Abstract
We characterized the structure and functioning of the food web associated to an intertidal rocky shore in the harbour of Brest (Western Brittany, France), through a seasonal δ13C and δ15N survey. The dominance of benthic-derived organic matter, and particularly resuspended sediment, in the local pool of suspended organic matter, is highlighted. This benthic-derived organic matter dominates the diet of filter-feeders, including a certain degree of selectivity displayed by various taxa belonging to this trophic group. The food web structure appeared very stable temporally, which suggested the dominance of mixed diets, rather than the consumption of pure sources. It was constituted of 4 trophic levels, including a large diversity of predators, likely favoured by the diversity of microhabitats that characterize this intertidal habitat. From our results, the trophic functioning of this peri-urban rocky shore community does not appear functionally simplified as previously reported for other anthropized and/or artificial rocky ecosystems. We suggest that the topological complexity of rocky habitats, including a diversity of microhabitats, might be responsible of the important animal diversity, in spite of the anthropogenic disturbances characterizing this peri-urban area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The structure and functioning of rocky shore-associated food webs subjected to anthropogenic disturbances is a topic raising increasing interest for a few years. Consumers inhabiting rocky shore habitats encounter a wide diversity of potentially available food sources, including several coexisting macroalgae, epilithic or epiphytic biofilms, detritus of various origins and phytoplankton (Bode et al. 2006). Within intertidal rocky habitats, recent findings have therefore highlighted the coexistence of distinct major trophic pathways, based either on benthic production or on pelagic production (Bode et al. 2006; Riera et al. 2009). This is an important difference between the trophic functioning of rocky shore and other marine ecosystems (e.g. Rau et al. 1990; Carlier et al. 2007). The consumption of macroalgae has been reported to occur mainly through the detrital pathway (Duggins et al. 1989; Bustamante and Branch 1996; Riera et al. 2009), which makes algal matter readily available to various trophic groups. For instance, on South African exposed rocky shores, this contribution was estimated at 60% of the diet of three filter-feeding species (Bustamante and Branch 1996). This consumption has been reported to be maximal during winter, when phytoplanktonic production is low (Duggins et al. 1989). Other benthic food sources may also be locally important for the food web, such as epiphytic or epilithic biofilms, which are the main food source of Trochidae and Patellidae gastropods (Peduzzi 1987; Jenkins and Hartnoll 2001). These gastropods represent an important biota on North Atlantic rocky shores (Little and Kitching 1996).
Hydrodynamics also affects the local availability of food sources. For instance, in the Bay of Arcachon, tide currents have been observed to enhance trophic transfers between an artificial rocky habitat and its adjacent environment, via the resuspension of seagrass detritus (Schaal et al. 2008). In the Seto Inland Sea, Takai et al. (2004) also observed a resuspension of epilithic microalgae by hydrodynamism, leading to the assimilation of this matter by local filter-feeders. Hydrodynamism can also affect food webs by introducing drifted macroalgae in barren grounds (Kang et al. 2008), therefore allowing the establishment of rich and diversified communities in poorly productive environments (Bustamante et al. 1995).
In addition, due to the concentration of human population along the coastal line, rocky shores are particularly subjected to disturbances related to anthropogenic activities (Benedetti-Cecchi and Chato Osio 2007). Although the effects of various human disturbances on the structure and functioning of soft-sediments communities have been widely studied (Grall and Glémarec 1997; Guyonnet et al. 2008), their impact on hard-bottom environments-associated food webs are to date still poorly understood. In particular, most of stable isotopes studies dealing with trophic functioning of rocky shore communities took place in relatively pristine environments (Fredriksen 2003; Riera et al. 2009). Recent investigations (Riera et al. 2004; Schaal et al. 2008) have, however, pointed out several characteristics of peri-urban areas-associated foods webs and the need for studies assessing their structure and functioning. These studies are necessary for the development of conceptual and predictive models for sustainable use and conservation of such environments (Humborg et al. 2000).
The use of stable isotopes (δ13C and δ15N) provides new insights for the study of trophic interactions in littoral rocky environment. Several studies have highlighted the possibility to discriminate, especially with δ13C, between pelagic and benthic primary producers (Fry and Sherr 1984) and among benthic primary producers (Raven et al. 2002). Due to the low 13C-enrichment occurring between a food source and its consumer, it is possible to track trophic transfers in rocky environments, where the food sources diversity is important. Moreover, the large δ15N fractionation occurring at each trophic transfer allows to infer important structural features of food webs, such as the number of trophic levels (Cabana and Rasmussen 1996), or the prevalence of omnivory (Bode et al. 2006).
In this ecological context, the aim of this study was to assess the structural and functional features of an intertidal rocky shore food web in a heavily impacted harbour. Based on δ13C and δ15N, the study focussed on seasonal and taxonomic variability of major trophic pathways in this habitat. Particular attention was paid to study whether the diversity of food sources available had an effect on the temporal stability of the food web.
Materials and methods
Study site and sampling
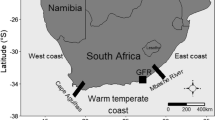
The Bay of Brest is a shallow semi-enclosed embayment located on the west coast of Brittany (France). The maximal tidal amplitude in this area is 8 m. This Bay is affected by nitrogen inputs from the two main tributaries, the Aulne and the Elorn, draining an extended catchment of 2,800 km2, where intensive pig, cattle and poultry farming occur (Grall and Glémarec 1997). Sampling was carried out in the low mediolittoral/high infralittoral fringe during spring tides in Brest harbour (Fig. 1). The rocky shore in this area is a rocky shore nearby the external breakwater. This site was chosen due to its proximity to the important harbour complex of Brest, which includes a marina, a commercial harbour and a military harbour. Brest is the most important city of Western Brittany, with more than 140,000 residents. Although there is a lack of recent published data concerning the pollution status of this area, Grall and Glémarec (1997) have observed the presence of highly impacted soft-bottom communities close to our site. Although some evidence for water quality improvement exist (Grall, unpublished data), this site is characterized by high anthropogenic impact due to the vicinity of the harbour facilities and the artificial nature of the shore.
The sampling took place in November 2006 and in January and May 2007. The more representative algae and consumers were collected by hand during low tides. Epiphytic and epilithic biofilms were also sampled by gently scrapping the substrate with a thin brush, and then resuspended in filtrated (0.45 μm) sea water. Sedimented organic matter (SOM) cover from underneath the underside of the rocks was sampled in the same way as biofilms. At each sampling, water (10 l) was collected to assess suspended particulate organic matter (SPOM) isotopic signature from the site. The δ13C and δ15N of offshore suspended particulate organic matter (OPOM) was obtained by referring to Lorrain et al. (2002).
Sample processing
Once back to the laboratory, algae were cleaned of any eventual macroepiphytes, rinsed with distilled water and dried (48 h, 60°C). Animals were starved overnight in filtered sea water (0.45 μm) and killed by freezing. They were extracted from their shell or cuticle, quickly acidified (HCl, 1 N), rinsed with distilled water and dried (48 h, 60°C). For large animals, only muscle was analysed, while small organisms were analysed entirely. All dried samples were ground into a fine and homogeneous powder using a mortar and a pestle and stored frozen (−20°C) until subsequent analysis.
Samples of epiphytic and epilithic biofilms and site water were prefiltered on a 63-μm mesh to remove large detritus and small animals, and then filtered on precombusted GF/F filters (4 h, 500°C). The filters were then quickly acidified (HCl, 1 N), rinsed with distilled water and dried (24 h, 60°C). They were then stored frozen (−20°C) until subsequent analysis.
Although several studies have reported possible stable isotope ratios modifications associated with samples acidifications (e.g. Bunn et al. 1995), the duration chosen for acidification was clearly too short to cause significant alteration of both δ13C and δ15N (a few seconds in most cases and never more than 5 min).
Stable isotopes measurements
Carbon and nitrogen isotope ratios were determined using a Flash EA CN analyser coupled with a Finnigan Delta Plus mass spectrometer, via a Finnigan Con-Flo III interface. Data are expressed in the standard δ unit.
with R = 13C/12C for carbon and 15N/14N for nitrogen. These abundances were calculated in relation to the certified reference materials Vienna Pee Dee Belemnite-limestone (V-PDB) and atmospheric dinitrogen (at-air). The V-PDB and at-air scaling were achieved using in-house protein standards that have been calibrated against NBS-19 and IAEA N3 reference materials. The standard deviation of repeated measurements of δ13C and δ15N values of a laboratory standard was 0.10‰ versus V-PDB and 0.05‰ versus at-air, respectively.
SPOM composition
The isotopic composition of SPOM was estimated by using the IsoSource model (Phillips and Gregg 2003). Five end-members were identified: (1) offshore POM, which may be assimilated to “pure” phytoplankton as sampled far from the study site, (2) SOM which has been observed in other environments to be subject to resuspension (Sarà et al. 2007)), and would thus contribute to the SPOM pool, (3) detritus from macroalgae, which were assimilated to the mean of δ13C and δ15N of all co-occurring macroalgae, (4) epilithic biofilm, which has also been observed to be subject to resuspension events (Takai et al. 2004) and (5) epiphytes. IsoSource calculations were performed using various tolerance threshold values, to take into account isotopic variability of end-members. Since the results did not vary much according to the tolerance value used, all the results presented here were acquired using a standard value of 0.1 (Phillips and Gregg 2003).
Statistical analyses
As the number of individuals analysed for each species (i.e. 3 or 5 when possible) was not sufficient to meet the conditions of normality, nonparametric tests were carried out. For species sampled at the three sampling dates, Kruskal–Wallis followed by post hoc tests were performed. For species sampled only twice, Mann–Whitney tests were performed. No statistical comparisons were carried out for species sampled only at one occasion or for counts lower than three individuals per species.
A hierarchical cluster analysis using the Ward’s minimum variance method was performed on δ13C and δ15N of filter-feeding species to identify within this trophic group species with similar trophic characteristics.
Results
Stable isotopes ratios of food sources
The δ13C of the potential food sources sampled for this study ranged from −33.1‰ (Lomentaria articulata, January) to −11.7‰ (Himanthalia elongata, May) (Fig. 2). The range of δ13C displayed by food sources at each sampling date was 12.2, 15.8 and 13.1‰ for November, January and May, respectively. However, this wide range of δ13C was mostly due to a few species displaying extreme δ13C values (e.g. Lomentaria articulata, Gelidium latifolium and Himanthalia elongata). The majority of macroalgae displayed similar δ13C comprised between −17 and −15‰. The range of δ15N displayed by macroalgae was 6, 5 and 5.1‰ at the three dates, respectively. The values of δ15N displayed by macroalgae were comprised between 6.3 and 10.2‰, which is much lower than the values reported for macroalgae sampled in a highly polluted environment (Riera et al. 2000).
δ13C versus δ15N of potential food sources and consumers sampled in November 2006, January 2007 and May 2007 in Brest harbour. Polygons represent the total areas encompassed by trophic groups, namely carnivores, grazers and filter-feeders. Arrows represent the mean trophic fractioning occurring between a food source and its consumer, according to Vander Zanden and Rasmussen (2001) and Vanderklift and Ponsard (2003). OPOM offshore particulate organic matter, SPOM site particulate organic matter, SOM sedimented organic matter, Eφ epiphytes, El epilithic biofilm, Ent Enteromorpha sp., Ulv Ulva sp., Gl Gelidium latifolium, Ms Mastocarpus stellatus, Pp Palmaria palmata, Fs Fucus serratus, Fv Fucus vesiculosus, He Himanthalia elongata, Ls Laminaria saccharina
The SPOM displayed δ13C ranging from −19.8 to −18.7‰, which was systematically 13C-enriched for at least 4‰ compared to OPOM values reported by Lorrain et al. (2002). Whatever the sampling date, the isotopic signatures of SPOM and SOM were very similar.
The temporal variations of isotopic ratios were consistent among macroalgae. Indeed, the δ13C of the four main species (i.e. Ulva sp., Palmaria palmata, Fucus serratus and Himanthalia elongata) was similar in November and in May, while the mean δ13C measured in January for these species was significantly lower (Kruskal–Wallis test, P < 0.05). For these species, the variations of δ15N were less important and not statistically significant (P > 0.05), excepted for Palmaria palmata (H = 7.26, P = 0.0265). In contrast to macroalgae, no consistent temporal pattern emerged for the isotopic signatures of other potential food sources (i.e. SPOM, SOM, Epiphytes and Epilithic biofilm).
SPOM composition
The SPOM composition varied greatly seasonally. The results strongly suggest that SOM is the main contributor to the pool of SPOM, with a contribution comprised between 0 and 53% in November, between 28 and 66% in January and between 73 and 95% in May (Table 1). The contribution of OPOM appeared small, excepted in November 2006 (between 32 and 42%). Other sources were of minor importance in their contribution to SPOM, excepted for the epilithic biofilm in January, which contributed between 17 and 48%.
Stable isotope ratios of consumers
Seventy-three species were sampled as representative of the whole food web (Table 2). Crustacea (20 species) and Mollusca (19 species) were the most represented taxa, followed by Annelida (15 species) and Chordata (9 species). Predators were the most diversified trophic group, comprising 30 species, while 22 filter-feeders, 15 deposit-feeders and 7 grazers were sampled. The range of δ13C displayed by the consumers remained unchanged (5.5, 5.8 and 5.5‰ for November, January and May, respectively), while the range of δ15N was slightly more variable (7.3, 6.3 and 8.5‰ for November, January and May, respectively). In addition, all the consumers δ13C were comprised between −19.9‰ (Ascidia mentula, January) and −13.5‰ (Littorina obtusata, November) for all the sampling dates.
Filter-feeders were characterized by δ13C and δ15N values lower than other trophic groups (i.e. grazers, deposit-feeders and predators) (Fig. 2). However, their variability according to δ15N was important and was comprised between 4.1‰ (May) and 4.8‰ (November and January). Within this trophic group, consumers clustered according to their taxonomic group (Fig. 3). Ascidians displayed the most 13C-depleted values, while crustaceans were the most 13C-enriched. Bivalves displayed intermediate δ13C.
In contrast to the wide range of δ15N displayed by filter-feeders, the isotopic variability showed by grazers was greater according to their δ13C than to their δ15N. This trophic group was represented mainly by trochids gastropods (Gibbula spp., Jujubinus striatus) and limpets (Patella vulgata), which were among the most abundant consumers on this shore (G.S. pers. obs.). Predators displayed the most 15N-enriched δ15N, comprised between 9.4‰ (Perinereis cultrifera, May) and 13.7‰ (Cancer pagurus, May), which is consistent with a higher trophic level.
In contrast to food sources, the temporal variation of consumers isotopic signatures did not display any consistent pattern. The δ13C and δ15N of consumers did not rise or drop uniformly for all the sampling dates. However, some species displayed important seasonal variations in their isotopic ratios, such as Cancer pagurus (δ13C = −15.4, −17.7 and −14‰ for October, January and May, respectively), Patella vulgata (δ13C = −16.7, −14.4 and −16.1‰ for October, January and May, respectively) or Necora puber (δ15N = 12.9, 11.3 and 12.9‰ for October, January and May, respectively).
Discussion
Food sources
The macroalgae sampled in this study were representative of the dominant species of the infralittoral fringe from the Atlantic French coastal waters (Castric-Fey et al. 1997). These species are well discriminated according to their δ13C, as previously observed in various rocky environments (Fredriksen 2003; Bode et al. 2006; Riera et al. 2009). The δ13C of marine algae is mainly related to their ability to use HCO3 − rather than dissolved CO2 as carbon source during primary production (Raven et al. 2002). Macroalgae displaying low δ13C likely use dissolved CO2 as source of inorganic carbon, while δ13C close to −10‰ uses HCO3 − for that purpose. The temporal variation of macroalgae δ13C highlights a 13C-depletion in January. The source of 13C-depleted inorganic carbon may come from the catchment water inputs, where C3 plants dominate the surrounding area. In fact, a strong flood event occurred one month before the sampling of January 2007 (French Ministry for Ecology and Sustainable Development 2007), and could be responsible for these temporal variations. A similar modification of the δ13C of the DIC pool incorporated by intertidal macroalgae, as already observed in the Charente River by Riera and Richard (1997).
The comparison of macroalgae δ15N with the measurements carried out simultaneously in a pristine environment of Northern Brittany (Batz Island) (Schaal et al. in press and unpublished data), indicates consistent 15N-enrichment for similar species of the Brest harbour (Table 3). This result suggests that anthropogenic nitrogen discharged in the Bay of Brest by the tributaries influences macroalgae growing locally, as previously observed in other impacted coastal environments (McClelland and Valiela 1998). The δ15N observed here are, however, much lower than those observed by Riera et al. (2000) in the Westerschelde, suggesting a moderate impact of anthropogenic nitrogen in Brest harbour. The importance of the tidal oscillating water volume (40% of the Bay renewed per tide) (Le Pape et al. 1996) may greatly dilute the nitrogen inputs, thus limiting its impact on local primary producers.
The stable isotope ratios measured for SOM are 13C-enriched compared to offshore POM (between −25 and −24‰) and to values typically obtained for river POM (e.g. between −32 and −26‰, Riera and Hubas 2003). High δ13C in coastal sediments is often associated to a high abundance of microphytobenthos, which typically displays δ13C close to −17‰ (Riera and Hubas 2003). However, the sediment analysed here was sampled under the rocks, and it is unlikely that photosynthetic cells contribute in great proportion to the SOM pool. Therefore, the 13C-enriched values measured here pointed out the contribution of detritus from macroalgae to the pool of SOM.
The composition of SPOM may be inferred from the isotopic signatures of its potential contributors (Maksymowska et al. 2000). This composition appears to be very variable in time, with a contribution of phytoplankton (OPOM) almost negligible, excepted in November (around 35%). The remainder of the local pool of SPOM is composed by resuspended benthic-derived organic matter (up to 85% of the SPOM composition in May), as previously reported for coastal ecosystems (Bustamante and Branch 1996; Dunton 2001; Kaehler et al. 2006). These studies on the composition of coastal SPOM focused on the importance of detritus from macroalgae (especially brown algae). At our sampling site, the pool of resuspended dentritic organic matter pool likely originates largely from brown algae, which are predominating on the rocky shore studied (G.S., pers. obs.). However, while macroalgae display high temporal variability, the SOM isotopic signature remains relatively unchanged seasonally, suggesting that SOM is constituted of detritus from various origins, without any clear dominance. The results also highlight a sporadic contribution of epilithic biofilm to the SPOM and are in accordance with the conclusions of Takai et al. (2004).
Importance of resource partitioning among filter-feeders
The substantial 13C-enrichment displayed by filter-feeding species compared to OPOM is in such proportion (up to 7‰) that it cannot be solely ascribe to an above average isotopic fractionation (Vander Zanden and Rasmussen 2001). This enrichment indicates that these species do not feed selectively on phytoplankton, which would have resulted in more 13C-depleted values. The similarity between filter-feeders δ13C and those of SPOM strongly suggests that filter-feeders assimilate the local pool of suspended organic matter without selecting the phytoplanktonic component. In spite of the temporal variability observed in SPOM composition, this trophic group appears to feed on the different sources composing suspended organic matter. This observation was in accordance with the conclusions of Coma et al. (2001), who described filter-feeders as “ultimate opportunists”. This capacity to feed on a composite pool of suspended matter, characteristic of urban marine environments (Grall and Glémarec 1997), may be responsible of the ecological success of filter-feeders in such artificial habitats.
However, when considering the variability within the filter-feeders group, a clear contrast can be observed between ascidians, that displayed the most 13C-depleted isotopic ratios, and crustaceans which were more 13C-enriched (Fig. 3). This contrast might result from a preferential assimilation within the SPOM pool which differs among taxa. For instance, the slight 13C-depletion observed for ascidians may reveal a preferential assimilation for phytoplankton, while the 13C-enrichment observed for crustaceans may reveal a higher contribution of 13C-enriched macroalgal detritus.
Filter-feeders display also an important δ15N variability (between 4 and 5‰), which reveals a dominance of omnivory for several species. In particular, our results clearly indicate that barnacles (mean δ15N = 10.9‰) assimilate a large part of their diet from zooplankton and have to be considered as secondary consumers rather than as primary consumers. These results strongly suggest a resource partitioning among filter-feeder species, as already observed in a maërl bed in the Bay of Brest (Grall et al. 2006).
Food web structure
The isotopic pattern displayed by the whole food web remained unchanged seasonally, especially for the δ13C, because most of consumers stable isotope ratios did not vary significantly. Insofar, the pure food sources display an important seasonal variation, this strong seasonal stability suggests the dominance of mixed diets for consumers inhabiting this environment. However, the isotopic ratios of some species (e.g. Perinereis cultrifera, Necora puber and Trivia monacha) varied significantly, revealing a greater trophic specificity for these taxa. The temporal evolution of their stable isotope ratios could also be explained by factors other than diet, such as their lipid content or the reproductive cycle of these consumers (Tieszen et al. 1983), although this hypothesis appears to be unlikely since an effort was done to analyse only muscle tissues, whose lipid content is low (Pinnegar and Polunin 1999).
The consumers δ13C range is often reported to be representative of the diversity of food sources supporting the food web (Layman et al. 2007). The seasonal stability of consumers δ13C range (around 5.5‰) observed here suggests a relative temporal stability in the diversity of food sources at the base of the food web. This range is relatively similar to those reported by other studies in rocky environments of North-east Atlantic (Sarà et al. 2007; Riera et al. 2009; Steinarsdottir et al. 2009), suggesting also a spatial stability in the diversity of food sources supporting rocky shore-associated food webs.
On this rocky shore subjected to anthropogenic influences, calculating trophic levels would be of low relevance due to the important variability of the δ15N of sources (δ15N range between 5‰ in January and 6‰ in November) as recently reported for an other intertidal rocky shore habitat (Riera et al. 2009). However, considering a mean trophic enrichment of about 3‰ at each trophic transfer and other studies in comparable habitats (Fredriksen 2003; Sarà et al. 2007; Riera et al. 2009; Steinarsdottir et al. 2009), our results suggest that this food web might contain at least 4 trophic levels. The identification of benthic top predators (arbitrarily defined as species displaying δ15N of 12‰ or more) revealed the presence of various taxa within this functional group. In addition, an important diversity in foraging behaviour can be observed among predators in this habitat. Polychaetes (e.g. Lepidonotus clava, Sthenelais boas and Glycera tridactyla) were found under the rocks and in the underlying sediment. They may forage at the interface between this sediment and the surrounding boulders. Large crabs (e.g. Necora puber, Cancer pagurus) are found during emersion under the rocks but are highly mobile predators, and able to forage on all the microhabitats of the shore. Gastropods (e.g. Calliostoma zizyphinum and Diodora graeca) are more specialized on grazing rocks surface, where they consume encrusting invertebrates (Ascidians, Sponges) (Graham 1988). Consequently, the diversity of microhabitats composing this rocky shore is clearly associated with a diversity of predators and grazers.
The δ13C and δ15N difference observed between filter-feeders and grazers points out the existence of at least two distinct major trophic pathways in this habitat. The first is based on detritus of various origins that are assimilated in different proportions by filter-feeders. The second is based on fresh food sources (macroalgae, biofilms) consumed by grazers, inducing more 13C-enriched values than for filter-feeders. This conclusion is in accordance with the findings of Riera et al. (2009), who described the co-occurrence of various major trophic pathways in a single Ascophyllum nodosum habitat. In the present study, the large range of δ13C displayed by predators, which overlapped the δ13C distribution of filter-feeders and grazers suggests that predators may play a role of coupling between these two trophic pathways, in accordance with the hypothesis of Rooney et al. (2006). According to these authors, the coupling of distinct energy channels by high trophic level consumers can contribute to the stability of the community against anthropogenic disturbances.
Here, the large animal diversity, together with the main food web features, did not reveal any functional simplification related to the anthropogenic disturbances affecting this area. An annual survey carried out in soft-sediments close to the study site indicated that the AMBI index (Borja et al. 2000) was higher than 4 in 2000 and decreased under 2 from 2006 (Grall J, unpublished data). The recent improvement of the ecological quality of this site, together with the high diversity and functional complexity of the food web, emphasizes that intertidal rocky ecosystems are resilient. Our results suggest that the ability of rocky shore communities to stand anthropogenic disturbances might be related to the food web complexity, including the coexistence of several trophic pathways, coupled through the presence of higher trophic levels.
Conclusions
So far, most studies dealing with the functioning of intertidal rocky shores have focused on trophic interactions between macroalgae and grazers (Davies et al. 2008). Results from the present survey, carried out in low intertidal rocky shore, contrast with previous studies since the dominant fucoid (i.e. Fucus serratus) does not really represent a significant food source for associated consumers. Our results suggest that macroalgae characterizing rocky shores contribute to a pool of dentritic suspended organic matter which represents the actual basis of the food web, via its consumption by a wide diversity of filter-feeders and deposit-feeders. This central trophic pathway should be more specifically considered for a thorough understanding of mechanisms underlying the functioning of rocky shore-associated food webs.
References
Benedetti-Cecchi L, Chato Osio G (2007) Replication and mitigation of effects of confounding variables in environmental impact assessment: effect of marinas on rocky-shore assemblages. Mar Ecol Prog Ser 334:21–35
Bode A, Alvarez-Ossorio MT, Varela M (2006) Phytoplankton and macrophyte contributions to littoral food webs in the Galician upwelling estimated from stable isotopes. Mar Ecol Prog Ser 318:89–102
Borja A, Franco J, Pérez V (2000) A marine biotic index to establish the ecological quality of soft-bottom benthos within European estuaries and coastal environments. Mar Pollut Bull 40:1100–1114
Bunn SE, Loneragan NR, Kempster MA (1995) Effects of acid washing on stable isotope ratios of C and N in penaeid shrimp and seagrass: implications for food-web studies using multiple stable isotopes. Limnol Oceanogr 40:622–625
Bustamante R, Branch G (1996) The dependance of intertidal consumers on kelp-derived organic matter on the west coast of South Africa. J Exp Mar Biol Ecol 196:1–28
Bustamante R, Branch G, Eekhout S (1995) Maintenance of an exceptional intertidal grazer biomass in South Africa: subsidy by subtidal kelps. Ecology 76:2314–2329
Cabana G, Rasmussen JB (1996) Comparison of aquatic food chains using nitrogen isotopes. Proc Natl Acad Sci USA 93:10844–10847
Carlier A, Riera P, Amouroux J, Bodiou J, Grémare A (2007) Benthic trophic network in the Bay of Banyuls sur Mer (northwest Mediterranean, France): an assessment based on stable carbon and nitrogen isotopes analysis. Est Coast Shelf Sci 72:1–15
Castric-Fey A, Girard-Descatoire A, Gentil F, Davoult D, Dewarumez J-M (1997) Macrobenthos des substrats durs intertidaux et subtidaux. Museum National d’Histoire Naturelle, Paris
Coma R, Ribes M, Gili J-M, Hughes RN (2001) The ultimate opportunists: consumers of seston. Mar Ecol Prog Ser 219:305–308
Davies AJ, Johnson MP, Maggs CA (2008) Subsidy by Ascophyllum nodosum increases growth rate and survivorship of Patella vulgata. Mar Ecol Prog Ser 366:43–48
Duggins DO, Simenstad C, Estes JA (1989) Magnification of secondary production by kelp detritus in coastal marine ecosystems. Science 245:270–273
Dunton KH (2001) δ15N and δ13C measurements of Antarctic Peninsula fauna: trophic relationships and assimilation of benthic seaweeds. Am Zool 41:99–112
Fredriksen S (2003) Food web studies in a Norwegian kelp forest based on stable isotope (d13C and d15N) analysis. Mar Ecol Prog Ser 260:71–81
Fry B, Sherr E (1984) δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems. Contrib Mar Sci 27:13–47
Graham AFRS (1988) Molluscs: Prosobranch and pyramidellid gastropods. In: Kermack DM, Barnes RSK (eds) Synopses of the British Fauna. Leiden, New York, Kobenhavn, Köln, p 662
Grall J, Glémarec M (1997) Using biotic indices to estimate macrobenthic community perturbations in the Bay of Brest. Est Coast Shelf Sci 44A:43–53
Grall J, Le Loc’h F, Guyonnet B, Riera P (2006) Community structure and food web based on stable isotopes (d15N and d13C) analysis of a North Eastern Atlantic maerl bed. J Exp Mar Biol Ecol 338:1–15
Guyonnet B, Grall J, Vincent B (2008) Modified otter trawl legs to reduce damage and mortality of benthic organisms in North East Atlantic fisheries (Bay of Biscay). J Mar Syst 72:2–16
Humborg C, Fennel K, Pastuszka M, Fennel W (2000) A bow model approach for a long-term assessment of estuarine eutrophication, Szczecin lagoon, Southern Baltic. J Mar Syst 25:387–403
Jenkins S, Hartnoll R (2001) Food supply, grazing activities and growth rate in the limpet Patella vulgata L.: a comparison between exposed and sheltered shores. J Exp Mar Biol Ecol 258:123–139
Kaehler S, Pakhomov E, Kalin RM, Davis S (2006) Trophic importance of kelp-derived suspended particulate matter in a through-flow sub-Antarctic system. Mar Ecol Prog Ser 316:17–22
Kang C-K, Choy EJ, Son Y, Lee J-Y, Kim JK, Kim Y, Lee K-S (2008) Food web structure of a restored macroalgal bed in the eastern Korean peninsula determined by C and N stable isotope analyses. Mar Biol 153:1181–1198
Layman CA, Arrington DA, Montana CG, Post D (2007) Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 88:42–48
Le Pape O, Del Amo Y, Menesguen A, Aminot A, Quequiner B, Treguer P (1996) Resistance of a coastal ecosystem to increasing eutrophic conditions: the Bay of Brest (France), a semi-enclosed zone of Western Europe. Cont Shelf Res 16:1885–1907
Little C, Kitching JA (1996) The biology of rocky shores. Oxford University Press, Oxford
Lorrain A, Paulet Y-M, Chauvaud L, Savoye N, Donval A, Saout C (2002) Differential δ13C and δ15N signatures among scallop tissues: implications for ecology and physiology. J Exp Mar Biol Ecol 275:47–61
Maksymowska D, Richard P, Piekarek-Jankowska H, Riera P (2000) Chemical and isotopic composition of the organic matter sources in the Gulf of Gdansk (Southern Baltic Sea). Est Coast Shelf Sci 51:585–598
McClelland JW, Valiela I (1998) Linking nitrogen in estuarine producers to land-derived sources. Limnol Oceanogr 43:577–585
Peduzzi P (1987) Dietary preferences and carbon absorption by two grazing gastropods, Gibbula umbilicalis (Linné) and Jujubinus striatus (Linné). PSZN I: Mar Ecol 8:359–370
Phillips DL, Gregg JW (2003) Source partitioning using stable isotopes. Coping with too many sources. Oecologia 136:261–269
Pinnegar JK, Polunin N (1999) Differential fractionation of δ13C and δ15N among fish tissues: implications for the study of trophic structure. Func Ecol 13:225–231
Rau GH, Teyssie J-L, Rassoulzadegan F, Fowler SW (1990) 13C/12C and 15N/14N variations among size-fractionated marine particles: implications for their origin and trophic relationships. Mar Ecol Prog Ser 59:33–38
Raven JA, Johnston AM, Kübler JE, Korb R, McInroy SG, Handley LL, Scrimgeour CM, Walker DI, Beardall J, Vanderklift MA, Fredriksen S, Dunton KH (2002) Mechanistic interpretation of carbon isotope discrimination by marine macroalgae and seagrasses. Func Plant Biol 29:355–378
Riera P, Hubas C (2003) Trophic ecology of nematodes from various microhabitats of the Roscoff Aber Bay (France): importance of stranded macroalgae evidenced through d13C and d15N. Mar Ecol Prog Ser 260:151–159
Riera P, Richard P (1997) Temporal variations of δ13C in particulate organic matter and oyster Crassostrea gigas in Marennes-Oléron Bay (France): effect of freshwater inflow. Mar Ecol Prog Ser 147:105–115
Riera P, Stal LJ, Nieuwenhuize J (2000) Heavy δ15N in intertidal benthic algae and invertebrates in the Scheldt estuary (The Netherlands): effects of river nitrogen inputs. Est Coast Shelf Sci 51:365–372
Riera P, Stal LJ, Nieuwenhuize J (2004) Utilization of food sources by invertebrates in a man-made intertidal ecosystem (Westerschelde, the Netherlands): a δ13C and δ15N study. J Mar Biol Ass U K 84:323–326
Riera P, Escaravage C, Leroux C (2009) Trophic ecology of the rocky shore community associated to the Ascophyllum nodosum zone (Roscoff, France): a δ13C vs δ15N investigation. Est Coast Shelf Sci 81:143–148
Rooney N, McCann KS, Gellner G, Moore JC (2006) Structural asymmetry and the stability of diverse food webs. Nature 442:265–269
Sarà G, De Pirro M, Romano C, Rumolo P, Sprovieri M, Mazzola A (2007) Sources of organic matter for intertidal consumers on Ascophyllum-shores (SW Iceland): a multi-stable isotope approach. Helgol Mar Res 61:297–302
Schaal G, Riera P, Leroux C (2008) Trophic coupling between two adjacent benthic food webs within a man-made intertidal area: A stable isotopes evidence. Est Coast Shelf Sci 77:523–534
Schaal G, Riera P, Leroux C (2009) Trophic ecology in a Northern Brittany (Batz Island, France) kelp (Laminaria digitata) forest, as investigated through stable isotope and chemical assays. J Sea Res (in press)
Steinarsdottir MB, Ingolfsson A, Olafsson E (2009) Trophic relationships on a fucoid shore in south-western Iceland as revealed by stable isotope analyses, laboratory experiments, field observations and gut analyses. J Sea Res 61:206–215
Takai N, Yorozu A, Tanimoto T, Hoshika A, Yoshihara K (2004) Transport pathways of microphytobenthos-originating organic carbon in the food web of an exposed hard bottom shore in the Seto Inland Sea, Japan. Mar Ecol Prog Ser 284:97–108
Tieszen LL, Boutton TW, Tesdahl KG, Slade NA (1983) Fractionation and turnover of stable carbon isotopes in animal tissues: implications for δ13C analysis of diet. Oecologia 57:32–37
Vanderklift MA, Ponsard S (2003) Sources of variation in consumer-diet δ15N enrichment: a meta-analysis. Oecologia 136:169–182
Vander Zanden MJ, Rasmussen JB (2001) Variation in δ15N and δ13C trophic fractionation. Implications for aquatic food web studies. Limnol Oceanogr 46:2061–2066
Acknowledgments
This work is partly funded by the ANR (National Research Agency) ECOKELP. G. Schaal is funded by a French Ministry of Research and Technologies grant. The authors thank Erwan Amice, Caroline Broudin, Benjamin Guyonnet, Robert Marc and Renaud Michel for their valuable help during field sampling. The authors would like to thank Dr Fabio Bulleri and three anonymous reviewers for their helpful comments that greatly contributed to improve this manuscript. We also greatly thank Dr L. M. -L. J. Noël for improving the english.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bulleri.
Rights and permissions
About this article
Cite this article
Schaal, G., Riera, P., Leroux, C. et al. A seasonal stable isotope survey of the food web associated to a peri-urban rocky shore. Mar Biol 157, 283–294 (2010). https://doi.org/10.1007/s00227-009-1316-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-009-1316-9