Abstract
Reefs are diverse environments because of the structural complexity provided by the tridimensional coral-built framework. However, they are sensitive environments that face multiple stressors including global warming, which triggers bleaching and mortality episodes. After death, coral skeletons are overgrown by a microbial film, which degrades and erodes the reef framework. Although erosive processes have been investigated in the Caribbean and Indo-Pacific, they remain poorly addressed for the unique Southwestern Atlantic reefs. Therefore, we investigated through field surveys of three Brazilian reefs if colonies of the endemic and regionally dominant coral Mussismilia harttii underwent erosion after the 2019 bleaching episode. We also collected corallite fragments from healthy, mildly bleached, severely bleached and dead M. harttii colonies for microcomputed tomography and densitometry analyses to assess whether microporosity and skeletal mineral density are reduced following bleaching. Our findings show that > 90% of the colonies underwent bleaching and loss of live cover was higher than 60% for all three reefs. All reefs also underwent severe erosion, with an area loss of intact colonies ranging from 33.8 to 85.2%. Furthermore, we detected higher total microporosity for dead skeletons and, together with severely bleached colonies, lower skeletal mineral density. Our results also suggest that bleaching, mortality and erosion processes are connected. These findings show that Southwestern Atlantic reefs are facing unprecedented degradation, although they are often considered climate refugia. In addition, because M. harttii is among the most important reef-builders in the region, carbonate budgets and structural complexity may face declines in the Southwestern Atlantic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coral reefs are tridimensional structures produced through the deposition of calcium carbonate mainly by scleractinian corals (Cnidaria, Anthozoa). These organisms may produce more than 10 kg of CaCO3 m−2 yr−1, thus engaging in intensive biomineralization and reef-building (Buddemeier and Smith 1988; Hopley et al. 2007; Tambutté et al. 2011). However, reef growth is dynamic in space and time and the carbonate budget is mediated by several processes that may contribute to either net accretion or erosion. Among the main factors influencing erosion are the local hydrodynamic conditions, physical and chemical properties of seawater and the activity of bioeroding taxa (Tribollet and Golubic 2011; Perry et al. 2012; Bozec et al. 2015; Glynn and Manzello 2015; Januchowski-Hartley et al. 2017).
Although reef environments currently face impacts associated with local-scale stressors including overfishing and pollution (Dubinsky and Stambler 1996; Mumby et al. 2004; Bellwood and Choat 2011; van Dam et al. 2011), global-scale impacts such as ocean acidification and warming often produce stronger deleterious effects on their carbonate budget (see Bruno et al. 2019 and Cornwall et al. 2021). Ocean acidification limits coral calcification and growth by reducing the availability of carbonate ions in the water column (Hoegh-Guldberg et al. 2007; Anthony 2016), while global warming is responsible for the bleaching phenomenon. Under thermal stress, the photosynthetic dinoflagellates (family Symbiodiniaceae—see LaJeunesse et al. 2018) associated with the coral host produce cytotoxic reactive oxygen species (Lesser 2006; Weis 2008; Krueger et al. 2015). The adaptive response of the host is to expel its symbionts, which, together with pigment breakdown, leaves the white skeleton visible under a pale tissue layer (Glynn 1993). Because most shallow-water corals have an obligate mutualistic association with symbiodiniaceans, high mortality often ensues from severe bleaching episodes (Glynn 1993; 1996; Hoegh-Guldberg 1999; Hughes et al. 2018), which in turn prompts the degradation of the reef framework leading to declines in the carbonate budget (Eakin 1996; Roff et al. 2015; Couch et al. 2017; Lange and Perry 2019).
Once corals perish, their bare skeleton is encased by a microbial film composed mainly of turf algae and bacteria (Davey et al. 2008; Leggat et al. 2019). Shortly afterward, the metabolic activity of these microorganisms starts degrading the coral skeleton and rapidly increases its porosity, with a high Ca2+ removal rate of approximately 2.0 µmol cm−2 h−1 (Leggat et al. 2019). This weakens the reef framework and makes it more susceptible to erosion by both wave action and biological activity. The ultimate consequence is the coral skeleton breakdown and crumbling, causing the loss of structural complexity and microhabitats (Wild et al. 2011; Fordyce et al. 2019; Leggat et al. 2019). Numerous feeding, reproduction and shelter sites are therefore lost, thus reducing overall reef fish and invertebrate diversity (Graham and Nash 2012). There are several examples for this process in the Coral Triangle and Great Barrier Reef, where it was shown that after coral mortality and erosion, damselfish (Pomacentridae) no longer took shelter in dead colonies and became more vulnerable to predation (Coker et al. 2012; Boström-Einarsson et al. 2014; 2018—but see Wismer et al. 2019a,b). In the Caribbean, the loss of Orbicella annularis has likewise caused a significant reduction in the abundance of the cryptic fauna (Roff et al. 2015). Furthermore, the decay of the reef framework is often associated not only with a decrease in species richness of associated biota, but also in functional diversity (Sano et al. 1987; Bellwood et al. 2003; Garpe et al. 2006; McWilliam et al. 2020).
Erosive processes have been described for reefs in the Caribbean and Indo-Pacific for decades (Edinger et al. 2000; Sheppard et al. 2002; Alvarez-Filip et al. 2009; Perry et al. 2013; Bozec et al. 2015; Alvarado et al. 2016). However, for the unique reef environments found in the Brazilian coast in the Southwestern Atlantic, this topic has only more recently been assessed (Bastos et al. 2018; Dechnik et al. 2019; 2021; Pereira-Filho et al. 2021; Randi et al. 2021). Reef environments in this area and their fauna are biogeographically separated from the Caribbean by the freshwater discharges of the Amazon-Orinoco plume, that is also rich in sediment and nutrients (Leão et al. 2003; Floeter et al. 2008), and from the Eastern Atlantic by more than 3500 km (the mid-Atlantic barrier) (Floeter et al. 2008; Toonen et al. 2016). These oceanographic features led to the establishment of a reef fauna that is characterized by low diversity and high endemism (Castro and Pires 2001; Leão et al. 2003; 2016; Pinheiro et al. 2018). Distributed across nearly 3000 km in latitude, Southwestern Atlantic reef environments are also remarkable because of their higher tolerance to disturbances in general (Leão et al. 2003, 2010). In fact, these reefs are often considered climate change refugia because of their lower mortality associated with global warming (Mies et al. 2020).
Within this context, the present work investigated the erosive process for the endemic scleractinian Mussismilia harttii in three Brazilian reefs before, during and after the 2019 bleaching event. This species was chosen because it is a major reef-builder and has a phacelloid morphology, which contributes significantly to structural complexity (Nogueira et al. 2015). Specifically, we (i) assessed bleaching incidence, (ii) quantified loss of live coral cover, (iii) quantified erosion through the loss of area covered by intact colonies (without fallen, broken or tumbled corallites) and (iv) investigated whether skeletal integrity is related to the colony health condition. Investigating these topics allows for the evaluation of the climate change impacts in the Southwestern Atlantic from an unexplored perspective. In addition, monitoring erosion is particularly important because reefs function as underwater barriers that buffer the coast from damage associated with storms and other weather phenomena (Sheppard et al. 2005).
Materials and methods
Field surveys
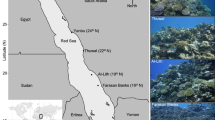
Surveys of M. harttii colonies were performed in three reefs near Porto Seguro (Bahia State, Brazil), located approximately 150 km north of the Abrolhos Bank (which harbors the largest reef complex in Brazil): Araripe, Mucugê and Recife de Fora. Three fixed sites, containing M. harttii, at approximately 4 m of depth were randomly chosen within each reef (16°10′55.27″ S, 38°55′15.14″ W; 16°10′56.55″ S, 38°55′13.30″ W; and 16°10′56.80″ S, 38°55′12.86″ W for Araripe; 16°29′41.09″ S, 39°04′01.47″ W; 16°29′40.59″ S, 39°04′01.47″ W; and 16°29′42.45″ S, 39°04′02.10″ W for Mucugê; and 16°24′39.84″ S, 38°59′07.05″ W; 16°24′39.86″ S, 38°59′07.28″ W; and 16°24′39.76″ S, 38°59′07.63″ W for Recife de Fora; Fig. 1) and monitored every two months between October 2018 and February 2020, with the exception of a few occasions on which weather conditions prevented access to the reef and caused for us to skip a survey. At each site, a fixed area of 2.25 m2 (1.5 × 1.5 m) was delimited and subdivided into nine 0.5 × 0.5 m quadrats, which were photographed at every survey with a fitted PVC frame. A total of 55 colonies were surveyed at the three reefs (13, 15 and 27 colonies for Araripe, Mucugê and Recife de Fora, respectively).
Bleaching, mortality and erosion data
The heat accumulation for the Porto Seguro area (4 × 4 km) was measured with the use of DHW (degree heating weeks), which is defined by the accumulation of temperature anomalies exceeding the maximum monthly mean over a period of 12 weeks (see Liu et al. 2014). Mean and maximum DHW data for the Porto Seguro region were downloaded from the National Oceanic and Atmospheric Administration’s Coral Reef Watch database (NOAA 2021).
The bleaching condition of each colony was assessed using the Coral Watch Health Chart (see Siebeck et al. 2006; Leiper et al. 2009; Fig. S1), which is a tool that allows for in situ rapid assessment of bleaching intensity. The reference chart was attached to the PVC frame and photographed along with M. harttii during surveys. Colonies were considered healthy when matching colors E4–E6 and bleached when matching E1–E3. Mortality was calculated by quantifying the loss of area occupied by live M. harttii colonies within each of the 2.25 m2 sites, with the use of the photoQuad software. This software calculates the area occupied by benthic organisms by comparing its measurements against a metric reference point (in this case, the 0.5 × 0.5-m PVC frame) (Trygonis and Sini 2012). Both the absolute and relative area losses were recorded. Likewise, erosion was calculated by quantifying the loss of area occupied by M. harttii colonies that were structurally intact and standing upright, without fallen or tumbled corallites, regardless of being dead or alive.
Skeleton integrity
To evaluate the relationship between colony health and skeleton integrity, we collected corallite samples at one of the Recife de Fora sites in October 2019 in four conditions (n = 3 samples per condition, Fig. S2): healthy (E5 in the reference chart), mildly bleached (E3), severely bleached (E1) and recently dead (covered in a microbial film, but less than two weeks after death). The corallite microporosity was analyzed on a SkyScan 1172 high-resolution (2000 × 1332 pixels) microtomograph comprised of a microfocus X-ray tube with voltages 80 kV and a current of 124 mA. A 10 Mp (4000 × 2300 pixels) CCD camera detector and a computer cluster were used to reconstruct the images. Microporosity data were divided into three variables: closed microporosity, which accounts for micropores contained within the skeleton; open microporosity, which assesses micropores that are on the corallite wall; and total microporosity, which is the sum of the two previous parameters with a correction factor.
Skeletal mineral density was investigated with a DXA X-ray densitometer (iDXA-Lunar, enCORETM 2008 software, version 12.30, GE Healthcare®). Image processing was performed with enCORETM 2008 to obtain sections of superficial skeletal density. Several regions of interest were obtained in the samples, measuring 1.51 × 1.78 cm. Skeletal mineral density was calculated for each sample by comparing against the densitometer calcium carbonate internal standard. Both microporosity and skeletal mineral density procedures were performed only in the top 5 cm of the corallite, to guarantee that analyses were performed on corallite regions that were previously in contact with living coral tissue.
Statistical analyses
We performed McNemar’s tests (which is a repeated measures variation of a chi-square test) to investigate whether there were differences in the relative live cover for M. harttii before and after the bleaching event. Before data points (prior to thermal stress) were considered at Oct 2018, Nov 2018 and Jan 2019 for Araripe, Mucugê and Recife de Fora, respectively. After data points (six months after the end of thermal stress) were Feb 2020, Jan 2020 and Jan 2020, respectively. The same procedures were performed to assess whether there was a reduction in the cover of colonies that were intact and standing upright.
To answer if there is a relationship between colony health condition (healthy, mildly bleached, severely bleached and dead) and skeleton integrity, we ran one-way analyses of variance (ANOVA) with closed microporosity, open microporosity, total porosity and skeletal mineral density as the dependent variable. Whenever statistical differences were detected (at p < 0.05) by ANOVA tests, a post hoc Tukey’s HSD test was employed to compare difference between category pairs. All data were checked for normality using Shapiro–Wilk’s test and for homoscedasticity using Levene’s test.
Results
Bleaching and mortality
DHW data show that thermal stress in the Porto Seguro area lasted for approximately 6 months (Fig. 2). Temperature anomalies started in February and increased until May, when the highest value of 15.1 °C-weeks was recorded. Although it decreased afterward, thermal stress only ceased in August. Bleaching incidence (considering both mildly and severely bleached colonies) was high for all three reefs, with 92.2%, 93.3% and 100% of M. harttii colonies undergoing bleaching in Araripe, Mucugê and Recife de Fora, respectively. The live cover of M. harttii underwent a significant reduction (χ2 = 7.02, p < 0.005) at Araripe, from 25.4 to 2.2% (Fig. 3). At Mucugê and Recife de Fora, live M. harttii cover decreased from 8.9 to 2.7% and from 15.8 to 5.6%, respectively, but without significant differences in both cases (χ2 = 0.51, p > 0.05; and χ2 = 1.03, p > 0.05). Compared to the pre-bleaching status, live M. harttii cover was proportionally reduced by 91.3%, 69.6% and 64.5% for Araripe, Mucugê and Recife de Fora, respectively. Mortality was apparently higher at the end of the bleaching event, between May and July (Fig. 3).
Mean and maximum heat accumulation (expressed in degree heating weeks, DHW) to which Mussismilia harttii colonies were exposed during the 2019 bleaching event in Porto Seguro (Bahia State, Brazil). DHW data were obtained from the National Oceanic and Atmospheric Administration’s Coral Reef Watch database (NOAA 2021). Red and dotted line represents time interval during which bleached colonies were observed in the three surveyed reefs (Araripe, Mucugê and Recife de Fora). Mass bleaching and mortality thresholds are depicted according to those described in Kayanne 2017)
Total live area (left Y axis and gray line) and relative live cover (right Y axis and black line) for Mussismilia harttii during the 2019 bleaching event in three reefs in Porto Seguro (Bahia State, Brazil): A Araripe, B Mucugê and C Recife de Fora. Red and dotted line represents time interval during which colonies underwent thermal stress (DHW > 4.0 °C-weeks). Data are presented as mean ± standard error
Erosion
The area covered by intact M. harttii colonies also decreased dramatically over time and followed a similar trend to the loss of live cover (Fig. 4). At Araripe, intact area was reduced significantly from 29.9 to 4.4% (χ2 = 6.85; p < 0.005). Mucugê and Recife de Fora also underwent reductions in the cover of intact M. harttii, from 8.9 to 2.8% and from 20.1 to 13.3%, respectively, albeit not significantly in both cases (χ2 = 0.46, p > 0.05; and χ2 = 0.19, p > 0.05). Compared to the pre-bleaching status, the area occupied by intact M. harttii colonies proportionally decreased by 85.2%, 68.5% and 33.8% for Araripe, Mucugê and Recife de Fora, respectively. Erosion was also more intense between May and July, but it continued in the following months (Fig. 4). In general, the data show a succession of events that went from bleaching to mortality to erosion, promoting changes in the local benthos (Figs. 5 and S3).
Total intact area (left Y axis and gray line) and relative intact cover (right Y axis and black line) for Mussismilia harttii during the 2019 bleaching event in three reefs in Porto Seguro (Bahia State, Brazil): A Araripe, B Mucugê and C Recife de Fora. Red and dotted line represents time interval during which colonies underwent thermal stress (DHW > 4.0 °C-weeks). Data are presented as mean ± standard error
The degradation process of a Mussismilia harttii colony at Recife de Fora (Porto Seguro, Bahia State, Brazil) during the 2019 bleaching event. A intact and healthy colony with only a few dead polyps in the center in January 2019; B intact and mildly bleached colony with an increased quantity of dead polyps in the center in March 2019; C and D intact and severely bleached colony in May and July 2019; E partially dead colony undergoing erosion in the top left corner in November 2019; and F partially dead colony with the left half eroded in January 2020
Health condition and skeleton integrity
The microcomputed tomography analysis showed no significant difference among the four health conditions for both the closed microporosity (F = 3.30, df = 3, p = 0.07; Fig. 6A) and the open microporosity (F = 1.16, df = 3, p = 0.38). However, total microporosity was different among conditions (F = 5.46, df = 3, p = 0.024). Healthy and mildly bleached colonies present significantly lower total microporosity when compared to dead colonies (Tukey’s HSD test: p = 0.042 and 0.029, respectively). As for skeletal mineral density, it was different across the four health conditions (F = 6.22, df = 3, p = 0.017; Fig. 6B). Healthy coral fragments had significantly higher skeletal mineral density than severely bleached and dead fragments (Tukey’s HSD test: p = 0.032 and 0.021, respectively).
The skeletal integrity of Mussismilia harttii after the 2019 bleaching event was assessed through quantification of A microporosity and B skeletal mineral density. Four different health conditions were tested: healthy, mildly bleached, severely bleached and dead colonies. Different superscript letters for the same response variable (closed porosity, open porosity or total porosity) denote statistically different groups (post hoc Tukey’s HSD test)
Discussion
The 2019 bleaching event in the Southwestern Atlantic affected nearly the whole Brazilian coast and was largely associated with the warm phase of the El Niño–Southern Oscillation (ENSO). To date, this was the most severe bleaching event in the Southwestern Atlantic, with the highest recorded levels of heat accumulation, bleaching incidence and mortality (Banha et al. 2020; Duarte et al. 2020; Ferreira et al. 2021; Gaspar et al. 2021). Our data show that more than 90% of M. harttii colonies bleached in all three reefs monitored in this study. This is the highest bleaching incidence ever recorded for this species, greater than the 55% detected for Abrolhos in 2019 and the 80% for the same region in 2016, during the third global mass bleaching event (Teixeira et al. 2019; Duarte et al. 2020). A DHW value of 4.0 °C-weeks is the threshold for the occurrence of mass bleaching, while more than 8.0 °C-weeks usually triggers mass mortality (Kayanne 2017). Therefore, the elevated degree of bleaching and mortality observed in this case can be explained by the intense heat stress of 15.1 °C-weeks to which M. harttii was subjected to.
The loss of live M. harttii cover, greater than 60% for all three reefs investigated, was also the highest ever recorded for the species. Previous losses of 13% and < 1% had been recorded at Abrolhos during the 2019 and 2016 bleaching events, respectively (Teixeira et al. 2019; Duarte et al. 2020). Despite experiencing a heat accumulation of 19.6 °C-weeks in 2019, Abrolhos may have suffered lower mortality because it is a well-preserved marine protected area and much less subject to urban influence than Porto Seguro reefs (Werner et al. 2010; Leão and Kikuchi 2001; Moura et al. 2013). In fact, similar reasons may explain why the Araripe reefs underwent considerably higher mortality than Mucugê and Recife de Fora. They are under the strong influence of the Santo Antônio River, which discharges an elevated quantity of sediment and pollutants (Coral Vivo Institute, unpublished data). In this case, the higher turbidity may not have been enough to buffer the effects from the exceptionally high thermal stress (see Cacciapaglia and van Woesik 2016; Sully and van Woesik 2020). Furthermore, fisheries and tourism are not regulated as in Recife de Fora, which likely increases anthropogenic pressure (de Paula et al. 2018; Lima et al. 2021).
Our data confirm that there was a considerable loss of intact M. harttii living cover for all three reefs (Fig. 4). However, statistical differences between pre- and post-bleaching time points were detected only for the Araripe reefs, suggesting that, although cover was reduced in more than 60%, the loss of area occupied by intact M. harttii at Mucugê and Recife de Fora may not have been as drastic. Additionally, it was confirmed that the skeleton integrity is directly related to colony health—dead colonies (with fallen, broken and tumbled corallites) displayed higher total microporosity and, together with severely bleached colonies, lower skeletal mineral density. This confirms they were undergoing erosion. It is unclear why no differences were detected among health conditions for closed and open microporosity, but it may be related to the specific circumstances that each colony experienced. For instance, external bioerosion and open microporosity may be associated with fish bites, while internal bioerosion and closed microporosity can be associated with the activity of boring and infaunal organisms (Ong and Holland 2010; Tribollet and Golubic 2011; Bozec et al. 2015). Hydrodynamics likely also play a significant role, although unclear. Nonetheless, the dynamics of the physical and biochemical processes surrounding the increase in porosity in coral skeletons still require more investigation (see Leggat et al. 2019).
The field surveys show that M. harttii colonies began eroding shortly after death. This is in accordance with Leggat et al. (2019), where it was demonstrated that coral skeletons may undergo significant ultrastructural changes less than two weeks after death due to microbial activity. In this way, the bleaching, mortality and erosive processes seem to be well connected. It is therefore possible that the Porto Seguro reefs may have suffered a swift decline in their carbonate budgets. It has been reported that reefs may not be able to accrete if coral cover is reduced to less than 10% (Perry et al. 2013). All three reefs investigated in this study had their live M. harttii cover reduced to less than 10%, which raises a severe alert. On a Caribbean reef, a coral cover loss of 9%, which is considerably lower than the figures we report, was enough to prevent reef accretion and growth (Roff et al. 2015). The Porto Seguro reefs face additional problems to maintain its structural integrity. Aragonite saturation state (ΩAR) levels below 3.0 have been detected in sections of the nearby Coroa Vermelha reefs (Longhini et al. 2015). However, levels of 3.3 or higher are required for reef development (Kleypas et al. 1999; Hoegh-Gudberg et al. 2007). Therefore, it is possible that reefs in this area may currently be unable to accrete. In addition, the presence of key species such as herbivorous parrotfish is usually lower in Porto Seguro reefs (Chaves et al. 2010). Together with the traditionally low functional redundancy associated with Brazilian reef fishes (Mouillot et al. 2014), herbivorous species, despite also contributing to bioerosion, may not fully control algal overgrowth and thus possibly facilitate phase shifts and reef erosion (see Done 1992; McManus and Polsenberg 2004; Bruno et al. 2009; Hughes et al. 2007a, b). Therefore, because the present work is limited by addressing the eroded area only, investigations on the carbonate budgets, specifically on the loss of carbonate mass, are warranted for Porto Seguro reefs.
In general, the main consequence of our findings is the potential loss of structural complexity in Porto Seguro reefs following the 2019 bleaching event. Branching coral species are usually the main providers of structural complexity, but there are no branching scleractinians in the Southwestern Atlantic. This niche is occupied by Millepora alcicornis, a branching and calcifying hydrozoan (Coni et al. 2013) distributed from Maranhão to Rio de Janeiro. However, branching species are usually more susceptible to bleaching (Loya et al. 2001; Pratchett et al. 2020; Morais et al. 2021) and M. alcicornis suffered more than 90% mortality during the 2019 bleaching event in the Southwestern Atlantic (Duarte et al. 2020; Ferreira et al. 2021). In the absence of branching corals, the species that provides the highest structural complexity is M. harttii, due to its phacelloid morphology and space between corallites (Nogueira et al. 2015; 2021). However, unlike M. alcicornis, M. harttii is a slow-growing species (Leão et al. 2003) and recovery may take decades. In fact, M. harttii populations have been declining in the past 50 yrs (Laborel-Deguen et al. 2019) and high cover is now restricted to a few locations such as Bahia State and the marine protected area Costa dos Corais. Also worrisome is that M. harttii populations in that location also suffered high mortality (32.6%) during the 2019 bleaching episode (Pereira et al. 2022). The conservation status for M. harttii was listed as “endangered” in 2013 after a major evaluation of the natural stocks (ICMBio 2018). However, our findings suggest that its status may require a careful revision after the recent mortality episodes. Therefore, the two main structural complexity providers in Southwestern Atlantic reefs are currently facing high mortality, and the impacts on overall reef biodiversity need urgent assessment. On the other hand, during field assessments performed few years after the 2019 bleaching episode, we observed that M. harttii rubble was colonized by small colonies of faster-growing coral species such as Favia gravida and Agaricia humilis. Therefore, eroded M. harttii skeletons are serving as suitable substrate for coral recruitment.
Current definitions of climate change refugia describe them as areas where impacts from climate change are occurring at a slower pace, providing short-term partial protection against its impacts (Morelli et al. 2020). In that sense, Southwestern Atlantic reefs may be considered climate change refugia because they have historically suffered fewer mass mortality episodes at the community level than other areas such as the Caribbean and Indo-Pacific, especially during the three previous global mass bleaching events (Mies et al. 2020). This is important to clarify, as previous investigations in the Southwestern Atlantic have misinterpreted refugia as immune to climate change, which is certainly not the case (Soares et al. 2021; Pereira et al. 2022). The reasons for this increased resilience in the Southwestern Atlantic include: (i) predominance of massive coral species, (ii) an increased heterotrophic capacity, (iii) protection from heat and irradiance due to highly turbid conditions, (iv) ability to inhabit deeper areas where heat impacts are reduced, (v) symbiosis flexibility and capability of reshuffling symbiont communities, (vi) the framework of local biogenic reefs display a high presence of non-scleractinian and more heat-resistant organisms, such as bryozoans, vermetids and calcareous algae and (vii) a history of lower frequency of severe heatwaves (Gherardi and Bosence 2001; Bastos et al. 2018; Mies et al. 2018, 2020; Marangoni et al. 2019; Skirving et al. 2019; Bleuel et al. 2021). In fact, (ii–v) are directly applicable to M. harttii (Marangoni et al. 2019; Mies et al. 2020). However, despite being more tolerant to climate change than other areas of the world, bleaching-associated mortality in the Southwestern Atlantic has been increasing severely in recent years (Duarte et al. 2020; Ferreira et al. 2021; Pereira et al. 2022). Our present findings show that, besides causing high mortality, bleaching may seriously erode the framework of Southwestern Atlantic reefs, mainly when regarding key species that provide high structural complexity.
Although reef environments have alternated between erosive and accretive states in past geological periods, this work documented an unprecedented erosive process during the Anthropocene of a Southwestern Atlantic coral species that contributes significantly to habitat complexity. In addition, we demonstrate that the skeleton of healthy corals displays lower porosity and higher skeletal mineral density when compared to severely bleached and dead ones. Our findings shed light on the dynamics of the erosive process that follows bleaching and may also serve as a red flag for the increasing degradation of Southwestern Atlantic reefs.
References
Alvarado JJ, Grassian B, Cantera-Kintz JR, Carvalho JL, Londoño-Cruz E (2016) Coral Reef Bioerosion in the Eastern Tropical Pacific. Coral Reefs of the Eastern Tropical Pacific 8:369–403
Alvarez-Filip L, Dulvy NK, Gill JA, Côté IM, Watkinson AR (2009) Flattening of Caribbean coral reefs: region-wide declines in architectural complexity. Proceedings of the Royal Society b: Biological Sciences 276:3019–3025
Anthony KR (2016) Coral reefs under climate change and ocean acidification: challenges and opportunities for management and policy. Annu Rev Environ Resour 41:59–81
Banha TN, Capel KC, Kitahara MV, Francini-Filho RB, Francini CL, Sumida PY, Mies M (2020) Low coral mortality during the most intense bleaching event ever recorded in subtropical Southwestern Atlantic reefs. Coral Reefs 39:515–521
Bastos AC, Moura RL, Moraes FC, Vieira LS, Braga JC, Ramalho LV, Amado-Filho GM, Magdalena UR, Webster JM (2018) Bryozoans are major modern builders of South Atlantic oddly shaped reefs. Sci Rep 8:9638
Bellwood DR, Choat JH (2011) Dangerous demographics: the lack of juvenile humphead parrotfishes Bolbometopon muricatum on the Great Barrier Reef. Coral Reefs 30:549–554
Bellwood DR, Hoey AS, Choat JH (2003) Limited functional redundancy in high diversity systems: resilience and function on coral reefs. Ecol Lett 6:281–285
Bleuel J, Pennino MG, Longo GO (2021) Coral distribution and bleaching vulnerability areas in Southwestern Atlantic under ocean warming. Sci Rep 11:1–2
Boström-Einarsson L, Bonin MC, Munday PL, Jones GP (2014) Habitat degradation modifies the strength of interspecific competition in coral dwelling damselfishes. Ecology 95:3056–3067
Bozec YM, Alvarez-Filip L, Mumby PJ (2015) The dynamic of architectural complexity on coral reefs under climate chance. Glob Change Biol 1:223–235
Bruno JF, Sweatman H, Precht WF, Selig ER, Schutte VG (2009) Assessing evidence of phase shifts from coral to macroalgal dominance on coral reefs. Ecology 90:1478–1484
Bruno JF, Côté IM, Toth LT (2019) Climate change, coral loss, and the curious case of the parrotfish paradigm: why don’t marine protected areas improve resilience? Ann Rev Mar Sci 11:307–334
Buddemeier RW, Smith SV (1988) Coral reef growth in an area of rapid rising sea level: predictions and suggestions for long-term research. Coral Reefs 7(51):56
Cacciapaglia C, van Woesik R (2016) Climate-change refugia: Shading reef corals by turbidity. Glob Change Biol 22:1145–1154
Castro CB, Pires DO (2001) Brazilian coral reefs: what we already know and what is still missing. Bull Mar Sci 69:357–371
Chaves LD, Nunes JD, Sampaio CL (2010) Shallow reef fish communities of south Bahia coast, Brazil. Braz J Oceanogr 58:33–46
Coker DJ, Pratchett MS, Munday PL (2012) Influence of coral bleaching, coral mortality and conspecific aggression on movement and distribution of coral-dwelling fish. J Exp Mar Biol Ecol 415:62–68
Coni EO, Ferreira CM, Moura RL, Meirelles PM, Kaufman L, Francini-Filho RB (2013) An evaluation of the use of branching fire-corals (Millepora spp.) as refuge by reef fish in the Abrolhos Bank, eastern Brazil. Environ Biol Fishes 96:45–55
Coral Reef Watch Satellite Monitoring and Modeled Outlooks (2022) NOAA Satellite and Information Service
Cornwall CE, Comeau S, Kornder NA, Perry CT, van Hooidonk R, DeCarlo TM, Pratchett MS, Anderson KD, Browne N, Carpenter R, Diaz-Pulido G, D’Olivo JP, Doo SS, Figueiredo J, Fortunato SAV, Kennedy E, Lantz CA, McCulloch MT, González-Rivero M, Schoepf V, Smithers SG, Lowe RJ (2021) Global declines in coral reef calcium carbonate production under ocean acidification and warming. Proceedings of the National Academy of Sciences of the USA 118:e2015265118
Couch CS, Burns JH, Liu G, Steward K, Gutlay TN, Eakin CM, Kosaki R (2017) Mass Coral Bleaching Due to Unpreceded Marine Heatwave in Papahānaumokuākea Marine National Monument (northwestern Hawaiian Islands) 12:1–27
Davey M, Holmes G, Johnstone R (2008) High rates of nitrogen fixation (acetylene reduction) on coral skeletons following bleaching mortality. Coral Reefs 1:227–236
Dechnik B, Bastos AC, Vieira LS, Webster JM, Fallon S, Yokoyama Y, Nothdurft L, Sanborn K, Batista J, Moura R, Amado-Filho G (2019) Holocene reef growth in the tropical southwestern Atlantic: evidence for sea level and climate instability. Quatern Sci Rev 218:365–377
Dechnik B, Bastos AC, Vieira LS, Webster JM, Fallon S, Yokoyama Y, Braga JC, Pereira MA, Nothdurft L, Sanborn K, Moura RL, Amado-Filho GM (2021) Environmental controls on holocene reef development along the eastern brazilian margin. Coral Reefs 40:1321–1337
Done TJ (1992) Phase shifts in coral reef communities and their ecological significance. Hydrobiologia 247:121–132
Duarte GAS, Villela HDM, Deocleciano M, Silva D, Barno A, Cardoso PM, Vilela CLS, Rosado P, Messias CSMA, Chacon MA, Santoro EP, Olmedo DB, Szpilman M, Rocha LA, Sweet M, Peixoto RS (2020) Heat waves are a major threat to turbid coral reefs in Brazil. Front Mar Sci 7:179
Dubinsky ZV, Stambler N (1996) Marine pollution and coral reefs. Glob Change Biol 2:511–526
Eakin CM (1996) Where have all the carbonates gone? A model comparison of calcium carbonate budgets before and after the 1982–1983 El Nino at Uva Island in the eastern Pacific. Coral Reefs 15:109–119
Edinger EN, Limmon GV, Jompa J, Widjatmoko W, Heikoop JM, Risk MJ (2000) Normal coral growth rates on dying reefs: are coral growth rates good indicators of reef health? Mar Pollut Bull 40:404–425
Ferreira LC, Grillo AC, Repinaldo Filho FP, Souza FN, Longo GO (2021) Different responses of massive and branching corals to a major heatwave at the largest and richest reef complex in South Atlantic. Mar Biol 168:54
Floeter SR, Rocha LA, Robertson DR, Joyeux JC, Smith-Vaniz WF, Wirtz P, Edwards AJ, Barreiros JP, Ferreira CEL, Gasparini JL, Brito A, Falcón JM, Bowen BW, Bernardi G (2008) Atlantic reef fish biogeography and evolution. J Biogeogr 35:22–47
Fordyce AJ, Ainsworth TD, Heron SF, Leggat W (2019) Marine heatwave hotspots in coral reef environments: Physical drivers, ecophysiological outcomes, and impact upon structural complexity. Front Mar Sci 6:498
Garpe KC, Yahya SA, Lindahl U, Öhman MC (2006) Long-term effects of the 1998 coral bleaching event on reef fish assemblages. Mar Ecol Prog Ser 315:237–247
Gaspar TL, Quimbayo JP, Ozekoski R, Nunes LT, Aued AW, Mendes TC, Garrido AG, Segal B (2021) Severe coral bleaching of Siderastrea stellata at the only atoll in the South Atlantic driven by sequential Marine Heatwaves. Biota Neotrop 12:21
Gherardi DF, Bosence DW (2001) Composition and community structure of the coralline algal reefs from Atol das Rocas, South Atlantic, Brazil. Coral Reefs 19:205–219
Glynn PW (1993) Coral reef bleaching: ecological perspectives. Coral Reefs 12:1–17
Glynn PW (1996) Coral reef bleaching: facts, hypotheses and implications. Glob Change Biol 2:495–509
Glynn PW, Manzello DP (2015) Bioerosion and coral reef growth: a dynamic balance. Coral reefs in the Anthropocene. Springer, Dordrecht
Graham NAJ, Nash KL (2012) The importance of structural complexity in coral reef ecosystems. Coral Reefs 32:315–326
Hoegh-Guldberg O (1999) Climate change, coral bleaching and the future of the world’s coral reefs. Mar Freshw Res 8:839–866
Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RD, Greenfield P, Gomez ED, Harvell CD, Sale PF, Edwards AJ, Caldeira K, Knowlton N, Eakin CM, Iglesias-Prieto R, Muthiga N, Bradbury RH, Dubi A, Hatziolos ME (2007) Coral reefs under rapid climate change and ocean acidification. Science 318:1737–1742
Hopley D, Smithers SG, Parnell K (2007) The geomorphology of the Great Barrier Reef: development, diversity and change. Cambridge University Press, Cambridge, UK
Hughes TP, Bellwood DR, Folke CS, McCook LJ, Pandolfi JM (2007a) No-take areas, herbivory and coral reef resilience. Trends Ecol Evol 22:1–3
Hughes TP, Rodrigues MJ, Bellwood DR, Ceccarelli D, Hoegh-Guldberg O, McCook L, Moltschaniwskyj N, Pratchett MS, Steneck RS, Willis B (2007b) Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr Biol 17:360–365
Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM, Baird AH, Baum JK, Berumen ML, Bridge TC, Claar DC, Eakin CM, Gilmour JP, Graham NAJ, Hobbs HH, JPA, Hoey AS, Hoogenboom M, Lowe RJ, McCulloch MT, Pandolfi JM, Schoepf V, Torda G, Wilson SK (2018) Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359:80–83
ICMBio (2018) Livro vermelho da fauna brasileira ameaçada de extinção. Brasília, Brazil
Januchowski-Hartley FA, Graham NA, Wilson SK, Jennings S, Perry CT (2017) Drivers and predictions of coral reef carbonate budget trajectories. Proceedings of the Royal Society b: Biological Sciences 284:20162533
Kayanne H (2017) Validation of degree heating weeks as a coral bleaching index in the northwestern Pacific. Coral Reefs 36:63–70
Kleypas JA, McManus JW, Meñez LAB (1999) Environmental limits to coral reef development: where do we draw the line? Am Zool 39:146–159
Krueger T, Hawkins TD, Becker S, Pontasch S, Dove S, Hoegh-Guldberg O, Leggat W, Fisher PL, Davy SK (2015) Differential coral bleaching — contrasting the activity and response of enzymatic antioxidants in symbiotic partners under thermal stress. Comp Biochem Physiol a: Mol Integr Physiol 190:15–25
Laborel-Deguen F, Castro CB, Nunes F, Pires DO (2019) Recifes Brasileiros: o Legado de Laborel. Museu Nacional, Rio de Janeiro, Brazil
LaJeunesse TC, Parkinson JE, Gabrielson PW, Jeong HJ, Reimer JD, Voolstra CR, Santos SR (2018) Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr Biol 28:2570–2580
Lange ID, Perry CT (2019) Bleaching impacts on carbonate production in the Chagos Archipelago: influence of functional coral groups on carbonate budget trajectories. Coral Reefs 4:619–624
Leão ZMAN, Kikuchi RKP, Testa V (2003) Corals and coral reefs of Brazil. In: Cortés J (ed) Latin American Coral Reefs. Elsevier, Amsterdam, Netherlands, pp 9–52
Leão ZMAN, Kikuchi RKP, Oleiveira MDM, Vasconcellos V (2010) Status of eastern Brazilian coral reefs in time of climate chances. Pan-American Journal of Aquatic Science 5:224–235
Leão ZMAN, Kikuchi RKP, Ferreira BP, Neves EG, Sovierzoski HH, Oliveira MD, Maida M, Correia MD, Johnsson R (2016) Brazilian coral reefs in a period of global change: A synthesis. Braz J Oceanogr 64:97–116
Leggat WP, Camp EF, Suggett DJ, Heron SF, Fordyce AJ, Gardner S, Deakin L, Turner M, Beeching LJ, Kuzhiumparambil U, Eakin CM, Ainsworth TD (2019) Rapid Coral Decay Is Associated with Marine Heatwave Mortality Events on Reefs. Curr Biol 16:2723–2730
Leiper IA, Siebeck UE, Marshal NJ, Phinn SR (2009) Coral health monitoring: linking coral colour and remote sensing techniques. Can J Remote Sens 35:276–286
Lesser MP (2006) Oxidative stress in marine environments: biochemistry and physiological ecology. Annu Rev Physiol 68:253–278
Lima AL, Zapelini C, Schiavetti A (2021) Governance of marine protected areas of the Royal Charlotte Bank, Bahia, east coast of Brazil. Ocean & Coastal Management 207:105615
Liu G, Heron SF, Eakin CM, Muller-Karger FE, Vega-Rodriguez M, Guild LS, De La Cour JL, Geiger EF, Skirving WJ, Burgess TF, Strong AE, Harris A, Maturi E, Ignatov A, Sapper J, Li J, Lynds S (2014) Reef-scale thermal stress monitoring of coral ecosystems: new 5-km global products from NOAA Coral Reef Watch. Remote Sensing 6:11579–11606
Longhini CM, Souza MF, Silva AM (2015) Net ecosystem production, calcification and CO2 fluxes on a reef flat in Northeastern Brazil. Estuar Coast Shelf Sci 166:13–23
Loya Y, Sakai K, Yamazato K, Nakano Y, Sambali H, van Woesik R (2001) Coral bleaching: the winners and the losers. Ecol Lett 4:122–131
Marangoni LF, Mies M, Güth AZ, Banha TN, Inague A, Fonseca JD, Dalmolin C, Faria SC, Ferrier-Pagès C, Bianchini A (2019) Peroxynitrite generation and increased heterotrophic capacity are linked to the disruption of the coral–dinoflagellate symbiosis in a scleractinian and hydrocoral species. Microorganisms 7:426
McManus JW, Polsenberg JF (2004) Coral-algal phase shifts on coral reefs: ecological and environmental aspects. Prog Oceanogr 60:263–279
McWilliam M, Pratchett MS, Hoogenboom MO, Hughes TP (2020) Deficits in functional trait diversity following recovery on coral reefs. Proceedings of the Royal Society Biological Sciences 287:1–9
Mies M, Güth AZ, Tenório AA, Banha TNS, Waters LG, Polito PS, Taniguchi S, Bícego MC, Sumida PYG (2018) In situ shifts of predominance between autotrophic and heterotrophic feeding in the reef-building coral Mussismilia hispida: an approach using fatty acid trophic markers. Coral Reefs 37:677–689
Mies M, Francini-Filho RB, Zilberberg C, Garrido AG, Longo GO, Laurentino E, Güth AZ, Sumida PYG, Banha TNS (2020) South Atlantic Coral Reefs Are Major Global Warming Refugia and Less Susceptible to Bleaching. Front Mar Sci 7:514
Morais J, Morais RA, Tebbett SB, Pratchett MS, Bellwood DR (2021) Dangerous demographics in post-bleach corals reveal boom-bust versus protracted declines. Sci Rep 11:18787
Morelli TL, Barrows CW, Ramirez AR, Cartwright JM, Ackerly DD, Eaves TD, Ebersole JL, Krawchuk MA, Letcher BH, Mahalovich MF, Meigs GW, Michalak JL, Millar CI, Quiñones RM, Stralberg D, Thorne JH (2020) Climate-change refugia: biodiversity in the slow lane. Front Ecol Environ 18:228–234
Mouillot D, Villéger S, Parravicini V, Kulbicki M, Arias-González JE, Bender M, Chabanet P, Floeter SR, Friedlander A, Vigliola L, Bellwood DR (2014) Functional over-redundancy and high functional vulnerability in global fish faunas on tropical reefs. Proceedings of the National Academy of Sciences USA 111:13757–13762
Moura RL, Secchin NA, Amado-Filho GM, Francini-Filho RB, Freitas MO, Minte-Vera CV, Teixeira JB, Thompson FL, Dutra GF, Sumida PYG, Guth AZ, Lopes RM, Bastos AC (2013) Spatial patterns of benthic megahabitats and conservation planning in the Abrolhos Bank. Cont Shelf Res 70:109–117
Mumby PJ, Edwards AJ, Arias-González JE, Lindeman KC, Blackwell PG, Gall A, Gorczynska MI, Harborne AR, Pescod CL, Renken H, Wabnitz CC, Llewellyn G (2004) Mangroves enhance the biomass of coral reef fish communities in the Caribbean. Nature 427:533–536
Nogueira MM, Neves E, Johnsson R (2015) Effects of habitat structure on the epifaunal community in Mussismilia corals: does coral morphology influence the richness and abundance of associated crustacean fauna? Helgol Mar Res 69:221–229
Nogueira MM, Neves E, Johnsson R (2021) Effects of habitat structure on the mollusc assemblage in Mussismilia corals: evaluation of the influence of different coral growth morphology. J Mar Biol Assoc UK 101:61–69
Ong L, Holland KN (2010) Bioerosion of coral reefs by two Hawaiian Parrotfishes: species size differences and fishery implications. Mar Biol 157:1313–1323
Paula YC, Schiavetti A, Sampaio CL, Caleron E (2018) The effects of feeding by visitors on reef fish in a Marine Protected Area open to Tourism. Biota Neotropica 18
Pereira PHC, Lima G, Pontes A, Silva S, Silva E, Sampaio CLS, Pinto TK, Miranda RJ, Tiego A, Caon J, Seoane JCS (2022) Unprecedented coral mortality on Southwestern Atlantic (SWA) coral reefs following major thermal stress. Front Mar Sci 9:725778
Pereira-Filho GH, Mendes VR, Perry CT, Shintate GI, Niz WC, Sawakuchi AO, Bastos AC, Giannini PC, Motta FS, Millo C, Paula-Santos GM, Moura RL (2021) Growing at the limit: Reef growth sensitivity to climate and oceanographic changes in the South Western Atlantic. Global Planet Change 201:103479
Perry CT, Edinger EN, Kench PS, Murphy GN, Smithers SG, Steneck RS, Mumby PJ (2012) Estimating rates of biologically driven coral reef framework production and erosion: a new census-based carbonate budget methodology and applications to the reefs of Bonaire. Coral Reefs 31:853–868
Perry CT, Murphy GN, Kench PS, Smithers SG, Edinger EN, Steneck RS, Mumby PJ (2013) Caribbean-wide decline in carbonate production threatens coral reef growth. Nat Commun 4:1402
Pinheiro HT, Rocha LA, Macieira RM, Carvalho-Filho A, Anderson AB, Bender MG, Di Dario F, Ferreira CEL, Figueiredo-Filho J, Francini-Filho RB, Gasparini JL, Joyeux J-C, Luiz OJ, Mincarone MM, Moura RL, Nunes JACC, Quimbayo JP, Rosa RS, Sampaio CLS, Sazima I, Simon T, Vila-Nova DA, Floeter SR (2018) South-western Atlantic reef fishes: zoogeographical patterns and ecological drivers reveal a secondary biodiversity centre in the Atlantic Ocean. Divers Distrib 24:951–965
Pratchett MS, McWilliam MJ, Riegl B (2020) Contrasting shifts in coral assemblages with increasing disturbances. Coral Reefs 783–793
Randi CB, Becker AC, Willemes MJ, Perry CT, Salgado LT, Carvalho RT, Motta FS, Moura RL, Moraes FC, Pereira-Filho GH (2021) Calcium carbonate production in the southernmost subtropical Atlantic coral reef. Mar Environ Res 172:105490
Roff G, Zhao JX, Mumby PJ (2015) Decadal-scale rates of reef erosion following El Niño-related mass coral mortality. Global Change Biology 21:4415–1124
Sano M, Shimizu M, Nose Y (1987) Long-term effects of destruction of hermatypic corals by Acanthaster planci infestation on reef fish communities at Iriomote Island. Japan Marine Ecology Progress Series 37:191–199
Sheppard CR, Spalding M, Bradshaw C, Wilson S (2002) Erosion vs. recovery of coral reefs after 1998 El Niño: Chagos reefs, Indian Ocean. AMBIO: A Journal of the Human Environment. 31:40–48
Sheppard C, Dixon DJ, Gourlay M, Sheppard A, Payet R (2005) Coral mortality increases wave energy reaching shores protected by reef flats: examples from the Seychelles. Estuar Coast Shelf Sci 64:223–234
Siebeck UE, Marshall NJ, Klüter A, Hoegh-Guldberg O (2006) Monitoring coral bleaching using a colour reference card. Coral Reefs 25(3):453–460
Siebeck UE, Logan D, Marshall NJ (2008) CoralWatch—a flexible coral bleaching monitoring tool for you and your group. Proceedings of the 11th International Coral Reef Symposium 1:7–11
Skirving WJ, Heron SF, Marsh BL, Liu G, De La Cour JL, Geiger EF, Eakin CM (2019) The relentless march of mass coral bleaching: a global perspective of changing heat stress. Coral Reefs 38:547–557
Soares MO, Rossi S, Gurgel AR, Lucas CC, Tavares TC, Diniz B, Feitosa CV, Rabelo EF, Pereira PHC, Kikuchi RKP, Leão ZMAN, Cruz ICS, Carneiro PBM, Alvarez-Filip L (2021) Impacts of a changing environment on marginal coral reefs in the Tropical Southwestern Atlantic. Ocean Coast Manag 210:105692
Sully S, van Woesik R (2020) Turbid reefs moderate coral bleaching under climate-related temperature stress. Glob Change Biol 26:1367–1373
Tambutté S, Holcomb M, Ferrier-Pagès C, Reynaud S, Tambutté E, Zoccola D, Allemand D (2011) Coral biomineralization: From the gene to the environment. Journal of Marine Biology and Ecology 408:58–78
Teixeira CD, Leitão RL, Ribeiro FV, Moraes FC, Neves LM, Bastos AC, Pereira-Filho GH, Kampel M, Salomon PS, Sá JA, Falsarella LN, Amario M, Abieri ML, Pereira RC, Amado-Filho GM, Moura RL (2019) Sustained mass coral bleaching (2016–2017) in Brazilian turbid-zone reefs: taxonomic, cross-shelf and habitat-related trends. Coral Reefs 38:801–813
Toonen RJ, Bowen BW, Iacchei M, Briggs JC (2016) Biogeography, Marine. In: Kliman RM (ed) Encyclopedia of evolutionary biology, vol 1. Oxford Academic Press, pp 166–178
Tribollet A, Golubic S (2011) Reef bioerosion: agents and processes. Coral Reefs: An Ecosystem in Transition. Springer, Dordrecht, pp 435–449
Trygonis V, Sini M (2012) PhotoQuad: a dedicated seabed image processing software, and a comparative error analysis of four photoquadrat methods. J Exp Mar Biol Ecol 424:99–108
van Dam JW, Negri AP, Uthicke S, Mueller JF (2011) Chemical pollution on coral reefs: exposure and ecological effects. In: Sánchez-Bayo F, van den Brink PJ, Mann RM (eds) Ecological Impacts of Toxic Chemicals. Bentham Science Publishers, pp 187–211
Weis VM (2008) Cellular mechanisms of Cnidarian bleaching: stress causes the collapse of symbiosis. J Exp Biol 19:3059–3066
Werner TB, Pinto LP, Dutra GF, Pereira PG (2010) Abrolhos 2000: conserving the Southern Atlantic’s richest coastal biodiversity into the next century. Coast Manag 28:99–108
Wild C, Hoegh-Guldberg O, Naumann MS, Colombo-Pallotta MF, Ateweberhan M, Fitt WK, Iglesias-Prieto R, Palmer C, Bythell JC, Ortiz JC, Loya Y, Woesik RV (2011) Climate change impedes scleractinian corals as primary reef ecosystem engineers. Mar Freshw Res 62:205–215
Wismer S, Tebbett SB, Streit RP, Bellwood DR (2019a) Spatial mismatch in fish and coral loss following 2016 mass coral bleaching. Sci Total Environ 650:1487–1498
Wismer S, Tebbett SB, Streit RP, Bellwood DR (2019b) Young fishes persist despite coral loss on the Great Barrier Reef. Communications Biology 2:1–7
Acknowledgements
We would like to thank Coral Vivo Project and its sponsors Petrobras (Programa Petrobras Socioambiental) and Arraial d’Ajuda Eco Parque and also Thomás Banha for providing assistance with some of the data.
Author information
Authors and Affiliations
Contributions
GBB, CHFL, HE, PLD and MM designed the study; CHFL performed fieldwork; AMBR and KCCC provided infrastructure/material/technical support; GBB and AZG analyzed the data; and GBB, HE, AZG, KCCC, PLD and MM contributed to the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of the authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topic Editor Lauren T. Toth
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Braz, G.B., Lacerda, C.H.F., Evangelista, H. et al. Unprecedented erosion of Mussismilia harttii, a major reef-building species in the Southwestern Atlantic, after the 2019 bleaching event. Coral Reefs 41, 1537–1548 (2022). https://doi.org/10.1007/s00338-022-02303-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-022-02303-1