Abstract
The 1997/1998 El Niño Southern Oscillation (ENSO) was the most severe coral bleaching event in recent history, resulting in the loss of 16 % of the world’s coral reefs. Mortality was particularly severe in French Polynesia, where unprecedented mortality of massive Porites was observed in lagoonal sites of Rangiroa Atoll. To assess the recovery of massive Porites 15 years later, we resurveyed the size structure and extent of partial mortality of massive Porites at Tivaru (Rangiroa). Surveys revealed an abundance of massive Porites colonies rising from the shallow lagoonal floor. Colony width averaged 2.65 m, reaching a maximum of 7.1 m (estimated age of ~391 ± 107 years old). The relative cover of recently dead skeleton within quadrats declined from 42.8 % in 1998 to zero in 2013, yet the relative cover of old dead skeleton increased only marginally from 22.1 % in 1998 to 26.1 % in 2013. At a colony level, the proportion of Porites dominated by living tissue increased from 34.9 % in 1998 to 73.9 % in 2013, indicating rapid recovery of recent dead skeleton to living tissue rather than transitioning to old dead skeleton. Such rapid post-bleaching recovery is unprecedented in massive Porites and resulted from remarkable self-regeneration termed the ‘Phoenix effect’, whereby remnant cryptic patches of tissue that survived the 1997/1998 ENSO event regenerated and rapidly overgrew adjacent dead skeleton. Contrary to our earlier predictions, not only are large massive Porites relatively resistant to stress, they appear to have a remarkable capacity for recovery even after severe partial mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The impact of climate change has resulted in substantial changes to the world’s oceans, with an increase in average sea surface temperatures (Lough 2012) and the frequency of extreme warm events (Lima and Wethey 2012) over the past decades. Such climate shifts have led to intensification of thermal stress events on coral reef ecosystems, with a corresponding increase in the frequency and intensity of coral bleaching events (McWilliams et al. 2005; Selig et al. 2010). The ecological impacts of these coral bleaching events have ranged from mild to severe, leading to local declines in diversity (Loya et al. 2001), catastrophic losses of coral cover (Edwards et al. 2001), shifts in community structure (van Woesik et al. 2011), and in some instances, localized species extinctions (Glynn 2011). Rates of recovery following coral bleaching events vary substantially across multiple scales, and may depend upon a multitude of factors, including pre-bleaching community structure, localized stresses, and previous disturbance history (e.g., Marshall and Baird 2000; Wooldridge 2009; Thompson and Dolman 2010). While rapid recovery has been observed at regional scales following severe bleaching events (e.g., Diaz-Pulido et al. 2009; Gilmour et al. 2013), some reefs have shown no signs of recovery over 10 years after bleaching-related mortality (e.g., Cheal et al. 2010).
The 1997/1998 El Niño Southern Oscillation (ENSO) event has been the single most severe global coral bleaching event observed in recent history (Hoegh-Guldberg 1999; Lough 2012). Extensive mortality was observed throughout the central Pacific region, including French Polynesia (Mumby et al. 2001). In general large, massive colonies of the genus Porites are found to be among the most resistant corals to bleaching, meaning that bleaching symptoms rarely result in significant mortality (Marshall and Baird 2000). However, in 1998, sea temperatures exceeded 36 °C in the lagoon of Rangiroa Atoll and led to the first reported major mortality event for this long-lived coral (Mumby et al. 2001, 2004). A quarter of colonies were categorized as ‘recently dead’, defined as having less than 19 % of the colony surface was living (and in >90 % of cases this was <5 % living) and any live tissue was usually confined to small patches (<20 cm2). Overall, ~40–80 % of living tissue in massive Porites colonies died in response to sustained elevated temperatures (Mumby et al. 2001). Given that the combination of severe bleaching mortality, unusually large colony sizes and the slow-growing nature of massive Porites (~1.1 cm per year, Bessat and Buigues 2001), recovery of Porites to pre-bleaching levels was estimated to take at least 100 years (Mumby et al. 2001).
Here, we returned to Motu Tivaru 15 years after the mortality event to quantify the level of recovery. We find surprisingly rapid recovery of Porites assemblages to pre-disturbance levels, primarily due to a previously underestimated yet significant mechanism of tissue regeneration termed ‘re-sheeting’ (Jordan-Dahlgren 1992), where remnant surviving tissue rapidly recolonized recently dead skeleton. Such surprise resurrection of coral has been termed a ‘Phoenix effect’ (sensu Krupp et al. 1992).
Methods
Study site
Situated approximately 350 km northeast of Moorea (Fig. 1), Motu Tivaru is located within the northwest side of the lagoon of Rangiroa Atoll. Rising from the shallow sandy lagoon floor (~8 m depth), the reef framework at Tivaru is dominated by massive Porites spp., covering an approximate area of 10 ha (Mumby et al. 2004). Surveys of Tivaru were conducted in October 1998, 6 months after the mass bleaching event (Mumby et al. 2001), and resurveyed in March 2013.
1998 Surveys
In 1998, a total of seven large quadrats (5 × 5 m2) were surveyed within randomly chosen locations at Tivaru (see Mumby et al. 2001, 2004 for further details). Briefly, six of the seven large quadrats were deployed within a rectangle located in satellite imagery through use of large white tarpaulins (Mumby et al. 2004), and the coordinates for each corner were measured in the field using GPS. To estimate benthic cover on each reef using the same sample unit, 1 m2 square areas were subsampled at random within each dataset, provided that they did not occur adjacent to one another. In total, 91 small quadrats (1 m2) were sampled, and the percent cover of benthic substrate recorded. Massive Porites substrates were categorized as either (1) living tissue, (2) ‘recently dead skeleton’ (corallite structure visible, usually colonized by filamentous algae), or (3) ‘old dead’ (corallite structure absent, grazing scars of herbivores often visible). The relative cover of these three categories for massive Porites substrates was calculated to determine the proportion of ‘living’, ‘recently dead’ and ‘old dead’ Porites.
In addition, five belt transects of approximately 6 m width and 20 m length were laid between white tarpaulin that were georeferenced (for further details, see Mumby et al. 2001). Each Porites colony along each transect was designated as ‘live coral’ if ≥80 % of the tissue was living; recently dead if ≤19 % (though typically 5 %) of the tissue was living and the remainder was recently dead; partially dead if the extent of living tissue fell between 20 and 79 %; and old dead if the colony simply comprised eroded substrate. As the surfaces of massive Porites tend to comprise of mosaics of living tissue and dead skeletal patches (Mumby et al. 2001), we defined a colony as an autonomous skeletal mass (Meesters et al. 1996).
2013 Surveys
In 2013, the same rectangular study site from 1998 was relocated using the corner GPS co-ordinates. Two parallel transects of 30 m length and separated by 30 m were laid haphazardly across the center of the site. Porites colonies adjacent to transects were systematically identified, and quadrats (0.25 m2) placed at random on each colony. In total, 63 small quadrats were sampled, and the percent cover of benthic substrate recorded. Massive Porites substrates were categorized as either (1) living tissue, (2) ‘recently dead skeleton’ (corallite structure visible, usually colonized by filamentous algae), or (3) ‘old dead’ (corallite structure absent, grazing scars of herbivores often visible) following the same methodology as 1998 surveys. The relative cover of these three categories for massive Porites substrates was calculated to determine the proportion of ‘living’, ‘recently dead’ and ‘old dead’ Porites.
In addition, using GPS co-ordinates, we located and revisited the longest of the five belt transects laid in 1998, and resurveyed 25 colonies that had been surveyed in 1998. As per the 1998 surveys, each Porites colony along each transect was designated as either ‘live coral’, ‘recently dead’ ‘partially dead’, or ‘old dead’. Thus, we were able to undertake two complementary analyses: first, comparing the overall change in coral state as measured at a fine scale within quadrats, and second, at a whole-colony scale for the 25 colonies that were resurveyed in 2013.
Statistical analysis
To assess of recovery of massive Porites from bleaching-related mortality at a quadrat scale, the relative cover of ‘living’, ‘recently dead’ and ‘old dead’ categories of massive Porites substrates was compared between 1998 and 2013 using a χ 2 test. The relationship between ‘living’ Porites and depth and colony size in 2013 was tested using linear regression. To assess of recovery of massive Porites from bleaching-related mortality at a colony scale, the proportion of colonies categorized as ‘live coral’, ‘recently dead’, ‘partially dead’ and ‘old dead’ was compared between 1998 and 2013 using a χ 2 test. All statistics were conducted in R (‘stats’ package, R 2013).
Results
A total of 114 colonies of massive Porites were surveyed at Tivaru in March 2013. The size of colonies ranged from 0.6 to 7.1 m, averaging 2.65 m. Surveys indicated an abundance of healthy colonies (Fig. 2a–e), and whole colony mortality (Fig. 2f) was infrequent. No significant relationship was observed between the percent cover of living tissue with depth (R 2 = 0.01, F = 1.246, p = 0.26) or colony size (R 2 = 0.01, F = 0.96, p = 0.33).
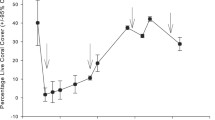
In 1998 surveys, the relative percent cover of living Porites tissue was relatively low (32.5 %, Fig. 3a), A high relative cover of recently dead skeleton (24.6 %) indicated recent mortality following the bleaching, while the remaining substrate was dominated by old dead skeleton (42.9 %). In contrast, 2013 surveys indicated a substantial and significant increase in the relative percent cover of living Porites tissue to 71.0 % (X 2 = 82.1, p < 0.001, Fig. 3b). Minimal signs of recent mortality were observed in 2013, with the cover of recently dead skeleton less than 1 %, and the cover of old dead skeleton declined to 28.9 % cover (Fig. 3a).
a Percent cover of massive Porites substrates within quadrats categorized as either (1) living tissue, (2) recently dead skeleton (corallite structure visible, usually colonized by filamentous algae), or (3) old dead (corallite structure absent, grazing scars of herbivores often visible) in 1998 and 2013 surveys (±standard error). b Relative percent cover of massive Porites substrates within quadrats following the above categories in 1998 and 2013 surveys
Analysis of individual colonies in 1998 revealed that 29.1 % of Porites were categorized as ‘live’ (>80 % living tissue), while 25 % were ‘recently dead’ (≤19 % living cover and remainder recently dead) and 16.7 % partially dead (Fig. 4). Resurveys of the exact colonies in 2013 revealed a significant difference in state among Porites than from 1998 (X 2 = 11.37, p > 0.01). The number of Porites categorized as ‘live’ more than doubled to 64 %, while the proportion of Porites categorized as ‘partially dead’ was reduced fourfold to 4 %. In contrast to 1998, no colonies were categorized as ‘recently dead’.
Proportion of Porites colonies categorized as ‘live coral’ (≥80 % of the tissue was living), recently dead (≤19 % living tissue and the remainder was recently dead); partially dead (20–79 % living tissue), and old dead (eroded substrate) from the longest transect surveyed in 1998 and resurveyed in 2012
To formally test whether the colonies exhibiting partial mortality in 1998 (‘recently dead’ and ‘partially dead’ categories) exhibited recovery in 2013, we compared the number of corals in the ‘live’ and ‘old dead’ categories between 2013 (observed) and 1998 surveys—assuming that all ‘recently dead’ and ‘partially dead’ colonies transitioned into the ‘live’ category (predicted). That no significant difference in the density of colonies was observed (X 2 = 1.027, p > 0.1) and supports the hypothesis that recovery to ‘live’ (>80 % living tissue) colonies in 2013 was driven by the regeneration of ‘recently dead’ (≤19 % living tissue) and ‘partially dead’ (20–79 % living tissue) colonies.
Discussion
Situated within a shallow sandy lagoon floor, Tivaru represents a distinctive reef habitat, with the reef framework formed entirely by massive Porites. With an average width of 2.65 m, these colonies are true giants, with the largest colony measuring 7.1 m wide. Following the calculations of Potts et al. (1985) and assuming a local growth rate of ~1.1 ± 0.3 cm per year over the past two centuries (Bessat and Buigues 2001), the largest colony recorded in our survey is between 284 and 497 years old. Indeed, the majority of colonies (66.1 %) within our surveys were >2 m in size, indicating that the Porites assemblages at Tivaru are stable and long-lived. Previous studies from multiple reefs across the Great Barrier Reef indicate that most Porites assemblages are dominated by substantially smaller size classes (<0.3 m), with only 0.9–2.7 % of colonies >2 m in size (Done and Potts 1992), and studies of three reef slope locations at Moorea (French Polynesia) revealed high densities of relatively small populations of massive Porites in shallow environments (6–12 m depth), with few large colonies existing only observed at deeper (18 m) sites (Adjeroud et al. 2007). The sheltered environment in the shallow lagoon likely protects these assemblages from catastrophic damage from cyclones observed in outer reef slope environments throughout the region (Harmelin-Vivien and Laboute 1986), resulting in such longevity and dominance.
Despite the persistence of massive Porites populations throughout the previous centuries, Tivaru was severely impacted by the 1998 ENSO event. Sustained thermal stress above the March average of 28.8 °C for a period of 3 months led to substantial bleaching at Rangiroa Atoll and throughout French Polynesia (Mumby et al. 2001). Surveys of Tivaru 6 months following the mass bleaching event revealed unprecedented recent mortality, with 44 % of area of living coral undergoing mortality subsequent to bleaching (Mumby et al. 2001). Fifteen years following the mass mortality, our surveys of Tivaru documented surprisingly high rates of recovery. The relative cover of living tissue increased from 32.5 % in 1998 to 71 % in 2013, while the relative cover of recently dead tissue declined from 24.6 % in 1998 to <1 % in 2013. At a colony scale, resurveys of individual colonies in 2013 first surveyed in 1998 revealed that the proportion of Porites categorized as ‘live’ (>80 % living tissue) increased from 29.2 % in 1998 to 64 % in 2013. This rapid recovery resulted from regeneration of existing living tissue within colonies previously categorized as either ‘recently dead’ (≤19 % living tissue) or ‘partially dead’ (20–79 % living tissue). Such recovery is remarkable considering the generally slow growth rates of Porites. Assuming that recovery of massive Porites would necessitate establishment of new colonies, following models of recruitment and recovery of massive Porites after crown-of-thorns starfish outbreaks (Done 1988); Mumby et al. (2001) estimated recovery to pre-disturbance size structure at Tivaru would be in excess of 50 years. By resurveying the exact transect in 1998 and 2013, we were able to confirm the generality of our results. While 1998 surveys revealed a significant negative relationship between Porites mortality and depth in that shallow colonies experienced higher rates of mortality (Mumby et al. 2001), we observed no correlation between cover of living tissue and depth in 2013, indicating that shallow colonies had undergone substantial recovery. Further, no significant relationship was observed between colony size and cover of living tissue, indicating that regeneration occurred in colonies of all sizes.
Our observations and surveys of colony sizes indicate that the rapid recovery of massive Porites at Tivaru was driven by regeneration of existing surviving tissue within colonies, rather than recruitment from planular larvae. Such unusually rapid recovery has been termed the ‘Phoenix effect’, (sensu Krupp et al. 1992), whereby corals, like the legendary Phoenix, rise from their ashes through rapid regeneration from cryptic tissues deep within the coral skeleton. Following the bleaching event of 1998, widespread tissue necrosis leads to extensive partial mortality within colonies (Fig. 5a). However, small remnant patches of tissue survived the mortality often by being shaded in cryptic areas of colonies (e.g., hollows in the bumpy surface of colonies, or skeletal depressions formed by the bioeroding bivalve Pedum spondyloideum, Fig. 5a, b). We hypothesize two mechanisms that may account for the rapid recovery following bleaching: Firstly, as Porites tissues extend deep within the skeleton (Davies 1991), it may be possible that colonies that appeared dead from the surface—even with overstorey canopies of algal turfs—may have recovered from remnant tissue surviving deep within the corallite structure. While such recovery from cryptic tissue has been observed in corals where no living tissue is visible from the surface and occurs on a timeframe ranging from weeks (Lirman et al. 2002) to months (Krupp et al. 1992) after apparent mortality, in the absence of repeated post-bleaching surveys, it is difficult to ascertain in retrospect whether such response occurred at Tivaru. Secondly, remnant patches of cryptic living tissue that survive mortality may coalesce through growth and fusion, resulting in recovery of colonies following partial mortality (Hughes and Jackson 1985; Loch et al. 2002). Our observations in 2013 documented massive Porites colonies in various stages of ‘re-sheeting’ recovery (Fig. 5c–g). While such overgrowth of adjacent dead skeleton in Acropora occurs often without investing energy or resources into vertical accretion (Jordan-Dahlgren 1992), re-sheeting in massive Porites seems to occur through the vertical and horizontal growth of residual surviving tissue into small hemispheres (Fig. 5 c–g), that eventually coalesce to re-sheet the colony with a new layer several cm thick.
a, b Still images from video footage captured 6 months after the bleaching event in 1998 showing ‘recently dead skeleton’ (corallite structure visible, usually colonized by filamentous algae), with small surviving patches of remnant living tissue (marked with white arrows), c–g representative images from 2013 surveys of stages of re-sheeting in the recovery in massive Porites following bleaching-related mortality (from dead skeleton through to 100 % regeneration), h latter stage recovery in a massive Porites colony in March 2013 (direction of recovery indicated by red arrows)
Either mechanism may account for our observation of rapid recovery at Tivaru. Regrowth by expansion of border polyps is less energetically costly than accreting growing new skeleton (Bak and Stewardvanes 1980). Re-sheeting allows rapid expansion of colony surface area, in turn increasing the energetic resources of the colony, and reducing the area of skeleton available for colonization by bioeroders (Peyrot-Clausade et al. 1995). As smaller patches continue to expand and cover larger areas of the colony, the surface to perimeter ratio of living tissue increases (Fig. 5b), further facilitating rates of recovery (van Woesik 1998). While this strategy has been commonly reported from Acropora species following extensive tissue loss (Jordan-Dahlgren 1992; Riegl and Piller 2001; Diaz-Pulido et al. 2009), to our knowledge, this is the first instance of mechanism occurring at such scales in massive corals such as Porites.
The rapid and extensive regenerative capacity of re-sheeting observed in the present study is unusual, and contrasts with previous experimental studies that show slower regeneration and limited recovery from larger lesions in massive Porites (van Woesik 1998; Denis et al. 2011). Yet, this unaccounted mechanism may explain previously documented recoveries of Porites, such as rapid increase in intertidal Porites spp. from 7 to 26 % cover within a single year following dredging mortality (Brown et al. 1990). The resilience of Porites assemblages at Tivaru is likely to be associated with multiple factors: (1) an intact herbivore assemblage means turf algae is kept to a minimum, reducing competitive interactions with algae and regenerating coral borders (Jompa and McCook 2003; but see Titlyanov and Titlyanova 2009), (2) the sheltered lagoonal environment reduces wave-driven turbidity and results in relatively homogenous light fields (Anthony et al. 2004), (3) the absence of fast growing branching or tabular corals at the site means that Porites are not diverting resources away from growth into competition for space and light (Lang and Chornesky 1990), (4) cyclones are relatively rare in French Polynesia, with only five major cyclones occurring since the beginning of the 20th century (1906, 1982, 1991, 1997, and 2010, Adjeroud et al. 2002), (5) when cyclones do occur, wave disturbance and dislodgment is infrequent compared with outer reef slope environments (Harmelin-Vivien and Laboute 1986), and finally (6) while crown-of-thorns starfish have decimated adjacent reefs in the Society and Austral Archipelagoes (Kayal et al. 2012), Rangiroa and other reefs in the Tuamotu Archipelago are yet to be impacted by crown-of-thorns outbreaks (Salvat et al. 2008), collectively implying that Tivaru has remained relatively unaffected by other recent episodic disturbances.
Thankfully, our projections for recovery of Porites at Tivaru to pre-bleaching levels of at least 100 years (Mumby et al. 2001) were overly pessimistic. Nonetheless, predicted increases in sea surface temperatures of 1.5 to 2.6 °C by the 2,100 (IPCC 2007) indicate that without an increase in thermal tolerance of 0.2–1.0 °C per decade, coral bleaching events similar to the 1998 mass bleaching event could become a biannual event in the next 30–50 years (Donner et al. 2005). Such necessary increases in thermal tolerance are unlikely to result from adaptation through successive generations in massive Porites, considering their long generation times and limited capacity for recruitment (Kojis and Quinn 1982; Potts et al. 1985). However, the contribution of somatic mutation to the adaptive capacity of corals under climate change has been significantly underestimated to date (van Oppen et al. 2011). While Porites is generally resistant to bleaching (Marshall and Baird 2000), the extent of bleaching at a colony level is often patchy (Baird and Marshall 2002). Such a heterogeneous response may potentially indicate genetic mosaicism within colonies resulting from somatic mutations (van Oppen et al. 2011). Assuming an average polyp density of 75 polyps per cm−2 (Tanzil et al. 2009), and that colonies approximate a hemispherical shape (2πr 2), we estimate the largest Porites colony (radius 3.55 m) to contain ~80 million individual polyps. With an average polyp lifetime of 2–3 years (Darke and Barnes 1993), the scope for beneficial somatic mutation within colonies (van Oppen et al. 2011) is considerable. While partial mortality resulting from acute disturbance is often indiscriminate (e.g., crown-of-thorns, Done 1988), bleaching acts as a selective mortality on susceptible genotypes within colonies (van Oppen et al. 2011). As such, it may be possible that the surviving patches of tissue that survived the 1998 bleaching event (Fig. 5 a, b) represent mutant genotypes that are better adapted to thermal stress, rather than remnant existing genotypes. If this were the case, then the Phoenix-like recovery of these newly evolved genotypes would confer rapid thermal tolerance in massive Porites at a colony scale over short timescales (i.e., 15 years). Whether this mechanism does occur and whether it can sustain recovery of massive Porites at Tivaru through successive bleaching events is unknown. Regardless, our observations of remarkable regenerative capacity and the ‘Phoenix effect’ in massive Porites following mass coral bleaching-related mortality provides an optimistic scope for resilience in an otherwise gloomy future for the worlds coral reefs.
References
Adjeroud M, Augustin D, Galzin R, Salvat B (2002) Natural disturbances and interannual variability of coral reef communities on the outer slope of Tiahura (Moorea, French Polynesia): 1991 to 1997. Mar Ecol-Prog Ser 237:121–131
Adjeroud M, Pratchett MS, Kospartov MC, Lejeusne C, Penin L (2007) Small-scale variability in the size structure of scleractinian corals around Moorea, French Polynesia: patterns across depths and locations. Hydrobiologia 589:117–126. doi:10.1007/s10750-007-0726-2
Anthony KRN, Ridd PV, Orpin AR, Larcombe P, Lough J (2004) Temporal variation of light availability in coastal benthic habitats: effects of clouds, turbidity, and tides. Limnol Oceanogr 49:2201–2211
Baird AH, Marshall PA (2002) Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar Ecol-Prog Ser 237:133–141
Bak RPM, Stewardvanes Y (1980) Regeneration of superficial damage in the scleractinian corals agaricia-agaricites-F-purpurea and porites-astreoides. Bull Mar Sci 30:883–887
Bessat F, Buigues D (2001) Two centuries of variation in coral growth in a massive Porites colony from Moorea (French Polynesia): a response of ocean-atmosphere variability from south central Pacific. Palaeogeogr Palaeoclimatol Palaeoecol 175:381–392
Brown BE, Letissier MDA, Scoffin TP, Tudhope AW (1990) Evaluation of the environmental impact of dredging on intertidal coral reefs at Ko Phuket, Thailand, using ecological and physiological parameters. Mar Ecol-Prog Ser 65:273–281
Cheal AJ, MacNeil MA, Cripps E, Emslie MJ, Jonker M, Schaffelke B, Sweatman H (2010) Coral–macroalgal phase shifts or reef resilience: links with diversity and functional roles of herbivorous fishes on the Great Barrier Reef. Coral Reefs 29:1005–1015. doi:10.1007/s00338-010-0661-y
Darke WM, Barnes DJ (1993) Growth trajectories of corallites and ages of polyps in massive colonies of reef-building corals of the genus Porites. Mar Biol 117:321–326
Davies PS (1991) Effect of daylight variations on the energy budgets of shallow-water corals. Mar Biol 108:137–144. doi:10.1007/Bf01313481
Denis V, Debreuil J, De Palmas S, Richard J, Guillaume MMM, Bruggemann JH (2011) Lesion regeneration capacities in populations of the massive coral Porites lutea at Reunion Island: environmental correlates. Mar Ecol Prog Ser 428:105–117. doi:10.3354/Meps09060
Diaz-Pulido G, McCook L, Dove S, Berkelmans R, Roff G, Kline D, Weeks SJ, Evans RD, Williamson DH, Hoegh-Guldberg O (2009) Doom and boom on a resilient reef: climate change, algal overgrowth and coral recovery. PLoS ONE 4:e5239
Done TJ (1988) Simulation of recovery of pre-disturbance size structure in populations of Porites spp damaged by the crown of thorns starfish acanthaster-planci. Mar Biol 100:51–61. doi:10.1007/Bf00392954
Done TJ, Potts DC (1992) Influences of habitat and natural disturbances on contributions of massive Porites corals to reef communities. Mar Biol 114:479–493
Donner SD, Skirving WJ, Little CM, Oppenheimer M, Hoegh-Guldberg O (2005) Global assessment of coral bleaching and required rates of adaptation under climate change. Glob Change Biol 11:2251–2265
Edwards AJ, Clark S, Zahir H, Rajasuriya A, Naseer A, Rubens J (2001) Coral bleaching and mortality on artificial and natural reefs in Maldives in 1998, sea surface temperature anomalies and initial recovery. Mar Pollut Bull 42:7–15
Gilmour JP, Smith LD, Heyward AJ, Baird AH, Pratchett MS (2013) Recovery of an isolated coral reef system following severe disturbance. Science 340:69–71. doi:10.1126/Science.1232310
Glynn PW (2011) In tandem reef coral and cryptic metazoan declines and extinctions. Bull Mar Sci 87:767–794. doi:10.5343/Bms.2010.1025
Harmelin-Vivien ML, Laboute P (1986) Catastrophic impact of hurricanes on atoll outer reef slopes in the Tuamotu (French-Polynesia). Coral Reefs 5:55–62. doi:10.1007/Bf00270353
Hoegh-Guldberg O (1999) Climate change, coral bleaching and the future of the world’s coral reefs. Mar Freshw Res 50:839–866
Hughes TP, Jackson JBC (1985) Population dynamics and life histories of foliaceous corals. Ecol Monogr 55:141–166
IPCC (2007) Climate change 2007: the physical science basis. Cambridge University Press, Cambridge
Jompa J, McCook LJ (2003) Contrasting effects of turf algae on corals: massive Porites spp. are unaffected by mixed-species turfs, but killed by the red alga Anotrichium tenue. Mar Ecol Prog Ser 258:79–86
Jordan-Dahlgren E (1992) Recolonization patterns of acropora-palmata in a marginal environment. Bull Mar Sci 51:104–117
Kayal M, Vercelloni J, de Loma TL, Bosserelle P, Chancerelle Y, Geoffroy S, Stievenart C, Michonneau F, Penin L, Planes S, Adjeroud M (2012) Predator crown-of-thorns starfish (Acanthaster planci) outbreak, mass mortality of corals, and cascading effects on reef fish and benthic communities. PLoS ONE. doi:10.1371/journal.pone.0047363
Kojis BL, Quinn NJ (1982) Reproductive strategies in four species of Porites (Scleractinia). In: Gomez ED, Birkeland CE, Buddemeier RW, Johannes RE, Marsh JA Jr, Tsuda RT (eds) Proceedings of the 4th international coral reef symposium. Marine Science Center, University of the Philippines, Manila, Philippines, pp 145–151
Krupp DA, Jokiel PL, Chartrand TS (1992) Asexual reproduction by the solitary Scleractinian coral Fungia scutaria on dead parent corall in Kaneohe Bay, Oahu, Hawaiian Islands. In: Richmond BM (ed) Proceedings of the seventh international coral reef symposium, Hawaii, pp 527–534
Lang JC, Chornesky EA (1990) Competition between scleractinian reef corals—a review of mechanisms and effects. In: Dubinsky Z (ed) Ecosystems of the world: coral reefs. Elsevier, Amsterdam, pp 209–252
Lima FP, Wethey DS (2012) Three decades of high-resolution coastal sea surface temperatures reveal more than warming. Nat Commun. doi:10.1038/Ncomms1713
Lirman D, Manzello D, Macia S (2002) Back from the dead: the resilience of Siderastrea radians to severe stress. Coral Reefs 21:291–292
Loch K, Loch W, Schuhmacher H, See WR (2002) Coral recruitment and regeneration on a Maldivian reef 21 months after the coral bleaching event of 1998. Mar Ecol-Pubbl Della Stn Zool Di Nap I 23:219–236
Lough JM (2012) Small change, big difference: sea surface temperature distributions for tropical coral reef ecosystems, 1950–2011. J Geophys Res-Ocean. doi:10.1029/2012jc008199
Loya Y, Sakai K, Yamazato K, Nakano Y, Sambali H, van Woesik R (2001) Coral bleaching: the winners and the losers. Ecol Lett 4:122–131
Marshall PA, Baird AH (2000) Bleaching of corals on the Great Barrier Reef: differential susceptibilities among taxa. Coral Reefs 19:155–163
McWilliams JP, Cote IM, Gill JA, Sutherland WJ, Watkinson AR (2005) Accelerating impacts of temperature-induced coral bleaching in the Caribbean. Ecology 86:2055–2060
Meesters EH, Wesseling I, Bak RPM (1996) Partial mortality in three species of reef-building corals and the relation with colony morphology. Bull Mar Sci 58:838–852
Mumby PJ, Chisholm JRM, Edwards AJ, Clark CD, Roark EB, Andrefouet S, Jaubert J (2001) Unprecedented bleaching-induced mortality in Porites spp. at Rangiroa Atoll, French Polynesia. Mar Biol 139:183–189
Mumby PJ, Hedley JD, Chisholm JRM, Clark CD, Ripley H, Jaubert J (2004) The cover of living and dead corals from airborne remote sensing. Coral Reefs 23:171–183. doi:10.1007/s00338-004-0382-1
Peyrot-Clausade M, LeCampionAlsumard T, Hutchings P, LeCampion J, Payri C, Fontaine MC (1995) Initial bioerosion and bioaccretion on experimental substrates in high island and atoll lagoons (French Polynesia). Oceanol Acta 18:531–541
Potts DC, Done TJ, Isdale PJ, Fisk DA (1985) Dominance of a coral community by the genus Porites (Scleractinia). Mar Ecol Prog Ser 23:79–84
R (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Riegl B, Piller WE (2001) “Cryptic” tissues inside Acropora frame works (Indonesia): a mechanism to enhance tissue survival in hard times while also increasing framework density. Coral Reefs 20:67–68
Salvat B, Aubanel A, Adjeroud M, Bouisset P, Calmet D, Chancerelle Y, Cochennec N, Davies N, Fougerousse A, Galzin R, Lagouy E, Lo C, Monier C, Ponsonnet C, Remoissenet G, Schneider D, Stein A, Tatarata M, Villiers L (2008) Monitoring of French Polynesia coral reefs and their recent development. Rev D Ecol-La Terre Et La Vie 63:145–177
Selig ER, Casey KS, Bruno JF (2010) New insights into global patterns of ocean temperature anomalies: implications for coral reef health and management. Glob Ecol Biogeogr 19:397–411. doi:10.1111/J.1466-8238.2009.00522.X
Tanzil JTI, Brown BE, Tudhope AW, Dunne RP (2009) Decline in skeletal growth of the coral Porites lutea from the Andaman Sea, South Thailand between 1984 and 2005. Coral Reefs 28:519–528. doi:10.1007/S00338-008-0457-5
Thompson A, Dolman AM (2010) Coral bleaching: one disturbance too many for near-shore reefs of the Great Barrier Reef. Coral Reefs 29:637–648. doi:10.1007/s00338-009-0562-0
Titlyanov EA, Titlyanova TV (2009) The dynamics of the restoration of mechanical damage to colonies of the scleractinian coral Porites lutea under conditions of competition with algal settlers for substratum. Russ J Mar Biol 35:230–235. doi:10.1134/S1063074009030067
van Oppen MJH, Souter P, Howells EJ, Heyward A, Berkelmans R (2011) Novel genetic diversity through somatic mutations: fuel for adaptation of reef corals? Diversity 3:405–423. doi:10.3390/d3030405
van Woesik R (1998) Lesion healing on massive Porites spp. corals. Mar Ecol Prog Ser 164:213–220. doi:10.3354/Meps164213
van Woesik R, Sakai K, Ganese A, Loya Y (2011) Revisiting the winners and the losers a decade after coral bleaching. Mar Ecol Prog Ser 434:67–76
Wooldridge S (2009) Water quality and coral bleaching thresholds: formalising the linkage for the inshore reefs of the Great Barrier Reef, Australia. Mar Pollut Bull 58:745–751
Acknowledgments
We thank the Khaled bin Sultan Living Oceans Foundation and crew of the Golden Shadow for the opportunity to revisit Tivaru on the French Polynesia leg of the Global Reef Expedition.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by T. L. Goulet.
Rights and permissions
About this article
Cite this article
Roff, G., Bejarano, S., Bozec, YM. et al. Porites and the Phoenix effect: unprecedented recovery after a mass coral bleaching event at Rangiroa Atoll, French Polynesia. Mar Biol 161, 1385–1393 (2014). https://doi.org/10.1007/s00227-014-2426-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-014-2426-6