Abstract
The assessment of relevant spatial scales at which ecological processes occur is of special importance for a thorough understanding of ecosystem functioning. In coastal ecosystems, the variability of trophic interactions has been studied at different spatial scales, but never at scales from centimetres to metres. In the present study, we investigated the link between habitat structure and small-scale variability of food web functioning on intertidal boulder field ecosystems. Two microhabitats, boulder-top and boulder-bottom, were considered, and the trophic ecology of invertebrate consumers was studied using stable isotope tracers. We found for two of the main suspension feeders of northern Atlantic rocky shores (the sponges Halichondria panicea and Hymeniacidon sanguinea) consistent 15N enrichment for individuals sampled under boulders, suggesting that these consumers relied on different trophic resource according to the microhabitat inhabited, at a centimetre scale. The high δ15N signatures found underneath boulders suggested higher use of highly decomposed organic matter in this microhabitat. The isotopic difference between the two microhabitats decreased in higher trophic level consumers, which likely foraged at a spatial scale including both microhabitats. Finally, our results reveal that in highly heterogeneous habitats such as boulder fields, trophic interactions are likely to vary strongly in space, which should be considered in future researches. The link between habitat physical structure and food web variability might also contribute to the high biological diversity characterizing heterogeneous ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The study of food webs and trophic interactions in natural environments has risen increasing interest over the past 20 years. The development of methods such as stable isotope (δ13C, δ15N, δ34S) analysis allows now researchers to investigate the diet of consumers using a time-integrated method, thereby bypassing the different biases inherent to food choice experiments in controlled conditions. Several studies have shown that trophic interactions can be affected by numerous factors, resulting in a variability occurring at different spatial scales (e.g. Mallela and Harrod 2008; Carlier et al. 2009). In coastal ecosystems, consumers are often characterized by an important trophic plasticity, thereby limiting the possibility to generalize patterns observed on a specific site (Riera 2008). Different factors have been proposed to explain the plasticity of food web functioning at different scales, such as resource availability (Kanaya et al. 2007), anthropogenic disturbance (Mallela and Harrod 2008) or hydrodynamic exposure (Bode et al. 2006; Schaal et al. 2009). However, most of these studies addressed spatial scales from local to regional, and few studies addressed issues on small-scale variability of trophic links. Identifying relevant spatial scales is a major concern in ecology. In highly heterogeneous habitats, the pattern observed at a given spatial scale may in fact result from the combination of several patterns driven by factors acting at smaller scales (Levin 1992). Our perception of the main processes driving patterns observed in the nature is therefore strongly dependant of the observation scale chosen for the sampling strategy (Levin 1992; Bishop et al. 2002). Hence, there is a clear need to identify ecological processes affecting small spatial scales in food webs.
Guest et al. (2004) have shown in Australian mangroves that carbon source of the crabs Australoplax tridentata and Parasesarma erythrodactyla differed at a scale of metres according to the dominant local primary producer. In another mangrove forest, Kon et al. (2007) also observed that the diet of the bivalve Laternula truncata changed according to the microhabitat (i.e. tidal creeks, forest, forest gaps) where it was found. These examples illustrate the difficulty to generalize trophic patterns obtained from studies that often do not consider spatial variability on the whole range of its possible extend. Further studies are therefore required to clarify the consistence of trophic interactions at different small spatial scales.
During the five last years, the trophic functioning of intertidal rocky ecosystems has been extensively studied (Bode et al. 2006; Sarà et al. 2007; Schaal et al. 2008; Riera et al. 2009; Steinarsdottir et al. 2009; Schaal et al. 2010a). However, most of these studies were carried out on a single site (but see Bode et al. 2006; Sarà et al. 2007), and few is known about the spatial consistence of the patterns observed. These ecosystems are constituted by numerous microhabitats, which have been proposed to be an important driver of biodiversity (Le Hir and Hily 2005). Moreover, the diversity of microhabitats in rocky shore habitats has recently been proposed to enhance trophic complexity in highest trophic levels, through the co-occurrence of predators specialized on the different microhabitats (Schaal et al. 2010a). It is however still unknown whether the physical structure of habitat affects low trophic levels, and in particular, the trophic resource of filter feeders, which are key consumers of rocky shore communities.
The present study aimed at establishing whether the trophic resource of consumers belonging to various trophic groups was affected by small-scale variability in different microhabitats of Northern Brittany (France) intertidal rocky shores.
Materials and methods
Study sites, sampling and sample processing
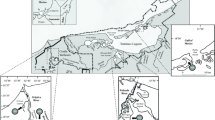
Samples were taken from two contrasting sites of Northern Brittany (Fig. 1). Ar Pourven site is located in the inner Bay of Morlaix (Northern Brittany, France) and is characterized by a granite boulder field with a high cover of the kelp Laminaria digitata, and the presence of a typical Fucus serratus—Mastocarpus stellatus—Corallina elongata understory algal assemblage. To test for the spatial consistency of microscale patterns observed at this first site, measurements were also carried out at the Western end of Batz Island, about five kilometres north-west of Ar Pourven. This second study site is a boulder field characterized by similar algal assemblages, but very exposed hydrodynamic conditions.
All field excursions were carried out during spring tides in February, August and November 2007 at Ar Pourven, and in February at Batz Island. Two microhabitats, boulders top and boulders bottom, were selected according to Le Hir and Hily (2005) as the most significant for rocky shore-associated communities. Several species belonging to different trophic groups were collected from these two microhabitats. A majority of boulders from where samples were collected measured between 50 cm and 1 m of diameter. Animals analysed for stable isotopes were (1) the most representative filter feeders and (2) other species occurring simultaneously at the surface and underneath the boulders. Twenty-eight species were collected, including mainly filter feeders (20 species), predators (5 species, Trivia arctica, Calliostoma zizyphinum, Nassarius incrassatus, Ocenebra erinacea, Nucella lapillus), grazers (2 species, Gibbula cineraria, Gibbula pennanti) and deposit feeders (1 species, Anapagurus hyndmanni). In the laboratory, animals were starved overnight to empty their gut and then rapidly frozen. Although we are aware that each step of the sample preparation procedure for stable isotopes analysis, including freezing (Feuchtmayr and Grey 2003), is likely to affect stable isotope ratios, this alteration will affect our samples in he same way, since the same procedure was used for samples of the two microhabitats. Our conclusions concerning the relative differences associated with the exploitation of different microhabitats will therefore not be affected by any bias linked to sample preparation procedure. Animals were extracted from their shell (molluscs) or cuticle (crustaceans), and the flesh was quickly acidified (HCl, 1 N) to remove any carbonated debris. Because sponges are often covered by large amounts of organic and inorganic particles, they were cut into thin slices using a scalpel and all exogenous particles were removed under a dissecting microscope. The surface of these organisms was then discarded, and only the core was considered for stable isotope analyses. Because most of animals analysed in this study were small or calcifying, physical removal of carbonated parts was impossible. However, we tried, as much as possible, to shorten the duration of acidification (typically less than 1 min), to limit its possible effects on consumers’ stable isotope signature. The flesh was rinsed with distilled water and dried (60°C, 48 h). When possible, only muscle tissues were analysed, but small specimens were analysed entirely. Dried samples were ground into a fine and homogeneous powder using a mortar and a pestle and weighed in tin capsules for isotopic analysis. Most species were analysed in triplicates, except some species for which we only managed to perform a single measurement (Table 1), and for the sponges Halichondria panicea and Hymeniacidon sanguinea, for which 5 replicates were analysed.
Stable isotope measurements
Carbon and nitrogen isotope ratios were determined using a Flash EA CN analyser coupled with a Finnigan Delta Plus mass spectrometer, via a Finnigan Con-Flo III interface. Data are expressed in the standard δ unit.
with R = 13C/12C for carbon and 15N/14N for nitrogen. These abundances were calculated in relation to the certified reference materials Vienna Pee Dee Belemnite-limestone (V-PDB) and atmospheric N2 (at-air). The V-PDB and at-air scaling were achieved using in-house protein standards that have been calibrated against NBS-19 and IAEA N3 reference materials. The standard deviation of repeated measurements of δ13C and δ15N values of a laboratory standard was 0.10 ‰ versus V-PDB and 0.05 ‰ versus at-air, respectively.
Statistical analyses
Because the small number of individuals analysed for each species was not sufficient to satisfy the conditions of normality, non-parametric Mann–Whitney U tests were used to compare the stable isotope signatures of species found both on the top and underneath boulders. The same test was also used to compare the general stable isotope ratios of the entire filter-feeder communities inhabiting the two microhabitats. A linear regression was performed, and Pearson’s correlation index was used to assess the relationship between the δ15N of consumers from the different trophic groups between the two microhabitats. Basic assumption of the linear regression (linearity of the relationship, independence and homoscedasticity of the errors, normality of the error distribution) was met and checked through the visualization of residuals versus predicted values plot, and normality probability plot of residuals plot.
Results
Specific focus was aimed at two sponges, Halichondria panicea and Hymeniacidon sanguinea, which are among the most abundant consumers of rocky environments in Brittany and specifically at the two field sites (G. Schaal, personal observation). Halichondria panicea was collected on two occasions at the same site, and Hymeniacidon sanguinea was collected at two different sites on the same date (Fig. 2). For both species and both sites, individuals sampled underneath boulders were characterized by a significant 15N enrichment compared to those sampled on the top (Mann–Whitney U tests, P < 0.05). This δ15N difference ranged from 1.1‰ (Halichondria panicea, February, Ar Pourven) to 2.5 ‰ (Hymeniacidon sanguinea, February, Ar Pourven). In contrast, δ13C values did not differ between the two microhabitats for these two species (data not shown).
Stable isotope ratios of the main filter feeders at Ar Pourven remained relatively unchanged through time (from −20 to −16 ‰ and from 6 to 10 ‰ for δ13C and δ15N, respectively). Considering the microhabitat where these consumers were sampled, the pattern obtained from the two microhabitats in February differed markedly from August and November (Fig. 3; Table 1). In February, all filter feeders found underneath the rocks displayed higher δ15N than those collected from the top (ranged from 8 to 9.7 ‰ from boulder to bottom and from 7.2 to 8 ‰ from boulder to top) (Mann–Whitney U test, P = 0.006). However, due to marked overlap of δ15N signatures between suspensivores from the two microhabitats, this pattern less marked in August and November than in February (Mann–Whitney U tests, P = 0.54 and P = 0.15 for February and August, respectively). Similar to the results obtained for the two sponge species, no clear distinction was observed between the δ13C of filter feeders of the two microhabitats.
For all species found simultaneously both on the top and underneath boulders, δ15N was plotted against the δ15N difference between the two microhabitats (Fig. 4). Filter feeders, which displayed the lowest δ15N, were found to display the highest difference between the two microhabitats, while predator species, which displayed the highest δ15N, displayed little or no difference according to the microhabitat. Considering the different functional trophic groups, a significant negative relationship was observed between the δ15N and the δ15N difference between the two microhabitats (y = −0.31x + 3.17; R² = 0.43; P < 0.05). This relationship suggested that the isotopic difference between the two microhabitats was greater for species displaying more 15N-depleted stable isotope ratios.
Discussion
Our results indicate a relationship between fine scale habitat structure and community trophic organization. This study is, to our knowledge, the first to highlight food web variations occurring in several benthic species at such a small scale in coastal rocky environments.
Sponges are key consumers in several coastal ecosystems and have a paramount functional role owing to the large water volumes filtered by these organisms (Bell 2008). In coastal areas, sponges feed on composite pool of suspended organic matter. In macroalgae-dominated ecosystems, this pool is dominated by algal detritus that likely constitutes a substantial part of the diet of filter feeders (Bustamante and Branch 1996). In the present study, the δ15N values of the Hymeniacidon sanguinea and Halichondria panicea were significantly higher for individuals found under the rocks than for those inhabiting boulder top (Fig. 2). Although animals fed with the same food source can sometimes display different isotopic signatures due to differing fractionation coefficients during assimilation (Vanderklift and Ponsard 2003), the spatial and temporal consistency of this difference strongly suggests that sponges living on the top and bottom surfaces of boulders do not rely on the same trophic resource.
The 15N enrichment occurring during degradation is likely to vary substantially among macroalgae species, thereby making it difficult to assess detritus isotopic signatures on the sole basis of fresh plants (Hill and McQuaid 2009). However, most studies dealing with changes in algal isotopic ratios during degradation reported some 15N enrichment but little or no change in δ13C signatures (Stephenson et al. 1986; Schaal et al. 2010b). Consequently, δ15N variations observed in the present study suggest that sponges living under rocks consume an organic matter source that is more degraded than organic matter available to boulder-top animals. A second hypothesis for the 15N enrichment found in animals underneath the rocks is that sponges assimilate detritus of different origin according to the microhabitat they inhabit. However, this latter hypothesis would imply that the identity of macroalgae constituting the pool of suspended particulate organic matter differs between these two microhabitats, which is very unlikely. The consumption of macroalgae-derived detritus by filter feeders has been reported in similar ecosystems along the eastern Atlantic coasts (Fredriksen 2003; Bode et al. 2006; Schaal et al. 2009).
In fact, the accumulation of highly degraded detritus under the rocks could arise from higher particle retention due to decreased water motion and water retention during low tide. Macroalgae detritus produced in boulder fields are trapped in the numerous cavities under the rocks and then decomposed by bacteria, leading to 15N enrichment due to the preferential mineralization of 14N by bacteria (Thornton and McManus 1994). This 15N-enriched pool of organic matter may be, then, transferred to upper trophic levels through its consumption by filter feeders. Accordingly, the cavities underneath the rocks would represent hotspots of bacterial activity, playing a crucial role in the fate of algal detritus on rocky shores. However, further studies should be necessary to confirm this hypothesis, for instance through performing fatty acid analyses and bacterial production measurements in the water.
Although less marked than for sponges, a similar trend was found for most of the different co-occurring filter feeders (Fig. 4). The suspended particles retained by suspensivores vary according to their feeding apparatus (Decottignies et al. 2007). Among this trophic group, sponges are known to most efficiently retain small (<10 μm) particles (Thomassen and Riisgård 1995). Preferential assimilation of this size class, which is the typical size range of bacteria (Rolff 2000), may explain why the isotopic difference observed is more pronounced in sponges than in other suspensivores, which select preferentially larger particles (Lesser et al. 1992).
The wide δ13C and δ15N ranges displayed by the most common suspensivores reveal the ability of benthic consumers to differentially assimilate different components of the suspended organic matter (e.g. Duggins and Eckman 1997; Schaal et al. 2008). In February, suspensivores from boulder tops were more 15N-enriched than those inhabiting boulder bottom (Fig. 3). This result indicates that individuals underneath the rocks and on their surface do not use the same trophic niche and that available food source differs between the two microhabitats. Resource partitioning between these two microhabitats is thus observed for the entire community. The widespread 15N enrichment supports the idea that water underneath boulders is abundant with detritus-decomposing bacteria and highlights the role of habitat structure on the trophic functioning of rocky marine communities. This pattern was less clear in August and in November, due to partial isotopic overlap between suspensivores of the two microhabitats (Fig. 3). For these two dates, the δ15N difference was, however, still significant in the sponges. This finding suggests that seasonality of resource partitioning may be related to temporal changes in food source availability. For instance, in the western English Channel, the lowest phytoplankton abundances occur in February (Not et al. 2004), which might create a necessity for filter feeders underneath boulders to seek alternate food sources, hence leading to an increased consumption of detritus.
Since all species were collected simultaneously from the two microhabitats, the significant relationship observed between δ15N signatures and the difference between animals inhabiting the top and bottom of rocks (Fig. 4) may indicate an ecologically consistent trend. The use of δ15N as trophic level indicator is relevant in Laminaria digitata beds due to the homogeneity of δ15N found in basal food sources (Schaal et al. 2010b). The overall trend in δ15N suggests a negative relationship between consumer trophic level and the isotopic difference associated with microhabitats, that is, resource partitioning. This pattern is likely sustained by the mobility of intertidal communities. Most of the primary consumers sampled in this study are indeed sessile or display low mobility, while predators are more likely to forage at larger spatial scales, thereby exploring different microhabitats. This pattern is strengthened by the ability of suspensivores to select small-sized particles, which are likely to be the most 15N-enriched, due to putatively higher abundance of bacteria in this particular microhabitat. According to this relationship, low trophic level species closely reflect their feeding habitat, while predators, by foraging at wider spatial scales, reflect an average of the heterogeneous stable isotope signatures arising from a variety of microhabitats within boulder fields. Hence, by feeding on prey in both boulder-top and boulder-bottom habitats, predators may represent an important coupling between spatially separated trophic pathways.
Stable isotope data are often used to calculate the contribution of different potential food sources to the diet of a consumer, through the use of mixing models (e.g. Phillips and Gregg 2001). The differences associated with the different microhabitats found in the present study (up to 2.5 ‰) are likely to strongly affect the outcomes of such models and hence the conclusions of stable isotope studies in highly complex habitats. Even though recent studies proposed, through a Bayesian approach, to incorporate sources of uncertainty in isotope mixing models (e.g. Parnell et al. 2010), the direct assessment of habitat heterogeneity-induced variability is necessary because (1) recent isotope mixing models only consider normally distributed sources of variability (e.g. trophic-step fractionation), which might not be the case of habitat heterogeneity-linked variability (because of the potential diversity of microhabitat and trophic conditions associated), and (2) the variability in stable isotope ratios associated with the different microhabitats reveals ecologically relevant processes, which give information on the link between habitat structure and ecosystem functioning. An important step that should not be omitted in future studies addressing food web issues in heterogeneous habitats is therefore to prior identify the different microhabitats and their potential effects on the trophic resource of consumers, and then to define the relevant spatial scale to address their ecological questions. This step seems to be necessary to integrate the results of local studies at larger spatial scales.
Conclusion
This study clearly shows that in rocky shore ecosystems, food webs display a marked spatial structure at small scale and that microhabitats induce intra- and inter-specific trophic segregation. The differences observed here in the stable isotope rations of consumers reveal that habitat structure enhances the diversity of ecological conditions, leading to an increased trophic diversity in the food web. Future studies of food webs in highly heterogeneous habitats such as rocky shores should therefore incorporate a small-scale perspective of consumers’ stable isotope ratio.
Several studies have attempted to identify the ecological mechanisms underlying the relationship between habitat structure and biodiversity (e.g. McGuiness and Underwood 1986; Beck 2000). Our results show that the different microhabitats constituting rocky shores are associated with different trophic conditions, suggesting that highly complex habitats necessarily include more trophic niches than structurally simple ones. Identifying the relative importance of the “trophic factor” compared to other mechanisms linking habitat structure to biodiversity represents a promising perspective for the understanding, conservation and management of biodiversity within natural and artificial ecosystems.
References
Beck MW (2000) Separating the elements of habitat structure: independent effect of habitat complexity and structural components on rocky intertidal gastropods. J Exp Mar Biol Ecol 249:29–49
Bell JJ (2008) The functional roles of marine sponges. Est Coast Shelf Sci 79:341–353
Bishop MJ, Underwood AJ, Archambault P (2002) Sewage and environmental impacts on rocky shores: necessity of identifying relevant spatial scales. Mar Ecol Prog Ser 236:121–128
Bode A, Alvarez-Ossorio MY, Varela M (2006) Phytoplankton and macrophyte contributions to littoral food webs in the Galician upwelling estimated from stable isotopes. Mar Ecol Prog Ser 318:89–102
Bustamante R, Branch G (1996) The dependence of intertidal consumers on kelp-derived organic matter on the west coast of South Africa. J Exp Mar Biol Ecol 196:1–28
Carlier A, Riera P, Amouroux J-M, Bodiou J-Y, Desmalades M, Grémare A (2009) Spatial heterogeneity in the food web of a heavily modified Mediterranean coastal lagoon: stable isotope evidence. Aquat Biol 5:167–179
Decottignies P, Beninger PG, Rincé Y, Riera P (2007) Trophic interactions between two introduced suspension-feeders, Crepidula fornicata and Crassostrea gigas, are influenced by seasonal effects and qualitative selection capacity. J Exp Mar Biol Ecol 342:231–241
Duggins DO, Eckman JE (1997) Is kelp detritus a good food for suspension feeders? Effect of kelp species, age and secondary metabolites. Mar Biol 128:489–495
Feuchtmayr H, Grey J (2003) Effect of preparation and preservation procedures on carbon and nitrogen stable isotope determinations from zooplankton. Rapid Commun Mass Spectrom 17:2605–2610
Fredriksen S (2003) Food web studies in a Norwegian kelp forest based on stable isotope (δ13C and δ15N) analysis. Mar Ecol Prog Ser 260:71–81
Guest MA, Connolly RM, Loneragan NR (2004) Carbon movement and assimilation by invertebrates in estuarine habitats at a scale of metres. Mar Ecol Prog Ser 278:27–34
Hill JM, McQuaid CD (2008) δ13C and δ15N biogeographic trends in rocky intertidal communities along the coast of South Africa. Evidence of strong environmental signatures. Est Coast Shelf Sci 80:261–268
Hill JM, McQuaid CD (2009) Variability in the fractionation of stable isotopes during degradation of two intertidal red algae. Est Coast Shelf Sci 82:397–405
Kanaya G, Takagi S, Nobata E, Kikuchi E (2007) Spatial dietary shift of macrozoobenthos in a brackish lagoon revealed by carbon and nitrogen stable isotope ratios. Mar Ecol Prog Ser 345:117–127
Kon K, Kurokura H, Hayashizaki K (2007) Role of microhabitats in food webs of benthic communities in a mangrove forest. Mar Ecol Prog Ser 340:55–62
Le Hir M, Hily C (2005) Macrofaunal diversity and habitat structure in intertidal boulder fields. Biodivers Conserv 14:233–250
Lesser MP, Shumway SE, Cucci T, Smith J (1992) Impact of fouling organisms on mussel rope culture: interspecific competition for food among suspension-feeding invertebrates. J Exp Mar Biol Ecol 165:91–102
Levin S (1992) The problem of pattern and scale in ecology. Ecology 73:1943–1967
Mallela J, Harrod C (2008) δ13C and δ15N reveal significant differences in the coastal foodwebs of the seas surrounding Trinidad and Tobago. Mar Ecol Prog Ser 368:41–51
McGuiness KA, Underwood AJ (1986) Habitat structure and the nature of communities on intertidal boulders. J Exp Mar Biol Ecol 104:97–123
Not F, Latasa M, Marie D, Cariou T, Vaulot D, Simon N (2004) A single species Micromonas pusilla (Prasinophyceae) dominates the eukaryotic picoplankton in the Western English Channel. Appl Environ Microbiol 70:4064–4072
Parnell AC, Inger R, Bearhop S, Jackson AL (2010) Source partitioning using table isotopes: coping with too much variation. PLoS ONE 5:e9672
Phillips DL, Gregg JW (2001) Uncertainty in source partitioning using stable isotopes. Oecologia 127:171–179
Riera P (2008) Trophic plasticity in similar habitats: an example which severely limits generalization among ecosystems. JMBA2—Biodiversity Records
Riera P, Escaravage C, Leroux C (2009) Trophic ecology of the rocky shore community associated to the Ascophyllum nodosum zone (Roscoff, France): a δ13C vs δ15N investigation. Est Coast Shelf Sci 81:143–148
Rolff C (2000) Seasonal variation in δ13C and δ15N of size-fractionated plankton at a coastal station in the northern Baltic proper. Mar Ecol Prog Ser 203:47–65
Sarà G, De Pirro M, Romano C, Rumolo P, Sprovieri M, Mazzola A (2007) Sources of organic matter for intertidal consumers on Ascophyllum-shores (SW Iceland): a multi-stable isotope approach. Helgol Mar Res 61:297–302
Schaal G, Riera P, Leroux C (2008) Trophic coupling between two adjacent benthic food webs within a man-made intertidal area: a stable isotopes evidence. Est Coast Shelf Sci 77:523–534
Schaal G, Riera P, Leroux C (2009) Trophic significance of the kelp Laminaria digitata (Lamour.) for the associated food web: a between-sites comparison. Est Coast Shelf Sci 85:565–572
Schaal G, Riera P, Leroux C, Grall J (2010a) A seasonal stable isotope survey of the food web associated to a peri-urban rocky shore. Mar Biol 157:283–294
Schaal G, Riera P, Leroux C (2010b) Trophic ecology in a Northern Brittany (Batz Island, France) kelp (Laminaria digitata) forest, as investigated through stable isotopes and chemical assays. J Sea Res 63:24–35
Steinarsdottir MB, Ingolfsson A, Olafsson E (2009) Trophic relationships on a fucoid shore in south-western Iceland as revealed by stable isotope analyses, laboratory experiments, field observations and gut analyses. J Sea Res 61:206–215
Stephenson RL, Tan FC, Mann KH (1986) Use of stable carbon isotope ratios to compare plant material and potential consumers in a seagrass bed and a kelp bed in Nova Scotia, Canada. Mar Ecol Prog Ser 30:1–7
Thomassen S, Riisgård HU (1995) Growth and energetics of the sponge Halichondria panicea. Mar Ecol Prog Ser 128:239–246
Thornton SF, McManus J (1994) Application of organic carbon and nitrogen stable isotope and C/N ratios as source of organic matter provenance in estuarine systems: evidence from the Tay Estuary, Scotland. Est Coast Shelf Sci 38:219–233
Vanderklift MA, Ponsard S (2003) Sources of variation in consumer-diet δ15N enrichment: a meta-analysis. Oecologia 136:169–182
Acknowledgments
This work was funded by the French National Research Agency (ANR) ECOKELP. G.S. received benefits from a South African National Research Foundation (NRF) and Rhodes University postdoctoral research fellowship. The authors would like to thank Dr Nicole Richoux and two anonymous reviewers, whose constructive comments greatly improved this manuscript. C. Broudin, C. Houbin, V. Ouisse and A. Vettoretti are also thanked for their help during field sampling.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bulleri.
Rights and permissions
About this article
Cite this article
Schaal, G., Riera, P. & Leroux, C. Microscale variations of food web functioning within a rocky shore invertebrate community. Mar Biol 158, 623–630 (2011). https://doi.org/10.1007/s00227-010-1586-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-010-1586-2