Abstract
During the past 5 decades, the large-sized biota inhabiting dark marine caves has attracted the attention of many marine biologists; in contrast, studies concerning the meiofaunal organisms of these peculiar biotopes remain scanty and mostly with a taxonomic aim. In this study, the nature and abundance of meiofaunal taxa living in a Mediterranean, semi-submerged sea cave was surveyed in relation to distance from the entrance and over two different seasonal periods. Particular attention was paid to the Gastrotricha taxocene. Research was carried out in a cave along the Ionian coast of Apulia (southern Italy), the “Grotta Piccola del Ciolo” which opens for approximately 120 m on the north-eastern side of a shallow fjord and has a bottom blanketed by fine to very fine sand, occasionally rich in detritus. Quantitative samples in four replicates were collected by SCUBA diving, in November 2000 and June 2001, coring the sediment with a hand-held piston corer in three light-free sites (stations 1–3) located at increasing distances from the entrance. At each site, two additional 500-ml sediment samples were collected for an in vivo study of the Gastrotricha. Faunistic analysis found a fairly high meiobenthic diversity, identifying representatives of more than 12 major groups, with total abundances ranging from 656 ind./10 cm2 (10 cm2) in November to 1,069 ind./10 cm2 in June. Station 1, the closest to the entrance invariably hosted the most abundant meiofaunal community (851 ind./10 cm2 in November and 1932 ind./10 cm2 in June), followed by station 2 or 3 depending on the season. While nematodes and harpacticoids appear as the most abundant taxa when the cave is considered as a whole, other taxa may prevail numerically in selected stations, e.g. priapulids, which are the second most abundant taxon at station 1 (30 ind./10 cm2 in November and 83 ind./10 cm2 in June). Although the density of total meiofauna and that of the single groups may not be very high, the cave is interesting by virtue of the peculiarity of the hosted fauna, e.g., species and genera new to science or new to the Mediterranean Sea. Regarding the Gastrotricha, we found 16 species, accounting for 1.3–2.6% of the total meiobenthos (density = 8.4 ind./10 cm2 in November and 27.4 ind./10 cm2 in June). Analysis of the gastrotrich community found, particularly in June, an assemblage of taxa quite different from those found in open habitats, even at the family level; differences that are probably due to the exploitation of different food resources by animals populating the two environments, i.e. algae in the open sea versus bacteria in the caves. Results indicate that for meiofauna, as happens for macrofauna, the marine caves may represent hotspots of biodiversity and endemism; the driving forces at the base of the trophic depletion hypothesis seem to be responsible for structuring the meiofauna community inside the cave.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the seminal works by Riedl (1959, 1966) life of the sea caves (dark caves in particular) has attracted the attention of many marine biologists. A series of articles (e.g., Harmelin et al. 1985; Harmelin and Vacelet 1997; Chevaldonne and Lejeusne 2003; Marti et al. 2004) have shown that by virtue of their particular environmental conditions (e.g., light gradient and water confinement), submerged and semi-submerged marine caves host a rich and diversified biota which bear several faunistic peculiarities including batial forms (Harmelin 1997), ‘relict’ species (Ohtsuka et al. 2002) and genuinely troglobial taxa (Stock 1993; Pansini 1996; Bussotti et al. 2003; Lejeusne and Chevaldonne 2005).
It is a pity that despite the great scientific interest that surrounds marine cave environments, the study of the meiobenthic communities of these systems has not yet gained a real foothold. Some attention has been paid in the past (Wieser 1954; Pesta 1959; Riedl 1966) and also more recently (Palacin and Masalles 1986; Sandulli et al. 1999), but its goal has been essentially taxonomic, with only few groups investigated: tardigrades (Villora-Moreno 1996; Boesgaard and Kristensen 2001; Gallo-D’Addabbo et al. 2001), kinorhynchs (Sørensen et al. 2000), priapulids (Todaro and Shirley 2003) and few others (see also Sandulli et al. 2002).
The information gathered by studying larger-sized biota, suggests that sea caves may also constitute a favourable environment for a wide range of meiofaunal taxa, therefore, representing a highly stimulating habitat for a variety of researchers interested in studying the different aspects of the meiobenthic community, ranging from biodiversity to ecology to evolution, most of which have not been looked at closely in the past.
The present research aims at contributing to the knowledge of meiofaunal communities of submerged marine caves, by studying their structural characteristics in terms of the major taxa (see Higgins and Thiel 1988; Giere 1993). A more detailed study will be reserved for the phylum Gastrotricha. Over the last 10 years, the marine representatives of this group have been the subject of numerous studies focusing on taxonomy (e.g., Clausen 2000; Todaro 2002; Nicholas and Todaro 2005; Todaro et al. 2005); zoogeography (e.g., Todaro et al. 2003a, b Clausen 2004; Todaro and Rocha 2004, 2005); reproductive biology (Balsamo et al. 2002; Guidi et al. 2004); and also evolution based both on morphology and molecular traits (Todaro et al. 1996, 2003a, b; Hochberg and Litvaitis 2000, 2001; Hochberg 2005; Marotta et al. 2005); however, the scarcity of ecological works on these animals endures. It is in this wide context that part of the research has been carried out, with the aim of at least partially filling the existing void in the knowledge regarding the basic ecology of these organisms.
Materials and methods
Study area
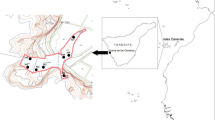
The cave, known as the “Grotta Piccola del Ciolo” is located at the south-eastern end of the Salento peninsula, near the cape of Santa Maria di Leuca (Apulia, southern Italy, long. 39°50′38′′N; lat. 18°23′11′′E). It is accessible from the sea and can only be explored by scuba diving, although the entrance is partially above sea level (Fig. 1a). The cave opens for about 120 m on the north-eastern side of a shallow fjord; its bottom is covered by sandy sediment, which is occasionally rich in detritus. The cave extends horizontally and is located on a N–S fracture, which has been slightly widened by the flow of water in the course of time; there are two small siphoned areas, at the beginning and towards the bottom, where there is considerable flow of fresh water, which is stratified above the saltwater (Onorato et al. 1999). The terminal chamber opens onto a small beach, which originated from collapsed materials.
The most abundant fauna is composed of encrusting organisms, such as sponges and cnidarians, which are present up to approximately 80 m from the mouth of the cave, after which the walls are totally bare, also due to the infiltration of fresh water. The sciaphilous cardinal fish, Apogon imperbis, is a well known inhabitant of the Ciolo (Bussotti et al. 2003), whereas recently Todaro and Shirley (2003) have described adults and larvae of a new meiobenthic priapulid, Tubiluchus troglodytes, found in the cave in astonishing numbers (see below).
Collection and sampling stations
The cave bottom is completely covered by a blanket of mobile sediment of various grain sizes, with a thickness of about 1 m. Quantitative samples in four replicates were collected by Scuba diving, coring the sediment with a hand-held piston corer (2.37 cm i.d.×10 cm h) at three light-free sites (stations 1, 2 and 3) located at 55, 75 and 90 m from the entrance of the caves, at water depths of 4.2, 3.6 and 3.2 m, respectively (Fig. 1). At each site, two additional 500-ml sediment samples were collected for in vivo analysis of the gastrotrichs. The fauna of quantitative samples was narcotised using a 7% magnesium chloride solution, fixed on site with a 10% buffered formalin solution pre-stained with rose bengal and stored for later checking; larger samples for in vivo study were taken to the laboratory and analysed within 10 days of collection. The samples were collected at two different times in different seasons, in November 2000 and June 2001. In June 2001, additional fresh material was also collected to study the fauna living in the chamber that was furthest inside the cave (station 4, Fig. 1) where, due to the partial mixing of fresh- and saltwater, the salinity was lower. At the time of sampling, the salinity and temperature of the interstitial water was measured, using a YSI digital salinometer (Yellow Spring Instruments, OH, USA).
Organism extraction and identification
In the laboratory, the fresh material was examined first. The fauna was extracted from the sediment (4–5 cm3 at a time) by narcotization with iso-osmotic (7%) magnesium chloride solution (Pfannkuche and Thiel 1988). The gastrotrichs were removed with a micropipette, freshly mounted on slides and observed using a Leitz, Dialux 20 microscope (DIC, Nomarski). During observation, the animals were measured with an ocular micrometer and photographed using a Nikon Coolpix 995 digital camera. After initial in vivo observation, if necessary, the animals were fixed and preserved for subsequent analysis, carried out mainly using scanning electron microscopy. To this end, formalin-fixed gastrotrichs were rinsed in tap water, dehydrated through a graded ethanol series, critical point-dried using CO2, mounted on aluminium stubs, sputter coated with gold–palladium and observed with a Philips XL 30 scanning electron microscope.
The meiofauna of the quantitative sample was separated from the sediment, generally by flotation and multiple decantations. For the samples collected at station 1, being richer in detritus, it was necessary to carry out the extraction using the silica gel gradient centrifugation technique (LUDOX AM, d = 1.210; Pfannkuche and Thiel 1988). In all cases, the supernatant was filtered using two sieves, 1 and 0.042 mm, respectively, laid one upon the other. The animals retained on the finer sieve were then transferred into Petri dishes and, with the aid of a stereomicroscope, subdivided into major groups, counted and preserved in formalin for any future checks. The gastrotrichs were identified to species directly using the stereomicroscope or with the aid of DIC optics.
The microscope inspection of the sandy pellets allowed us to ascertain that the efficacy of the extraction was almost 100% as far as almost all the taxa were concerned, with the exception of the foraminiferans that mostly stay in the sediment. For this reason, the foraminiferans were excluded from all subsequent analyses and, as other types of free-living protozoans were only found occasionally and always with an extremely low number of individuals, meiofauna, in this paper, only refers to the fraction formed by multicellular organisms.
Sediment analysis
The grain size analysis of the sediment was carried out on dry sediment using the classic method of sieving through 2, 1, 0.5, 0.250, 0.125, and 0.063 mm sieves (Folk 1958). The sediment fractions collected in the single sieves were weighed and the values of the different granulometric parameters calculated subsequently using Todaro’s (1992), computerised programme the algorithm of which is based on the Seward–Thompson and Hails (1973) equation. The calculation of the total organic content present in the sediment was obtained by analysing additional samples, which were collected at the same time as those used for the fauna analysis. The values, expressed as percentages of the dry weight, were calculated as loss of weight after combustion, in a muffle kiln at 480°C for 4 h, of 100 g of material that had previously been dried in an oven at 60°C for 48 h.
Statistical analysis
The taxa present with density ≥5 ind./10 cm2 were treated singly, the taxa present in lower density were pooled in the category ‘others’. Possible differences in the mean values were evaluated using analysis of variance (ANOVA) or the t-test. Before proceeding with the comparisons, the values were checked to be within the normal distribution and homogeneity of variance limits. If not, before continuing, the data were transformed using the equation y = log (x+1). All the analyses were carried out using the Sigmastat® application package (Jendel, Scientific, CA, USA).
Results and discussion
Habitat characteristics
Out of the physical and chemical characteristics taken into account (Table 1), salinity and temperature were relatively constant: the salinity remained at approximately 38‰, whereas the temperature varied throughout the season by only 2°C, from 19°C in November 2000 to 21°C in June 2001, while it remained constant among the three stations investigated. On the other hand, the grain size analysis of the sediment highlighted a difference in the mean grain diameter, both among the three stations and during the two periods investigated. Stations 1 and 3 were those with the finest sediments (Table 1). The sorting values were different at the three stations, but remained unvaried during the two seasons. The lowest value, 0.551 mm, was found at station 1, and the highest, 0.632 mm, at station 2. With reference to the Wentworth scale, the sediment was, depending on the sample, composed of fine to very fine and medium to fine sand, moderately sorted or moderately well sorted (Giere et al. 1988).
The total organic content, expressed as a percentage of the dry weight of the sediment, was invariably the highest at station 1, with a maximum value of 5.23% in June 2001, twice that of station 2, and approximately 1% higher compared to that of station 3 (Table 1).
At station 4, which was located at the edge of the internal chamber and was only involved in qualitative samples taken in June 2001, the water temperature was almost identical to that of the other stations, while the salinity was considerably lower, 32‰, due to the presence of resurgence/infiltrations of fresh water. The sediment was composed of fine to very fine moderately sorted sand, with little detritus.
The high salinity value recorded, 38‰, classifies the Ciolo cave as a decidedly marine habitat. The absence of appreciable fluctuations in the salinity value, at the three stations investigated, indicates that the bottom of the cave is reached by few freshwater infiltrations (if any), except for station 4, where the salinity of the interstitial water decreases by about 6‰ down to the value of 32‰.
Overall, the sediment at the three sampling sites can be considered to be constituted of fine sand; as it is well known that the substrate reflects the physiographical conditions of the habitat, and in particular, that the mean sediment grain diameter is positively correlated with the energy in the surrounding water, i.e. currents, waves etc. (cf. Giere 1993), our data indicate that the energy in the water column inside the cave is low. Therefore, it seems that the Ciolo cave is a habitat little influenced by external weather conditions, probably due to the fact that the cave mouth is quite protected. This observation seems to be confirmed by the fact that the sediment grain size characteristics at the different stations only changed slightly with the alternation of the seasons (Table 1) and also by the constant stratification of the percolation water on the seawater observed in the cave both during our investigation and on other occasions (G. Belmonte, personal communication). It is difficult to attribute the slight decrease in mean grain size recorded in June 2001 at station 2 (0.233 compared to 0.270 mm) to natural causes. It should be taken into account that the surface layers of the sediment may be disturbed by scuba divers who visit the cave often during the warm season causing the finer material to be displaced.
As could be expected, the finer grain sediments were found at the sites located deeper in the cave, with the exception of station 1. At this site, the sediment was composed of fine or very fine sand, always with a high quantity of detritus (approximately 5% organic content). It is likely that the conformation of the cave at this specific station causes sudden lowering of the energy in the water column; so the fine material that reaches it cannot be re-suspended and is deposited. The relative ‘immobility’ of the sediment at this station is confirmed by the fact that only the surface layer is a yellow colour, which is typical of oxidised substrates, while at just 1–2 cm from the surface the sediment is a grey colour, typical of reduced sediments.
In agreement with literature data (Higgins and Thiel 1988; Giere 1993), in the Ciolo cave also a greater quantity of organic content is associated with the finer sediments. At the moment, neither the composition (algal, microbial etc.) nor the origin (autochthonous, allochthonous) of such a substance at the different stations is known. The high value recorded at station 1, the nearest to the mouth, and the increase recorded in the month of June would seem to indicate that, at least at this station, part of the organic content is of allochthonous origin. The presence of abundant fauna and the particular types of species (see below), also at stations located deeper, leads one to suppose that the main energy source for these organisms depends on the contribution of autochthonous organic content, intended for the development of bacteria, production of detritus and agglutinated proteins. In fact, the total absence of light does not allow primary production connected with photosynthesis.
Meiofauna
Overall, in the cave the total meiofauna density found was 656.0 ind./10 cm2 in November 2000 and 1069.1 in June 2001 (Table 2; Fig. 2). The nematodes were always the dominant taxon with 596.0 and 952.4 ind./10 cm2 in November and June, respectively, followed by the harpacticoid copepods (adults + nauplii, 19.8 and 32.0 ind./10 cm2), priapulids (10.3 and 29.2 ind./10 cm2) and polychaetes (11.4 and 17.4 ind./10 cm2). The gastrotrichs, with a mean density of 8.4 and 27.4 ind./10 cm2 in November 2000 and June 2001, respectively, complete the list of the five taxonomic groups constantly present, and whose mean overall density exceeded 5 ind./10 cm2. There were always turbellarians, nemertines, ostracods and tanaidaceans present, but with very low densities, whereas the oligochaetes, tardigrades and amphipods were found sporadically and mostly in low numbers. These rarer meiobenthic groups present in the cave with low densities were pooled in a single category named ‘others’.
The percentage of each taxon’s contribution to the composition of the community does not show large variations with the changing of the seasons (Table 2). Above all, a decrease by 1.7% in the nematodes was recorded, which dropped from 90.8% in November 2000 to 89.1% in June 2001, and an increase in the gastrotrichs and priapulids, from 1.3 and 1.6% in November to 2.6 and 2.7% in June, with increases of 1.3 and 1.1%, respectively.
Station 1 was always the one with the highest total density (ANOVA, P<0.05), followed by station 2 or 3 depending on the season; for example, in November 2000, at station 1 a density of 851.3 ind./10 cm2 was calculated, compared to 625.5 ind./10 cm2 at station 2, and 525.3 at station 3; in June 2001, at station 1 the total density was 1932.8 ind./10 cm2 compared to 564.8 at station 2, and 709.6 ind./10 cm2 at station 3 (Tables 3, 4; Fig. 3).
Average meiofaunal densities in three collecting sites (Stations 1–3) located at increasing distance from the cave entrance. Graphs on the left (a, b, c) refer to samples collected in November 2000, whereas graphs on the right (d, e, f) pertain to samples collected in June 2001. a, d = station 1; b, e = station 2; c, f = station 3. Error bars represent ±1 standard deviations
Comparisons of the mean densities found at the single stations during the two periods investigated found that only those at station 1 were statistically different (ANOVA, P<0.05), and in particular, higher in the summer samples compared to the autumn ones: total meiofauna 1932.8 compared to 851.3 ind./10 cm2 (Tables 3, 4; Fig. 3a, d). On the contrary, the community structure was quite different at the three stations investigated, with small seasonal variations at a particular station (Tables 3, 4; Fig. 3c, f).
Station 1
In November 2000, at the site closest to the mouth of the cave, representatives of nine taxonomic groups were found, only four of which (nematodes, copepodes, priapulids and polychaetes) had densities higher than 5 ind./10 cm2 (Table 3; Fig. 3a). The nematodes were present with a density of 770.5 ind./10 cm2, composing 90.5% of the total meiofauna, and proving to be the dominant group; the priapulids were sub-dominant, with a density of 30.9 ind./10 cm2 (3.6%), followed by the polychaetes, 25.8 ind./10 cm2 (3%), the harpacticoids, 11.8 ind./10 cm2 (1.4%), and their larval stages, 3.9 ind./10 cm2 (0.5%). At this station, the gastrotrichs reached a density of just 3.4 ind./10 cm2, contributing only 0.4% to the formation of the overall meiofauna. The other taxa combined reached a density of 5.1±4.4 ind./10 cm2, representing 0.6% of the overall population.
In June 2001 (Table 4; Fig. 3d), ten taxa were found (the amphipods appear), five of which exceeded the threshold of minimum density of 5 ind./10 cm2. Although the total meiofaunal density and that of the most abundant taxa increased significantly (see above) the percentage contribution of the single taxa to the community only varied slightly; among these, we observed a 0.7% increase in priapulids, from 3.6 to 4.3%, and a 0.9% decrease in the polychaetes population, from 3.0 to 2.1% (Tables 3, 4).
Station 2
In the sediment collected at station 2 in November 2000, eight main taxonomic groups were found, four of which had a density greater than 5 ind./10 cm2 (Table 3; Fig. 3b). The dominant group was that of the nematodes which, with a density of 578.3 ind./10 cm2, composed 92.5% of the overall meiofauna, followed by the harpacticoids with a density of 20.2 ind./10 cm2 (3.3%). The gastrotrichs were present with a density of 8.4 ind./10 cm2 and (1.3%), followed by the nauplii which, with 6.7 ind./10 cm2, composed 1.1% of the total meiofauna; the polychaetes were only present in small numbers, 3.4 ind./10 cm2, composing just 0.5% of the meiobenthic community. The other taxa were present overall with a density of 8.4 ind./10 cm2, and represented 1.3% of the total population.
In June 2001 at station 2, there were ten taxa; compared with the preceeding November, the priapulids, oligochaetes and nemertines appeared while the tardigrades disappeared (Table 4; Fig. 3e). Despite the fact that the number of taxa increased, the seasonal variations in density at station 2 were not significantly different (ANOVA, P>0.05) and also the percentage contribution of the single taxa to the overall community composition did not undergo large modifications. The most abundant taxon was the nematodes which, with a density of 502.6 ind./10 cm2, composed 89.2% of the total meiofauna, followed by the harpacticoid copepods (24.0 ind./10 cm2; 4.3%) and by the gastrotrichs which were very abundant (20.6 ind./10 cm2; 3.4%). Also the turbellarians were rather well represented reaching a density of 7.1 ind./10 cm2 or 1.3% of the total population. Other taxonomic groups were present in densities lower than 5 ind./10 cm2, and overall only represented 1.6% of the population.
Seasonally, there was a decrease by 3.3% in the nematodes, whose contribution to the total meiofauna decreased from 92.5 in November to 89.2% in June, and an increase in copepods and turbellarians from 3.3 and 0.2% in 2000 to 4.3 and 1.3% in 2001 respectively, with an increase in both cases of approximately 1%. The gastrotrichs increased by 2.1%, from 1.3 in November to 3.4% in June.
Station 3
At station 3, in November 2000, representatives of eight major taxonomic groups were found, out of which only four had densities greater than 5 ind./10 cm2 (Table 3; Fig. 3c). The dominant group, represented by the nematodes, had a density of 473.9 ind./10 cm2 making up 90.2% of the total meiofauna. It was followed, closely to each other, by three sub-dominant taxa represented by the copepods, with a density of 17.7 ind./10 cm2 (3.4%), the gastrotrichs (12.2 ind./10 cm2; 2.3%), and the turbellarians (11.8 ind./10 cm2; 2.2%). The polychaetes had considerably lower abundances, with a density of 4.6 ind./10 cm2 (0.9%), and so did the nauplii (1.7 ind./10 cm2; 0.3%). Overall, other taxa (among them the nemertines, which were found exclusively at this station) composed only 0.7% of the entire population, reaching a density of 3.4 ind./10 cm2.
In June 2001, there were eight major taxa represented and an apparent overall increase in total meiofauna density was recorded (Tables 3, 4; Fig 3c, f); however, the t-test could not exclude the possibility that the difference in the density mean value was due to random sampling variability. Also the contribution percentage of the single taxa to the total meiobenthic population did not change much, except for a few groups. Whilst the dominant nematodes fell slightly down, 86.6 versus 90.2% of the total population, a major change involved the gastrotrichs that at this station reached their maximum abundance throughout the investigation (60.2 ind./10 cm2); in June they represented in fact the numerically sub-dominant group, constituting 8.2% of the total meiobenthos at this station (Table 4, Fig. 3f). The higher abundance of the gastrotrichs (t-test, P<0.05) was due to the population ‘explosion’ of just two species: Cephalodasys turbanelloides and Paradasys sp. as will be explained in more detail below.
At a certain distance from nematodes and gastrotrichs, there was a group of three taxa with rather similar densities composed of polychaetes (8.0 ind./10 cm2; 1.1%), harpacticoids (6.7 ind./10 cm2; 0.9%), and nauplii (5.9 ind./10 cm2; 0.8%); followed by the ostracods for which a density of 4.6 ind./10 cm2 (0.7%) was recorded. As far as the percentage contribution of the single taxa to the total community was concerned, above all there was a constant increase (5.9%) in gastrotrichs, from 2.3 to 8.2%, a decrease by 3.6% in nematodes, from 90.2 to 86.6%, and a decrease by 2.5% of copepods, from 3.4 to 0.9%.
In the literature, until now there have been no quantitative data relating to cave meiofauna with which to compare the values found in the Ciolo cave. If the comparison is made with data from unconfined environments, the density values are within the range of values indicated for fine to medium sandy substrates (e.g., Coull 1988). Overall, even though it is not particularly abundant, the Ciolo meiofauna is still interesting due to its diversification and peculiarity, as at least 12 phyla are represented, with some completely new aspects, both on a worldwide level and regarding the Mediterranean Sea. The very high density of a new species of meiobenthic priapulids, found at station 1 in the Apulian cave (up to 83 ind./10 cm2) offers a valid example in this regard (Todaro and Shirley 2003).
As could be expected from the sediment characteristics, the nematodes were always the dominant group; the high percentage contribution of this taxon to the meiobenthic community was generally higher than 90%, which indicates that factors other than grain size may contribute to the massive presence of these organisms. It is well known, for example, that among the meiobenthic groups the nematodes include a high percentage of opportunistic feeders, which may change feeding strategies in response to available food (Moens and Vincx 1997). The darkness that characterises the cave environment certainly does not favour the growth of micro-algae, and it is, therefore, plausible that organisms like nematodes, whose diet may be tuned to be based mainly on bacteria, should be present with a higher percentage compared to the copepods for example, which feed mainly on micro-algae and do not show such a foraging plasticity. In the case of the Ciolo cave, copepods contribute a maximum of 4.3% to the composition of the total meiobenthos. In almost all cases, the dominant copepod group was that of the Harpacticoida, whose density decreased overall with increased distance from the mouth of the cave, following the overall meiofauna trend and, on a different level, also that of the macrofauna.
In accordance with the hypothesis on trophic depletion (Zabala et al. 1989), supported by a growing body of evidences (e.g. Fichez 1990, 1991), the main factor responsible for the impoverishment of macrobenthic populations inside submarine caves is the gradual qualitative and quantitative reduction of food. The trophic depletion of cave ecosystems mainly derives from the negative gradient of two physical factors: light and water confinement. Light, fading suddenly until absent, causes the disappearance of the algae (Cinelli et al. 1977) inhibiting autochthonous primary production; in turn the confinement, linked to the reduction in hydrodynamic factors, reduces the supply of nutritional substances from the outside (Harmelin 1980; Balduzzi et al. 1989). The progressive decrease in the mean densities of the total meiofauna, and in particular of the harpacticoid copepods, would seem to indicate that the meiobenthic community also, like the macrobenthic one, is structured in accordance with the trophic depletion hypothesis.
The change in season did not have statistically significant effects on the overall mean density of the cave meiofauna (ANOVA, P<0.05), and only the meiobenthic assemblage of the station nearest the mouth of the cave (station 1) showed significant increases, both overall and for the single taxa, changing from autumn to spring. The meiofauna community of the Ciolo cave, therefore, seems to a large extent to be decoupled from phenomena or characteristics that vary with season (temperature, phytoplankton bloom etc.) particularly as distance from the entrance increases; this along with physical and chemical data recorded lead us to think of Ciolo cave as a fairly stable habitat.
Gastrotricha
In the samples examined there were approximately 250 gastrotrich individuals, with an overall mean density of 19.2 ind./10 cm2, a value that means they compose 2.2% of the total meiofauna (Table 2; Figs. 2, 3). Lower values were found in November (8.4 ind./10 cm2; 1.3%), higher values in June (27.4 ind./10 cm2, 2.6% of the total population).
The taxonocoenosis was formed of 16 species, 12 of which are included in the order Macrodasyida (M) and 4 in the order Chaetonotidae (C). There were 15 genera present (12 M and 3 C) distributed over six families (4 M and 2 C; Table 5).
Station 1 had the lowest number of species (1), station 2 the highest (11) whereas station 3 had the highest number of gastrotrichs (60.2 ind./10 cm2 in June 2001).
Station 4, characterised by a salinity of only 32‰ hosted two species, one of which, Turbanella sp., was only found at this site (Table 5). The mean number of species per station was 4.8 with a minimum of one species at station 1 in both November and June, and a maximum of nine species at stations 2 and 3 in November and June, respectively.
Less than half the species (37.5%) were found in both the periods investigated and only exceptionally with high densities; ten species were present during one period or the other, but never with high numbers of individuals (Table 6). C. turbanelloides and Paradasys sp., with mean densities of 8.1 and 3.7 ind./10 cm2, respectively, and maximum densities of 37.5 and 9.7 ind./10 cm2, reached in June 2001, were the dominant gastrotrichs in the Ciolo cave overall.
The spatial and temporal distribution of the single species inside the cave and their relative abundance was, as also deduced from the high standard deviation values, quite diversified. At station 1, the only species present was Megadasys minor with 3.4 ind./10 cm2 in November 2000 and 1.3 ind./10 cm2 in June 2001 (Table 6). The species was also present at station 3 with a density of 2.9 ind./10 cm2 and 6.3 ind./10 cm2 in the November and June samples, respectively (Table 5).
Out of the 11 species found at station 2, Pseudostomella cataphracta, Platydasys sp., Chaetonotus sp. a, Chaetonotus sp. b and Xenotrichula intermedia were exclusive to this site, Draculiciteria tesselata was also found at station 4, while C. turbanelloides, Mesodasys laticaudatus, Macrodasys caudatus and Tetranchyroderma sp. were also common at station 3. At station 2, during the month of November, a total of nine species was found, mostly represented by single individuals, while in the June samples there were only six species, the most abundant of which (59.2% of the total) was M. caudatus with 12.2 ind./10 cm2 (Table 5).
At station 3, there was a total of nine species, only three of which were present in both seasons and six only during the month of June; in autumn the dominant species was Paradasys sp. with a density of 5.1 ind./10 cm2 (41.8% of the total). In spring, the community was dominated by C. turbanelloides, which reached the record density for the cave with 37.5 ind./10 cm2 (62.3%), followed by Paradasys sp. and M. minor, present with densities of 9.7 (16.1%) and 6.3 ind./10 cm2 (15.4%), respectively. Other species present in June, with a fair number of individuals, were Mesodasys adenotubulatus (2.1 ind./10 cm2), Urodasys acanthostilis (1.7 ind./10 cm2) and Lepidodasys martini (0.4 ind./10 cm2) while M. caudatus and Tetranchyroderma sp. were both represented by single individuals (Table 5).
In the Ciolo cave, the species diversity of the gastrotrich communities expressed as the Shannon–Wiener index (H′) was equal to 1.399, with a minimum (H=1.070) for station 3 during the month of November, and a maximum (H′= 2.197) at station 2 also during November (Table 6). Unanimously with the H′ index, also the Pielou equitability index (J) showed a maximum (J=0.693) at station 2 in November, whereas the minimum value of equitability (J=0.374) was for the samples from station 3 collected in June.
Out of the 16 species identified throughout this study, only four belong to the order Chaetonotida, which were also found occasionally and in small numbers (Table 5). Chaetonotidans, therefore, represent only 25% of the total gastrotrich species found in the Ciolo cave. This is cause for reflection as, in 256 other previously studied Italian locations (Todaro et al. 2001), it has been observed that the chaetonotidans on average contribute 40% to the total gastrotrich species, and this value rises even more if only subtidal sites in which the gastrotrichs were represented by more than 15 species are taken into consideration. As the locations where the chaetonotidans compose up to 45% of the total species are characterised by medium to fine sandy sediment, and considering the sampling effort involving the Ciolo cave, it can be stated that in the cave sediments, the order Chaetonotida is poorly represented. The reasons for which the chaetonotidans are present in very low numbers in fine to very fine sand are not known, but it can only be due to the morphological and physiological characteristics and the behaviour of the representatives of this order. Within the limits of the latter aspect, it must be remembered that the chaetonotidans (except Neodasys) are small gastrotrichs that in marine environments lead such distinctly interstitial lives that they can reach a depth within the sediment down to several centimetres (Todaro 1998). It is likely that the lacunar spaces that characterise the fine or very fine grain size sediment that may also be rich in biodetritus, like those of the Ciolo cave, are not wide enough to allow the free movement of these animals and, therefore, these types of substrate are ‘perceived’ as inhospitable and, therefore, avoided. It is not by chance that the four species of chaetonotidae were all found at station 2, which is characterised by a sediment whose grains have a larger mean diameter than the other two stations and is also relatively poor in biodetritus (Table 1). Although poor in chaetonotidans, the very fine grained substrates are of interest, as they frequently accommodate taxa that are exclusive to these environments or that are found only occasionally in others. For these reasons, it is not rare to come across species that have not yet been described (such as Chaetonotus sp. a and Chaetonotus sp. b, see below).
Draculiciteria tesselata is found throughout much of the Atlantic and its sub-seas, but apart from the upper Red Sea it has not yet been reported from the Indo-Pacific. It tends to occur in mobile, rather clean sands of medium size and moderate sorting, either at the shoreline or sublittorally in shallow waters. This cave find is unusual: Station 2 sediments some 80 m in from the cave mouth. Its previous discovery in sand from the groundwater a metre or more beneath the supralittoral of a beach situated 40 m back from the shore at el-’Arish, Egypt (Hummon 2004), leads us, perhaps, to look at a broader habitat paradigm rather than a ‘once off’ observation.
Out of the 12 species of Gastrotrich Macrodasyida found (Table 5), six species belong to the family Lepidodasyidae (C. turbanelloides, Lepidodasys martini, M. minor, M. adenotubulatus, M. laticaudatus and Paradasys sp.), three to the family Thaumastodermatidae (Platydasys sp., P. cataphracta and Tetranchyroderma sp.), two to the family Macrodasyidae (M. caudatus and Urodasys acanthostilis) and one to the family Turbanellidae (Turbanella sp.). Out of the last six taxa, M. caudatus is an eurytopic species with a wide geographical distribution, and is also quite common along the Italian coasts where it has been found in sediments of different origin and grain size, both in the littoral zone and the shallow sublittoral one (Todaro et al. 2001). P. cataphracta, discovered in the sublittoral sediments off North Carolina (Ruppert 1970) and later also found in the Mediterranean (Balsamo et al. 1995) was recorded on this occasion for the third time overall. Urodasys acanthostylis, recently described in the sands of the Cala Corvino tunnel cave, near Bari, Italy (Fregni et al. 1998), was recorded at this site for the second time overall. The finding of this species is of particular relevance as its presence also in the Ciolo cave could be an indication of its typical cave-dwelling nature. The remaining three species probably belong to taxa that are new to science, with the possible exception of Platydasys sp. for which, due to the finding of a single individual, all the necessary diagnostic characteristics to exclude affiliation with a known species are not available. However, as the ten known Platydasys species are preferentially found in medium-coarse sediments, it is more likely that Ciolo’s specimens belongs to a species that has not yet been described.
The family Lepidodasyidae in the Ciolo cave has both eurytopic species that are quite common along the Mediterranean coasts (Todaro et al. 2003a, b) and in particular the Italian ones, such as C. turbanelloides, M. laticaudatus and M. adenotubulatus, as well as more infrequent species tied to a particular substratum such as Lepidodasys martini, previously found only four times in Italy, in medium sandy sediment of the Tyrrhenian and Adriatic shores (Todaro et al. 2001). Also M. minor can be considered a rare species, as the one in the Ciolo is the third finding in Italian waters and the fourth overall (Todaro et al. 2001). The presence of this species in the Apulian cave is, in a certain sense, less problematic to explain since it has a preference for fine sediments, rich in organic content, corresponding to Ciolo’s stations 1 and 3. Unfortunately, the sampling strategy adopted did not allow us to check whether, as some authors sustain, gastrotrichs of this and other genera, characterised by an extremely elongated body (the individuals of Megadasys can reach a length of 3 mm) are able to penetrate into the sediment until reaching the layers where reducing processes prevail over oxidising ones (RPD-layer). Life in this extreme environment is possible by virtue of particular enzymes that are able to protect animals from the toxic effects of the sulfide created by the chemical reactions that take place there (Powel et al. 1979; Fox and Powell 1986). The name “thiobios” has been given to the community of organisms that are able to live in this particular type of microhabitat (for general comments see Giere 1993 and for the gastrotrichs see Boaden 1974; Maguire and Boaden 1975; Gagne 1977; Todaro et al. 2000). The presence of different specimens of M. minor in samples from station 1 where it was also possible to ascertain visually that the redox potential discontinuity layer was very near the surface, suggests that this species also has evolved mechanisms that can protect it from toxic sulphidic exhalation.
The presence in the Ciolo cave of individuals of an undescribed species of Paradasys; is also of considerable taxonomic and biogeographical interest, as the finding is the third record of this genus in the Mediterranean and follows those of Paradasys subterraneus found in the gulf of Lion (Fize 1963) and in the northern Adriatic sea by Tongiorgi et al. (1999).
Out of the 240 known macrodasyidans, the lepidodasyid species compose only 12.5% of the total with a more or less corresponding percentage contributing to the formation of the different gastrotrich communities investigated up to now (Todaro et al. 2001). The reason for the presence of such a high percentage (50%) of lepidodasyids inside the Ciolo cave is not entirely clear. Besides the factors linked to the physical and chemical characteristics of the substrate, it is also likely that trophism plays a key role in structuring the local community. The ecological role of gastrotrichs takes place inside the microphagous, detritivorous benthic community; like the nematodes, the gastrotrichs ingest food (biodetritus, microalgae, protozoa) using the suction action of their powerful pharynx, and in turn they are predated upon by turbellarians, nemertines and juvenile stages of the macrofauna. Excluding a selective predatory action, also because the number of possible predators in the Ciolo sediments is scant (Tables 3, 4), a working hypothesis to explain the high number of lepidodasyids, is that the species in the cave depend only slightly on the microalgal component for their foraging whereas they greatly exploit the microbial one (bacteria and fungi), unlike the worms of the open environment communities that depend more on the plant component for their survival. The darkness inside most of the cave limit primary production to the portion nearest the entrance, the absence of diatom frustules inside the intestines of the species examined and the lack of increase (ANOVA, P>0.05) in the gastrotrich population during the spring, when algae are more abundant, offer good reasons to support this hypothesis.
In addition to the strong presence of Lepidodasyidae in the Ciolo cave, there is the scarce representation of thaumastodermatids, and in particular of the genus Tetranchyroderma that, with a total of 55 species, is the most highly represented taxon among the macrodasyidans in unconfined marine environments (Todaro 2002). D’Hondt (1971) attributes a greater resistance to physical stresses to the members of the family Thaumastodermatidae in general, and in particular to the genus Tetranchyroderma, compared with other macrodasyids. They would, therefore, be at an advantage in environments subjected to large amounts of mechanical stress that can be identified by a coarser sediment grain size. The characteristics of the mobile substrate of the Ciolo cave (fine or very fine sands) bear witness to the low energy in the water column of this habitat, and consequently the low physical stress to which the individuals that inhabit it would be subjected. It is also likely that the low number of species belonging to the genus Tetranchyrodema derives from the lack of a more suitable food source for this animal (i.e., algae) in the caves.
The presence of five new gastrotrich species (a widely studied phylum in Italy) and a new priapulid species would indicate that also for the meiofauna, like the macrofauna, the submarine caves are a ‘hotspot’ for biodiversity and endemism. Widened research of other caves and other systematic groups in the future could ascertain whether the overall biodiversity of cave meiofauna is comparable to that found on deep-sea bottoms, a habitat with which caves share many physical and chemical characteristics (Vacelet et al. 1994).
References
Balduzzi A, Bianchi CN, Boero F, Cattaneo Vietti R, Pansini M, Sarà M (1989) The suspension-feeder communities of a Mediterranean sea cave. In: Ros JD (ed) Topics in marine biology. Sci Mar 53:287–395
Balsamo M, Fregni E, Tongiorgi P (1995) Marine Gastrotricha from the coasts of Sardinia (Italy). Boll Zool 62:273–286
Balsamo M, Ferraguti M, Guidi L, Todaro MA, Tongiorgi P (2002) Reproductive system and spermatozoa of Paraturbanella teissieri (Gastrotricha, Macrodasyida): implications for sperm transfer modality in Turbanellidae. Zoomorphology 121:235–241
Boaden PJS (1974) Three new thiobiotic Gastrotricha. Cah Biol Mar 15:367–378
Boesgaard TM, Kristensen RM (2001) Tardigrades from Australian marine caves. With a redescription of Actinarctus neretinus (Arthrotardigrada). Zool Anz 240:253–264
Bussotti S, Guidetti P, Belmonte G (2003) Distribution patterns of the cardinal fish, Apogon imberbis, in shallow marine caves in southern Apulia (SE Italy). Ital J Zool 70:153–157
Chevaldonne P, Lejeusne C (2003) Regional warming-induced species shift in north-west Mediterranean marine caves. Ecol Lett 6:371–379
Cinelli F, Fresi E, Mazzella P, Pansini M, Pronzato R, Svoboda A (1977) Distribution of benthic phyto- and zoocenoses along a light gradient in a superficial marine cave. In: Keegan BF, O’Ceidigh P, Boaden PJS (eds) Biology of benthic organisms (Proceedings of the 11th European Marine Biology Symposium 1976, Galway, Ireland). Pergamon Press, New York, pp 173–183
Clausen C (2000) Gastrotricha Macrodasyida from the Tromsø region, northern Norway. Sarsia 85:357–384
Clausen C (2004) Gastrotricha from the Faroe Bank. Sarsia 89:423–458
Coull BC (1988) Ecology of the marine meiofauna. In: Higgins RP, Thiel H (eds) Introduction to the study of Meiofauna. Smithsonian Institution Press, Washington, pp 18–38
Fichez R (1990) Decrease in allochthonous organic inputs in dark submarine caves, connection with lowering in benthic community richness. Hydrobiologia 207:61–69
Fichez R (1991) Benthic oxygen uptake and carbon cycling under aphotic and resource-limiting conditions in a submarine cave. Mar Biol 110:137–143
Fize A (1963) Contribution á l´étude de la microfaune des sables littoraux du Golfe d´Aigues-mortes. Vie Milieu 14:669–774
Folk RL (1958) Petrology of sedimentary rocks. Hemphills, Austin
Fox CA, Powell EN (1986) Meiofauna and the sulfide system: the effects of oxygen and sulfide on the adenylate pool of three turbellarians and a gastrotrich. Comp Biochem Physiol 85:37–44
Fregni E, Tongiorgi P, Faienza MG (1998) Two new species of Urodasys (Gastroricha, Macrodasyidae) with cuticular stylet. Ital J Zool 65:377–380
Gagne GD (1977) Dolichodasys elongatus n.g., n.sp., a new macrodasyid gastrotrich from New England. T. Am Microsc Soc 96:19–27
Gallo D’Addabbo M, De Zio Grimaldi S, Sandulli R (2001) Heterotardigrada of two submarine caves in San Domino island (Tremiti islands) in the Mediterranean sea. Zool Anz 240:361–369
Giere O (1993) Meiobenthology. The microscopic fauna in aquatic sediments. Springer, Berlin Heidelberg New York
Giere O, Eleftheriou A, Murison DJ (1988) Abiotic factors. In: Higgins RP, Thiel H (eds) Introduction to the study of meiofauna. Smithsonian Institution Press, Washington, pp 61–78
Guidi L, Pierboni L, Ferraguti M, Todaro MA, Balsamo M (2004) Spermatology of the genus Lepidodasys Remane, 1926 (Gastrotricha, Macrodasyida): towards a revision of the family Lepidodasyidae Remane, 1927. Acta Zool Stockholm 85:211–221
Harmelin JG (1980) Etablissement des communautés de substrat durs en milieu obscur. Résultats préliminaires d’une experience à long terme en Méditerranée. Mem Biol Mar Oceanogr 10(suppl.):29–52
Harmelin JG (1997) Diversity of bryozoans in a Mediterranean sublittoral cave with bathyal like conditions: role of dispersal processes and local factors. Mar Ecol Prog Ser 153:139–152
Harmelin JG, Vacelet J (1997) Clues to deep-sea biodiversity in a nearshore cave. Vie Milieu 47:351–354
Harmelin JG, Vacelet J, Vasseur P (1985) Les grottes sous-marines obscures: un milieu extreme et un remarquable biotope refuge. Tethys 11:214–229
Higgins RP, Thiel H (eds) (1988) Introduction to the study of Meiofauna. Smithsonian Institution Press, Washington
Hochberg R (2005) Musculature of the primitive gastrotrich Neodasys (Chaetonotida): functional adaptations to the interstitial environment and phylogenetic significance. Mar Biol 146:315–323
Hochberg R, Litvaitis MK (2000) Phylogeny of gastrotricha: a morphology-based framework of gastrotrich relationships. Biol Bull 198:299–305
Hochberg R, Litvaitis MK (2001) Macrodasyida (Gastrotricha): a cladistic analysis of morphology. Invertebr Biol 120:124–135
d’Hondt JL (1971) Gastrotricha. Oceanogr Mar Biol 9:141–192
Hummon WD (2004) Global database for marine Gastrotricha. Server at http://www.132.235.243.28 under Eastern Mediterranean and Red Seas
Lejeusne C, Chevaldonne P (2005) Population structure and life history of Hemimysis margalefi (Crustacea: Mysidacea), a ‘thermophilic’ cave-dwelling species benefiting from the warming of the NW Mediterranean. Mar Ecol Prog Ser 287:189–199
Maguire C, Boaden PJS (1975) Energy and evolution in the thiobios: an extrapolation from the marine gastrotrich Thiodasys sterreri. Cah Biol Mar 26:635–646
Marotta R, Guidi L, Pierboni L, Ferraguti M, Todaro MA, Balsamo M (2005) Sperm ultrastructure of Macrodasys caudatus (Gastrotricha: Macrodasyida) and a sperm based phylogenetic analysis of Gastrotricha. Meiofauna Mar 14:9–21
Marti R, Uriz MJ, Ballesteros E, Turon X (2004) Benthic assemblages in two Mediterranean caves: species diversity and coverage as a function of abiotic parameters and geographic distance. J Mar Biol Assoc UK 84:557–572
Moens T, Vincx M (1997) Observations on the feeding ecology of estuarine nematodes. J Mar Biol Assoc UK 77:211–227
Nicholas LW, Todaro MA (2005) Observations on Gastrotricha from a sandy beach in southeastern Australia with a description of Halichaetonotus australis sp. nov. (Gastrotricha: Chaetonotida). New Zeal J Mar Fresh 39:973–980
Onorato R, Denitto F, Belmonte G (1999) Le grotte marine del Salento: classificazione, localizzazione e descrizione. Thalassia Salentina 23:67–116
Ohtsuka S, Hanamura Y, Kase T (2002) A new species of Thetispelecaris (Crustacea: Peracarida) from submarine cave on Grand Cayman Island. Zool Sci 19:611–624
Palacin C, Masalles D (1986) Some data on the meiofauna of an underwater cave of the island of Majorca, Spain. Publ Dep Zool, Barcelona 12:15–26
Pansini M (1996) Petrosia pulitzeri n. sp. (Porifera, Demospongiae) from Mediterranean caves. Ital J Zool 63:169–172
Pesta O (1959) Harpacticoiden (Crustacea: Copepoda) aus submariner hohlen und den benachbarten Litoralbezirken am Zap von Sorrent (Neapel). Pubbl Staz Zool Napoli 30(suppl.):95–117
Pfannkuche O, Thiel H (1988) Sample processing. In: Higgins RP, Thiel H (eds) Introduction to the study of meiofauna. Smithsonian Institution Press, Washington, pp 134–145
Powell EN, Crenshaw MA, Rieger RM (1979) Adaptations to sulfide in the meiofauna of the sulfide system. I. 35S-sulfide accumlation and the presence of a sulfide detoxication system. J Exp Mar Biol Ecol 37:57–76
Riedl R (1959) Die Hydroiden des Golfes von Neapel und ihr Anteil an der Fauna unterseeischen Höhlen. In: Ergebnisse der Österreichischen Tyrrhenia-Expedition 1952, Teil xvi. Pubbl Staz Zool Napoli, 30 (suppl.):591–755
Riedl R (1966) Biologie der Meereshöhlen. Paul Parey, Hamburg
Ruppert EE (1970) On Pseudostomella Swedmark 1956 with descriptions of P. plumosa nov. spec., P. cataphracta nov. spec. and a form of P. roscovita Swedmark 1956 from the west Atlantic coast. Cah Biol Mar 11:121–143
Seward–Thompson BL, Hails JR (1973) An appraisal of the computation of statistical parameters in grain size analysis. Sedimentology 20:161–169
Sandulli R, Gallo D’addabbo M, De Lucia Morone MR, D’Addabbo R, Pietanza R, Grimaldi-De Zio S (1999) Preliminary investigations on meiofauna of two caves in San Domino island (Tremiti Archipelago, Adriatic Sea). Biol Mar Medit 6:437–440
Sandulli R, De Zio-Grimaldi S, Gallo D’addabbo M (2002) Meiofauna. In: Cicogna F, Bianchi CN, Ferrari G, Forti P (eds) Grotte Marine, cinquant’anni di ricerche in Italia. Ministero dell’Ambiente e della Tutela del Territorio, Roma, pp 273–278
Sørensen MV, Jørgensen A, Boesgaard TM (2000) A new echinoderes (Kinorhyncha, Cyclorhagida) from a submarine cave in new south Wales, Australia. Cah Biol Mar 41:167–179
Stock JH (1993) Some remarkable distribution patterns in stygobiont Amphipoda. J Nat Hist 27:807–819
Todaro MA (1992) Contribution to the study of the Mediterranean meiofauna: Gastrotricha from the Island of Ponza, Italy. Boll Zool 59:321–333
Todaro MA (1998) Meiofauna from the Meloria Shoals: Gastrotricha, biodiversity and seasonal dynamics. Biol Mar Medit 5:587–590
Todaro MA (2002) An interesting new gastrotrich from littoral meiobenthos (Long Beach Island, USA), with a key to species of Tetranchyroderma (Gastrotricha: Macrodasyida). J Mar Biol Assoc UK 82:555–563
Todaro MA, Shirley TC (2003) A new meiobenthic priapulid (Priapulida, Tubiluchidae) from a Mediterranean submarine cave. Ital J Zool 70:79–87
Todaro MA, Rocha CEF (2004) Diversity and distribution of marine Gastrotricha along the northern beaches of the state of São Paulo (Brazil), with description of a new species of Macrodasys (Macrodasyida, Macrodasyidae). J Nat Hist 38:1605–1634
Todaro MA, Rocha CEF (2005) Further data on marine gastrotrichs from the State of São Paulo and the first records from the State of Rio de Janeiro (Brazil). Meiofauna Mar 14:27–31
Todaro MA, Fleeger JW, Hu YP, Hrincevich AW, Foltz DW (1996) Are meiofauna species cosmopolitan? Morphological and molecular analyses of Xenotrichula intermedia (Gastrotricha: Chaetonotida). Mar Biol 125:735–742
Todaro MA, Bernhard JM, Hummon WD (2000) A new species of Urodasys (Gastrotricha, Macrodasyida) from dysoxic sediments of the Santa Barbara Basin (California, USA). Bull Mar Sci 66:467–476
Todaro MA, Hummon WD, Balsamo M, Fregni E, Tongiorgi P (2001) Inventario dei Gastrotrichi marini italiani: una checklist annotata. Atti Soc Tosc Sci Nat Mem Ser B 107:75–137
Todaro MA, Littlewood DTJ, Balsamo M, Herniou EA, Cassanelli S, Manicardi G, Wirz A, Tongiorgi P (2003a) The interrelationships of the Gastrotricha using nuclear small rRNA subunit sequence data, with an interpretation based on morphology. Zool Anz 242:145–156
Todaro MA, Matinato L, Balsamo M, Tongiorgi P (2003b) Faunistics and zoogeographical overview of the Mediterranean and Black Sea marine Gastrotricha. Biogeographia 24:131–160
Todaro MA, Balsamo M, Kristensen RM (2005) A new genus of marine chaetonotids (Gastrotricha), with a description of two new species from Greenland and Denmark. J Mar Biol Assoc UK 85:1391–1400
Tongiorgi P, Fregni E, Balsamo M (1999) Gastrotricha from Italian brackish environment with description of a new species of Chaetonotus. J Mar Biol Assoc UK 79:585–592
Vacelet J, Boury-Esnault N, Harmelin JG (1994) Hexactinellid cave, a unique deep-sea habitat in the scuba zone. Deep Sea Res 41:965–973
Villora-Moreno S (1996) New genus and species of the deep-sea family Coronarctidae (Tardigrada) from a submarine cave with a deep-sea like condition. Sarsia 81:275–283
Wieser W (1954) Beitrage zur Kenntnis der Nematoden submariner Hohlen. Österr Zool Ztschr 1/2:172–230
Zabala M, Riera T, Gili JM, Barange M, Lobo A, Penuelas J (1989) Water flow, trophic depletion, and benthic macrofauna impoverishment in a submarine cave from the Western Mediterranean. PSZNI Mar Ecol 10:271–287
Acknowledgments
We are grateful to G. Belmonte (University of Lecce) for providing us with invaluable information on Grotta del Ciolo and priceless logistic help during sampling. D. Mosci assisted by SCUBA divers of the ‘Gruppo Speleologico Neretino’ (Lecce) collected the samples. We thank W.D. Hummon and an anonymous reviewer for the insightful comments on an earlier draft of the manuscript. The research is within the framework of the project, biodiversity of inconspicuous organisms in Italian marine protected areas (BIOIMPA) and benefited from a grant by the Italian Ministry of Research (MIUR Prin-2004 “Contributo della meiofauna alla biodiversità marina italiana”) M.A. Todaro Co-PI.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Cattaneo-Vietti, Genova
Rights and permissions
About this article
Cite this article
Todaro, M.A., Leasi, F., Bizzarri, N. et al. Meiofauna densities and gastrotrich community composition in a Mediterranean sea cave. Mar Biol 149, 1079–1091 (2006). https://doi.org/10.1007/s00227-006-0299-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-006-0299-z