welch 1992). While we fully recognize that the isotopic niche is not necessarily equivalent to the ecological niche, the derivation of isotopic niches can greatly assist in understanding food web structure and function (Newsone et al. 2007).
The relationships described here can now be used to examine the existing structure within the food web and serve as a template and baseline with which to compare with other Arctic marine food webs and monitor the effects of environmental changes underway in this and other regions (e.g. Vizzini and Mazzola 2004). We advocate the continued use of a generalized TEF value of 3.8 ‰ because this makes for direct comparisons with other high Arctic marine food webs. However, we recognize that for monitoring purposes, consistency is required and encourage researchers to continually strive to improve TP estimates based on the stable isotope approach.
West Greenland is continuously experiencing major environmental challenges which affect the trophic ecology of organisms (diet and habitat) and could thereby impact the whole marine food web structure. For instance, Greenland has experienced a warming of the sea along its west coast and a subsequent regime shift in the fish community in the marine system in the 1920s and 1930s (Drinkwater 2006). The shift resulted in the retreat of cold-water fish species to the north and at the same time in an expansion of boreal species (most noticeably the Atlantic cod). Following this warm period, the last three decades of the 1900s experienced a series of anomalous cold years (Buch et al. 2004; Drinkwater 2004; Stein 2004, 2005) before a new regime shift during 1997 in Disko Bay. This latter resulted in a noticeable change in water characteristics and a change to a general warming trend in this century (Hansen et al. 2012b; Cappelen and Vinther 2014). Accordingly, a change in the diet of Greenland halibut has been observed during the period 1978–2003 with increasing importance of invertebrates (Dwyer et al. 2010). Similarly, Falk and Durinck (1993) studied stomach contents in Brünnich’s guillemots during the winter 1988–89 and found that they fed almost exclusively on fish from October to December. Brünnich’s guillemots are opportunistic feeders favouring fish when readily available. A TP of 3.1 for Brünnich’s guillemots sampled in November 2003 in this study suggests a diet mainly consisting of zooplankton in the preceding month. This contrasts to the study from 1988 to 1989, possibly indicating a change in the availability of fish. Conversely, little auks sampled for this study had a TP of 3.0 and a similar TP value was found for little auks wintering off Nuuk in 2007 (Fort et al. 2010a), suggesting that the base of the food web had not changed considerably between 2003 and 2007.
Invertebrates
The nine species of invertebrates analysed from West Greenland showed a wide range of both δ15N and δ13C values, reflected by a large isotopic niche width for this group in comparison with fish, seabirds and marine mammals. This result confirms that invertebrates show a wide range of trophic ecologies, occupying various habitats and feeding at several trophic levels. Furthermore, the calculated 40 % isotopic niches for the different species of invertebrates did not overlap, indicating a group mostly made up of specialists segregating in their environment. However, we need to acknowledge that an absence of overlap between species in this group could also be a result of a lack of species sampled. Values of δ15N for copepods ranged from 7.9 ‰ (TP 2) for Calanus finmarchicus to 9.2 ‰ for C. glacialis, suggesting that C. finmarchicus were primarily herbivorous during the sampling period and supporting findings by Stevens et al. (2004) and Campbell et al. (2009). This result also supports our choice of this species as representative of a primary herbivore at TP 2.
Being filter feeders, blue mussels play an important role in harvesting the plankton and seston in the water column, and thus we would have expected blue mussels to occupy the same trophic level as C. finmarchicus and Iceland scallop (Chlamys islandica) (i.e. at TP 2). Our model with a TEF of 3.8 ‰ (as well as a TEF of 3.4 ‰ and the scaled TEF), however, produced a TP of 1.5 (1.5 and 1.6, respectively) for blue mussel, which is below that expected for a filter feeder. Our model did not consider organisms that void nitrogenous wastes in different ways and could therefore be inappropriate for these organisms. As reviewed by Vanderklift and Ponsard (2003), molluscs and detritivores show the lowest diet–tissue isotopic discrimination for δ15N. This is related to their excretion of ammonia. Such lower discrimination would result in lower tissue δ15N values and hence lower TP estimates. Future refinements of marine TP models using δ15N values should consider the form of nitrogenous excretion and the relative dependence of some organisms on detritus (Vanderklift and Ponsard 2003).
As expected, the predator Boreoatlantic armhook squid foraged at the highest trophic level among invertebrates. For some individuals, these levels reached those occupied by some piscivorous marine mammals. In that respect, we found the total niche area of squids and marine mammals to have an overlap of 10.3 %. All squid individuals sampled were larger than 10 cm, which corresponds to an age of 1 year or older (Kristensen 1984). As with other predators, the diet of Boreoatlantic armhook squids has been found to change with size, with larger individuals having a more piscivorous diet (Wiborg 1980; Kristensen 1984). We did not have accurate information on individual size, but the large range in δ15N values (10.7–16.5 ‰) could be explained by large difference in individual size. Future studies should aim at collecting more species/age specific details in order for us to improve our understanding of interactions at lower trophic levels.
Fish
The fish community in West Greenland is characterized by demersal species. These include Greenland halibut, Atlantic and spotted wolffish, deepwater and golden redfish in the deeper waters, whereas, on the banks, sandeel is more prominent (Pedersen and Kanneworff 1995). Based on the δ13C data, the demersal species did not exclusively feed on benthic organisms, except for the two most 13C-enriched species of wolffish and shorthorn sculpin (Pedersen and Zeller 2001, Fig. 3; Table 1). As discussed for Boreoatlantic armhook squids, isotope results for Greenland halibut, polar cod and Atlantic cod showed these species to change their diet with increasing size from crustaceans towards a higher proportion of fish (Fig. 3, Pedersen and Riget 1993; Hovde et al. 2002; Ramsvatn and Pedersen 2012).
Sharks play an important role in food webs as apex predators by interacting at several trophic positions (Ferretti et al. 2010). The Greenland shark in this study had a TP of 4.4. This position is similar to findings by McMeans et al. (2013b) which revealed that Atlantic cod make up a large proportion of the diet of Greenland sharks from Kongsfjorden (Svalbard). Stable isotope results from our study (Fig. 3; Table 1) suggest that this is likely the case in West Greenland as well.
The sixteen species of fish analysed also showed a wide range of both δ15N and δ13C values. However, the core isotopic niche width for the group was considerably smaller than that of invertebrates and similar to that of marine mammals and seabirds, indicating a lower diversity of habitats and feeding ecologies. In contrast to the invertebrate group, all species except sandeel (and possibly great silver smelt) overlapped in their isotopic niche area with other species. This indicates a group consisting of many generalists and confirms a highly productive West Greenland ecosystem. Indeed, a higher degree of niche segregation would have been expected in a nutrient poor system to reduce competition between species. Such information is essential as it demonstrates that environmental change, if modifying specific habitats or specific prey species like Calanus copepods, could highly impact higher trophic levels and the entire food chain.
Seabirds
Large numbers of common eiders, king eiders, Brünnich’s guillemots, black guillemots and little auks winter in the open water of the West Greenland ecosystem (Merkel et al. 2002; Boertmann et al. 2004, 2013) where species were sampled in this study. The seabird community sampled showed a high degree of grouping in their isotopic niche areas (Fig. 4).
King and common eiders grouped together in their isotopic niches. They both occupied a TP of 2.9 and the most benthic position compared to the four other seabird species in West Greenland. Given the low TP found for mussels in this study, the diet of the eiders suggests a mixed diet such as polychaetes, echinoderms and crustaceans as found by Merkel et al. (2007a; 2007b) in Southwest Greenland and in West Iceland by Kristjansson et al. (2013). This is also supported by the isotopic niche area for common eider, which was the largest in the seabird group.
Little auks, Brünnich’s guillemots and black-legged kittiwakes all overlapped in their isotopic niche area, but with kittiwakes having a broader diet (according to the isotopic niche width). The little auk feeds predominantly on copepods during spring and summer (Stempniewicz 2001), but has, based on stomach analyses, been found to feed predominantly on krill during winter in Southwest Greenland (Rosing-Asvid et al. 2013), while isotopic analysis in another study suggested that copepods were still important during winter (Fort et al. 2010b). Little auks in our study were collected in March, during the copepod feeding phase, and our estimate of a TP of 3.0 supports this expectation. Similar results have been found in the North Water Polynya (TP 3.2, Hobson et al. 2002a) and in West Greenland (TP 3.2 using Eq. 3, Fort et al. 2010a). A TP of 3.1 found for Brünnich’s guillemots (November) and black-legged kittiwakes (September) in contrast to the higher values found in the North Water Polynya (Hobson et al. 2002a; Karnovsky et al. 2008), suggests a mixed diet of fish and crustaceans with more fish in the North Water Polynya.
The diet of black guillemots generally consists of a variety of fish and invertebrates (predominantly benthic species, Ewins 1990; Byers et al. 2010). The TP of 3.5, and the fact that the isotopic niche area of black guillemots did not overlap with any of the other species, is in agreement with a diet consisting of a higher percentage of fish than the other birds collected in this study. However, the δ13C data indicated little use of benthic fish species in West Greenland. This is in accordance with the winter and spring occurrence of black guillemots dispersed in the offshore pack ice (Mosbech and Johnson 1999) feeding mainly on polar cod (Bradstreet and Brown 1985).
Marine mammals
Due to the high productivity of West Greenland fjords, banks and shelves (Smidt 1979; Juul-Pedersen et al. 2015), this region is an important feeding area for marine mammals with seasonal occurrence of high-quality food patches (Heide-Jørgensen and Teilmann 1994). In addition to polar bear and walrus, at least 14 species of whale and five species of seals occur in the area (Boertmann et al. 2013). In this study we only sampled three whale species; thus, this group is highly underrepresented. This could possibly be an explanation for why we measured a relatively small isotopic niche width for the group and only a small or no overlap with the fish and seabird groups. Only narwhal and beluga overlapped in their isotopic niche area, but, in general, we expect that greater dietary overlap exist among marine mammals excluding polar bears and encourage more extensive sampling of this group.
Minke whale, harp seal and walrus were found to occupy the lowest TP in the marine mammal group. Capelin and sandeel have been found to be important food items for minke whales (Larsen and Kapel 1981) and harp seals (Kapel and Angantyr 1991; Boertmann et al. 2013) in West Greenland waters. However, a TP of 3.5 for harp seals and 3.1 for minke whales suggests a mixed diet of small schooling fish and crustaceans, also found by Boertmann et al. (2013). A TP of 3.6 is higher than expected for walruses if their sole diet was herbivorous clams but could be explained by walruses occasionally feeding on vertebrates like seals, birds and fish (Muir et al. 1995, Born 1997).
Ringed seals TP (4.0) was in good agreement with prior estimates of diet consisting of polar cod, Arctic cod, Liparis spp. and amphipods (Siegstad et al. 1998). Beluga (TP = 4.1) and narwhals (TP = 4.2) were also in good agreement as they are known to predominantly feed on fish (polar cod and Greenland halibut, respectively). Squid and shrimp have been found to contribute to their diet (Heide-Jørgensen and Teilmann 1994; Laidre and Heide-Jørgensen 2005); however, during the sampling period of this study fish appear to be the main prey items. Hooded seals occupied a TP of 4.4 also suggesting forage fish to be the main diet of the sampled individuals. A previous study analysing stomach contents indicated that capelin was the most important prey (ca. 93 % by weight) for hooded seals in Southwest Greenland (Kapel 2000). Our data support a diet consisting of capelin and/or other forage fish.
Implications and future perspectives
Our study, to our knowledge, is the first extensive investigation of the West Greenland marine food web structure. In this respect, our model is important in assisting future studies of the effects of climatic and anthropogenic stressors on West Greenland marine ecosystems, potentially causing changes in the structure of marine food webs. Nonetheless, future and expanded studies focused on additional species should provide complementary data and allow long-term investigations of modifications of Arctic marine food web structure under rapid environmental change. Moreover, it is well recognized that baseline isotopic values for primary production can vary spatially and seasonally in isotopic signals (e.g. Hansen et al. 2012a) and should be taken into account, or the variance in resulting trophic estimates modelled. To some degree, this can be accomplished by investigating a variety of tissues representing different periods of dietary integration (Hobson 1993; Hobson et al. 2002a; Hobson and Bond 2012). We recommend a generalized approach where more species and especially those representing lower trophic levels (i.e. invertebrates) are included, as they play a key role in transferring energy from primary producers to upper-level predators in marine ecosystems (McMeans et al. 2013a). An example is the amphipod Themisto libellula that, through SI modelling, is considered a keystone in the North Water Polynya high Arctic system (Hobson et al. 2002a). Likewise, organisms involved in the microbial loop (unicellular grazers such as ciliates and dinoflagellates) are potential key species in marine Arctic ecosystems (Levinsen and Nielsen 2002) and should therefore be considered in future investigations. In addition, we recommend carefully designed collection schemes where standard biological data (i.e. total length, weight, age, sexual status) are recorded in order to allow for a full interpretation of species stage-dependent diet within and between regions.
References
ABA (2013) Arctic biodiversity assessment—Status and trends in Arctic biodiversity: synthesis. Arctic Council CAFF—Conservation of Arctic flora and fauna
Arendt KE, Nielsen TG, Rysgaard S, Tonnesson K (2010) Differences in plankton community structure along the Godthabsfjord, from the Greenland Ice Sheet to offshore waters. Mar Ecol Prog Ser 401:49–62
Arrigo KR, van Dijken G, Pabi S (2008) Impact of a shrinking Arctic ice cover on marine primary production. Geophys Res Lett 35:L19603
Beaugrand G, Reid PC, Ibanez F, Lindley JA, Edwards M (2002) Reorganization of North Atlantic marine copepod biodiversity and climate. Science 296:1692–1694
Bergström B, Vilhjalmarsson H (2007) Cruise report and preliminary results of the acoustic/pelagic trawl survey off West Greenland for capelin and polar cod 2005. Pinngortitalerriffik, Greenland Institute of Natural Resources, Nuuk. Technical Report No. 6
Boecklen WJ, Yarnes CT, Cook BA, James AC (2011) On the use of stable isotopes in trophic ecology. Annu Rev Ecol Evol Syst 42:411–440
Boertmann D, Lyngs P, Merkel FR, Mosbech A (2004) The significance of Southwest Greenland as winter quarters for seabirds. Bird Conserv Int 14:87–112
Boertmann D, Mosbech A, Schiedek D, Dünweber M (2013) Disko West. A strategic environmental impact assessment of hydrocarbon activities. Scientific Report from DCE—Danish Centre for Environment and Energy No. 71. Aarhus University
Born EW (1997) Historical and present status of the Atlantic walrus (Odobenus rosmarus rosmarus) in eastern Greenland. Meddelelser om Grønland. Bioscience 46:1–73
Born EW, Outridge P, Riget FF, Hobson KA, Dietz R, Oien N, Haug T (2003) Population substructure of North Atlantic minke whales (Balaenoptera acutorostrata) inferred from regional variation of elemental and stable isotopic signatures in tissues. J Mar Syst 43:1–17
Bradstreet MSW, Brown RG (1985) Feeding ecology of the Atlantic Alcidae. In: Nettleship DN (ed) The Atlantic Alcidae. Academic Press, London, pp 263–318
Buch E, Pedersen SA, Ribergaard MH (2004) Ecosystem variability in West Greenland waters. J Northwest Atl Fish Sci 34:13–28
Byers T, Smith A, Mallory ML (2010) Diet of black guillemots and northern fulmars breeding beside a high Arctic polynya. Polar Biol 33:457–467
Campbell RG, Sherr EB, Ashjian CJ, Plourde S, Sherr BF, Hill V, Stockwell DA (2009) Mesozooplankton prey preference and grazing impact in the western Arctic Ocean. Deep Sea Res Part II 56:1274–1289
Cappelen J, Vinther BM (2014) SW Greenland temperature data 1784–2013. Technical Report 14–06, Danish Meteorological Institute, Copenhagen
Carl J, Burmeister A (2006) Assessment of snow crab in West Greenland. Greenland Institute of Natural Resources, Internal report, Nuuk
Comiso J, Parkinson C, Gersten R, Stock L (2008) Accelerated decline in the Arctic sea ice cover. Geophys Res Lett 35:L01703
Development Core Team R (2011) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Drinkwater KF (2004) Atmospheric and sea-ice conditions in the Northwest Atlantic during the decade, 1991–2000. J Northwest Atl Fish Sci 34:1–11
Drinkwater KF (2006) The regime shift of the 1920s and 1930s in the North Atlantic. Prog Oceanogr 68:134–151
Dünweber M, Swalethorp R, Kjellerup S, Nielsen TG, Møller EF, Hjort M, Arendt K, Tönnesson K (2010) Fate of the spring diatom bloom in Disko Bay, western Greenland. Mar Ecol Prog Ser 419:11–29
Dwyer KS, Buren A, Koen-Alonso M (2010) Greenland halibut diet in the Northwest Atlantic from 1978 to 2003 as an indicator of ecosystem change. J Sea Res 64:436–445
Ewins PJ (1990) The diet of black guillemots Cepphus grylle in Shetland. Holarctic Ecol 13:90–97
Falk K, Durinck J (1993) The winter diet of thick-billed murres, Uria lomvia, in Western Greenland, 1988–1989. Can J Zool 71:264–272
Ferretti F, Worm B, Britten GL, Heithaus MR, Lotze HK (2010) Patterns and ecosystem consequences of shark declines in the ocean. Ecol Lett 13:1055–1071
Fort J, Cherel Y, Harding AMA, Egevang C, Steen H, Kuntz G, Porter WP, Gremillet D (2010a) The feeding ecology of little auks raises questions about winter zooplankton stocks in North Atlantic surface waters. Biol Lett 6:682–684
Fort J, Cherel Y, Harding AMA, Welcker J, Jakubas D, Steen H, Karnovsky NJ, Gremillet D (2010b) Geographic and seasonal variability in the isotopic niche of little auks. Mar Ecol Prog Ser 414:293–302
Grebmeier JM, Overland JE, Moore SE, Farley EV, Carmack EC, Cooper LW, Frey KE, Helle JH, McLaughlin FA, McNutt SL (2006) A major ecosystem shift in Northern Bering Sea. Science 311:1461–1464
Halpern BS, Walbridge S, Selkoe KA, Kappel CV, Micheli F, D’Agrosa C, Bruno JF, Casey KS, Ebert C, Fox HE, Fujita R, Heinemann D, Lenihan HS, Madin EMP, Perry MT, Selig ER, Spalding M, Steneck R, Watson R (2008) A global map of human impact on marine ecosystems. Science 319:948–952
Hansen JH, Hedeholm RB, Sünksen K, Christensen JT, Grønkjær P (2012a) Assessment of carbon (δ13C) and nitrogen (δ 15N) in arctic marine food webs at several spatial scales. Mar Ecol Prog Ser 467:47–59
Hansen MO, Nielsen TG, Stedmon CA, Munk P (2012b) Oceanographic regime shift during 1997 in Disko Bay, Western Greenland. Limnol Oceanogr 57:634–644
Hedeholm R, Gronkjaer P, Rysgaard S (2012) Feeding ecology of capelin (Mallotus villosus Muller) in West Greenland waters. Polar Biol 35:1533–1543
Heide-Jørgensen MP, Teilmann J (1994) Growth, reproduction, age structure and feeding habits of white whales (Delphinapterus leucas) in West Greenland waters. Meddelelser om Grønland. Bioscience 39:195–212
Hobson KA (1993) Trophic relationships among high Arctic seabirds: insights from tissue-dependent stable-isotope models. Mar Ecol Prog Ser 95:7–18
Hobson KA, Bond AL (2012) Extending an indicator: year-round information on seabird trophic ecology from multiple-tissue stable-isotope analyses. Mar Ecol Prog Ser 461:233–243
Hobson KA, Clark RG (1992) Assessing avian diets using stable isotopes II: factors influencing diet-tissue fractionation. Condor 94:189–197
Hobson KA, Welch HE (1992) Determination of trophic relationships within a high Arctic marine food web using δ13C and δ15N analysis. Mar Ecol Prog Ser 84:9–18
Hobson KA, Piatt JF, Pitocchelli J (1994) Using stable isotopes to determine seabird trophic relationships. J Anim Ecol 63:786–798
Hobson KA, Ambrose WG, Renaud PE (1995) Sources of primary production, benthic-pelagic coupling, and trophic relationships within the Northeast Water Polynya: insights from δ13C and δ15N analysis. Mar Ecol Prog Ser 128:1–10
Hobson KA, Sease JL, Merrick RL, Piatt JF (1997) Investigating trophic relationships of pinnipeds in Alaska and Washington using stable isotope ratios of nitrogen and carbon. Mar Mammal Sci 13:114–132
Hobson KA, Fisk A, Karnovsky N, Holst M, Gagnon JM, Fortier M (2002a) A stable isotope (δ13C, δ15N) model for the north water food web: implications for evaluating trophodynamics and the flow of energy and contaminants. Deep Sea Res Part II 49:5131–5150
Hobson KA, Gilchrist G, Falk K (2002b) Isotopic investigations of seabirds of the North Water Polynya: contrasting trophic relationships between the eastern and western sectors. Condor 104:1–11
Hooper DU, Chapin FS III, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton JH, Lodge DM, Loreu M, Naeem S, Schmid B, Setälä H, Symstad AJ, Vandermeer J, Wardle DA (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecoll Monogr. 75(1):3–35
Hovde SC, Albert OT, Nilssen EM (2002) Spatial, seasonal and ontogenetic variation in diet of Northeast Arctic Greenland halibut (Reinhardtius hippoglossoides). ICES J Mar Sci 59:421–437
Hussey NE, MacNeil MA, McMeans BC, Olin JA, Dudley SFJ, Cliff G, Wintner SP, Fennessy ST, Fisk AT (2014a) Rescaling the trophic structure of marine food webs. Ecol Lett 17:239–250
Hussey NE, MacNeil MA, McMeans BC, Olin JA, Dudley SFJ, Cliff G, Wintner SP, Fennessy ST, Fisk AT (2014b) Corrigendum to Hussey et al. (2014). Ecol Lett 17:768
Jackson AL, Inger R, Parnell AC, Bearhop S (2011) Comparing isotopic niche widths among and within communities: SIBER—Stable Isotope Bayesian Ellipses in R. J Anim Ecol 80:595–602
Juul-Pedersen T, Arendt KE, Mortensen J, Blicher ME, Søgaard DH, Rysgaard S (2015) Seasonal and interannual phytoplankton production in a sub-Arctic tidewater outlet glacier fjord, SW Greenland. Mar Ecol Prog Ser 524:27–38
Kanneworff P, Wieland K (2003) Stratified-random trawl survey for Northern Shrimp (Pandalus borealis) in NAFO Subareas 0+1 in 2003. NAFO SCR Doc. 03/71, Serial No. N4910
Kapel FO (2000) Feeding habits of harp and hooded seals in Greenland waters, vol 2. NAMMCO scientific publications, pp 50–64
Kapel FO, Angantyr LA (1991) Grønlandssælernes fødevalg ved Vestgrønland: teknisk rapport. Grønlands Fiskeriundersøgelser
Karnovsky NJ et al (2008) Seasonal changes in diets of seabirds in the North Water Polynya: a multiple-indicator approach. Mar Ecol Prog Ser 357:291–299
Kjellerup S, Dünweber M, Møller EF, Schiedek D, Oskarsson GJ, Rigét F, Johansen KL, Mosbech A (2015) Vertical and horizontal distribution of zooplankton and polar cod in southern Baffin Bay (66–71ºN) in September 2009. Polar Biol 38(5):699–718
Kristensen TK (1984) Biology of the squid Gonatus fabricii (Lichtenstein, 1818) from West Greenland waters, Meddelelser om Grønland. Bioscience 13:1–20
Kristjansson TO, Jonsson JE, Svavarsson J (2013) Spring diet of common eiders (Somateria mollissima) in Breidafjordur, West Iceland, indicates non-bivalve preferences. Polar Biol 36:51–59
Kwok R, Rothrock DA (2009) Decline in Arctic sea ice thickness from submarine from submarine and ICESat records: 1958–2008. Geophys Res Lett 36:L15501
Laidre KL, Heide-Jørgensen MP (2005) Winter feeding intensity of narwhals (Monodon monoceros). Mar Mammal Sci 21:45–57
Larsen F, Kapel FO (1981) Collection of biological material of minke whales in West Greenland, 1979. Rep Int Whal Comm 31:279–285
Levinsen H, Nielsen TG (2002) The trophic role of marine pelagic ciliates and heterotrophic dinoflagellates in arctic and temperate coastal ecosystems: a cross-latitude comparison. Limnol Oceanogr 47:427–439
McMeans BC, Rooney N, Arts MT, Fisk AT (2013a) Food web structure of a costal Arctic marine ecosystem and implications for stability. Mar Ecol Prog Ser 482:17–28
McMeans BC, Arts MT, Lydersen C, Kovacs KM, Hop H, Falk-Petersen S, Fisk AT (2013b) The role of Greenland sharks (Somniosus microcephalus) in an Arctic ecosystem: assessed via stable isotopes and fatty acids. Mar Biol 160:1223–1238
Merkel FR, Mosbech A, Boertmann D, Grøndahl L (2002) Winter seabird distribution and abundance off south-western Greenland, 1999. Polar Res 21:17–36
Merkel FR, Jamieson SE, Falk K, Mosbech A (2007a) The diet of common eiders wintering in Nuuk, Southwest Greenland. Polar Biol 30:227–234
Merkel FR, Mosbech A, Jamieson SE, Falk K (2007b) The diet of king eiders wintering in Nuuk, Southwest Greenland, with reference to sympatric wintering common eiders. Polar Biol 30:1593–1597
Middelburg JJ (2014) Stable isotopes dissect aquatic food webs from the top to the bottom. Biogeosciences 11:2357–2371
Minagawa M, Wada E (1984) Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal range. Geochim Cosmochim Acta 48:1135–1140
Møller EF, Nielsen TG, Richardson K (2006) The zooplankton community in the Greenland Sea: composition and role in carbon turnover. Deep Sea Res Part I 53:76–93
Møller PR, Nielsen JG, Knudsen SW, Poulsen JY, Sunksen K, Jorgensen OA (2010) A checklist of the fish fauna of Greenland waters. Zootaxa 2378:1–84
Møller EF, Bohr M, Kjellerup S, Maae M, Møhl M, Swalethorp R (2016) Nielsen TG (2016) Calanus finmarchicus egg production at its northern border. J Plankton Res 00(00):1–9
Mosbech A, Johnson SR (1999) Late winter distribution and abundance of sea-associated birds in South-Western Greenland, the Davis Strait and southern Baffin Bay. Polar Res 18(1):1–17
Muir DG, Segstro MD, Hobson KA, Ford CA, Stewart REA, Olpinski S (1995) Can seal eating explain elevated levels of PCBs and organochlorine pesticides in walrus blubber from eastern Hudson Bay (Canada)? Environ Pollut 88:1–14
Munk P, Hansen BW, Nielsen TG, Thomsen HA (2003) Changes in plankton and fish larvae communities across hydrographic fronts off West Greenland. J Plankton Res 25:815–830
Newsone SD, Martinez del Rio C, Bearhop S, Phillips DL (2007) A niche for isotopic ecology. Front Ecol Environ 5:429–436
Nielsen TG, Hansen B (1995) Plankton community structure and carbon cycling on the western coast of Greenland during and after the sedimentation of a diatom bloom. Mar Ecol Prog Ser 125:239–257
Olin JA, Hussey NE, Grgicak-Mannion A, Fritts MW, Wintner SP, Fisk AT (2013) Variable δ15N diet-tissue discrimination factors among sharks: implications for trophic position, diet and food web models. PLoS One 8(10):e77567
Olive PJW, Pinnegar JK, Polunin NVC, Richards G, Welch R (2003) Isotope trophic-step fractionation: a dynamic equilibrium model. J Anim Ecol 72:608–617
Pedersen SA, Kanneworff P (1995) Fish on the West Greenland shrimp grounds, 1988–1992. ICES J Mar Sci 52:165–182
Pedersen SA, Riget F (1993) Feeding habits of redfish (Sebastes Spp) and Greenland halibut (Reinhardtius hippoglossoides) in West Greenland waters. ICES J Mar Sci 50:445–459
Pedersen SA, Zeller D (2001) A mass balance model for the West Greenland marine ecosystem. In: Guenette S, Christensen V, Pauly D (eds) Fisheries impacts on North Atlantic ecosystems: models and analyses. Fisheries Centre Research Reports 9(4)
Polito MJ, Trivelpiece WZ, Karnovsky NJ, Ng E, Patterson WP, Emslie SD (2011) Integrating stomach content and stable isotope analyses to quantify the diets of Pygoscelid penguins. PLoS One 6:e26642
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83(3):703–718
Quillien N, Nordström MC, Schaal G, Bonsdorff E, Grall J (2016) Opportunistic basal resource simplifies food web structure and functioning of a highly dynamic marine environment. J Exp Mar Biol Ecol 477:92–102
Ramsvatn S, Pedersen T (2012) Ontogenetic niche changes in haddock Melanogrammus aeglefinus reflected by stable isotope signatures, δ13C and δ15N. Mar Ecol Prog Ser 451:175–185
Rosing-Asvid A, Hedeholm R, Arendt KE, Fort J, Robertson GJ (2013) Winter diet of the little auk (Alle alle) in the Northwest Atlantic. Polar Biol 36:1601–1608
Siegstad H, Neve PB, Heide-Jørgensen MP, Härkönen T (1998) Diet of ringed seal (Phoca hispida) in Greenland. In: Heide-Jørgensen MP, Lydersen C (eds) Ringed seals in the North Atlantic, vol 1. NAMMCO Scientific Publications, pp 229–241
Simonsen CS, Munk P, Folkvord A, Pedersen SA (2006) Feeding ecology of Greenland halibut and sandeel larvae off West Greenland. Mar Biol 149:937–952
Smidt ELB (1979) Annual cycles of primary production and of zooplankton at Southwest Greenland—Meddelelser om Grønland. Bioscience (Geoscience, Man and Society) 1:57
Søreide JE, Tamelander T, Hop H, Hobson KA, Johansen I (2006) Sample preparation effects on stable C and N isotope values: a comparison of methods in Arctic marine food web studies. Mar Ecol Prog Ser 328:17–28
Stein M (2004) Climatic conditions around Greenland—2004. NAFO SCR Doc, No. 2, Serial No. N5075
Stein M (2005) North Atlantic subpolar gyre warming—impacts on Greenland offshore waters. J Northwest Atl Fish Sci 36:43–54
Stempniewicz L (2001) Little auk Alle alle. BWP update. J Birds Western Palearct 3:175–516
Stevens CJ, Deibel D, Parrish CC (2004) Copepod omnivory in the North Water Polynya (Baffin Bay) during autumn: spatial patterns in lipid composition. Deep Sea Res Part I 51:1637–1658
Swalethorp R, Kjellerup S, Dünweber M, Nielsen TG, Møller EF, Rysgaard S, Hansen BW (2011) Production of Calanus finmarchicus, C. glacialis and C. hyperboreus in Disko Bay, western Greenland, with emphasis on life strategy. Mar Ecol Prog Ser 429:125–144
Swart NC, Fyfe JC, Hawkins E, Kay JE, Jahn A (2015) Influence of internal variability on Arctic sea-ice trends. Nature Clim Change 5:86–89
Thompson RM, Brose U, Dunne JA, Hall ROJ, Hladyz S, Kitching RL, Martinez ND, Rantala H, Romanuk TN, Stouffer DB, Tylianakis JM (2012) Food webs: reconciling the structure and function of biodiversity. Trends Ecol Evol 27:689–697
Vanderklift MA, Ponsard S (2003) Sources of variation in consumer-diet δ15N enrichment: a meta-analysis. Oecologia 136:169–182
Vizzini S, Mazzola A (2004) Stable isotope evidence for the environmental impact of a land-based fish farm in the Western Mediterranean. Mar Pollut Bull 49(1–2):61–70
Votier S, Bearhop S, MacCormick A, Ratcliffe N, Furness R (2003) Assessing the diet of great skuas, Catharacta skua, using five different techniques. Polar Biol 26:20–26
Wassmann P, Duarte CM, Agustí S, Sejr MK (2011) Footprints of climate change in the Arctic marine ecosystem. Glob Chang Biol 17:1235–1249
Wiborg KF (1980) Undersøkelser av Gonatus fabricii (Lichtenstein) i Norskehavet og det vestlige Barentshavet i juni–september 1979. Gonatus fabricii (Lichtenstein) investigations in the Norwegian Sea and the western Barents Sea, June–September 1979, 1–7
Wiese FK, Robertson GJ (2004) Assessing seabird mortality from chronic oil discharges at sea. J Wildl Manag 68:627–638
Acknowledgments
This project was supported by the Danish Environmental Protection Agency as part of the environmental support programme DANCEA—Danish Cooperation for Environment in the Arctic and the Danish Ministry for Higher Education and Science. The authors are solely responsible for all results and conclusions presented. We acknowledge the assistance from the crew at Paamiut A/S, colleagues at the Greenland Institute of Natural Resources and the Danish National Environmental Research Institute during field collections. We wish to thank the local hunters and fishermen from Nuuk, Sisimiut, Ummanaq, Saqqaq and Qeqertarsuaq for their collaboration and for supplying us with samples from their local catches and especially Morten Frederiksen for help with the statistical analyses. Environment Canada made available their stable isotope facility in Saskatoon, Canada, for all sample preparation and analyses that were partly financed by an Environment Canada operating grant to KAH. We also thank the anonymous reviewers for improving an earlier draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Responsible Editor: C. Harrod.
Reviewed by Undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Linnebjerg, J.F., Hobson, K.A., Fort, J. et al. Deciphering the structure of the West Greenland marine food web using stable isotopes (δ13C, δ15N). Mar Biol 163, 230 (2016). https://doi.org/10.1007/s00227-016-3001-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-016-3001-0