Abstract
Submerged sea caves are priority areas for conservation according to the Habitat Directive 92/43/CEE because of their unique biodiversity. A limited number of publications exist about communities living on sediments inside caves, mostly focused on the macrofaunal fraction (>0.5-mm body size). Meiofaunal communities (0.062–0.5-mm body size) have been largely neglected in ecological studies about communities inhabiting sea caves. In the present study, we analysed meiofaunal communities from Los Cerebros cave, a shallow marine cave (3–8 m in depth, 80 m long), with secondary openings in the inner parts and freshwater infiltrations. Sediment samples were taken by scuba divers using cylinders (cores), with known inner diameter. Sampling stations were sampled from the different sections of the cave (entrance, twilight zone, dark zone and jameos). Five surveys were carried out, from June 2003 to February 2005. Nematodes, copepods, and polychaetes dominated overwhelmingly the meiofaunal composition, with the remaining taxonomic groups being scarce. Generalized linear models showed that the high spatial and temporal variability observed among on the abundance of major meiofaunal groups inside the cave was better explained by the surveys, the section of the caves and the presence of freshwater. Higher abundances are observed near the entrance and in the station with regular freshwater input. Nematodes and polychaetes were clearly dominated by species extensively recorded in shallow subtidal sandy sediments on the study area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sea-flooded caves are very particular marine environments from both evolutionary and ecological perspectives (Iliffe and Kornicker 2009). From an evolutionary point of view, sea-flooded caves often harbor unique lineages of organisms characterized by high levels of adaptation and endemism (Iliffe and Kornicker 2009; Juan et al. 2010) often with unknown marine relatives (Neiber et al. 2011). The most remarkable cases occur amongst arthropods, including Remipedia, Thermosbaenacea, Stygiomysida (Wagner 1994; Meland and Willassen 2007; Hoenemann et al. 2013) and several families of amphipods, copepods and isopods (Wägele 1985; Koenemann and Holsinger 1999; Fosshagen et al. 2001; Iliffe and Botosaneanu 2006; Bauzà-Ribot et al. 2012; Hou et al. 2014). Many of these lineages exhibit disjunt distribution patterns in caves separated by large geographical distance (Koenemann et al. 2009; Wilkens et al. 2009). These patterns have been explained addressing cave colonization events during the Miocene and followed by tectonic vicariance (Iliffe et al. 1984; Wilkens et al. 1986; Humphreys 2000), often with extinction of the marine ancestral populations. Other cave lineages belong to groups that are otherwise exclusively known from the deep sea, such as certain sponges (Vacelet et al. 1994; Vacelet and Boury-Esnault 1995; Vacelet 2006), annelids (Pettibone 1985; Núñez et al. 1997; Martínez et al. 2013; Martínez et al. 2014, 2016; González et al. 2015) and crustaceans (Wilkens et al. 1990; Ahyong et al. 2011; Iglikowska and Boxshall 2013). In these cases, cave colonization might be facilitated by the ecological similarities between caves and the deep sea.
Although often categorized as a single habitat, caves harbor a mosaic of ecological conditions with strong differences at both a macro- and micro-scale (Sket 1996; Martínez et al. 2009). Sea-flooded caves are often categorized as marine caves if they hold strong influence from the sea and receive the effects of waves and currents (Riedl and Ozretić 1969; Tilzer 1970); or anchialine if they are isolated from the sea and harbor stratified water bodies with long residence times, sometimes including freshwater layers (Stock et al. 1986; Sket 1996; Wilkens et al. 2009; Bishop et al. 2015). However, these two categories are not always easy to apply to real cases since many marine caves directly connected to the sea become progressively isolated as they extend inland becoming true anchialine environments (van Hengstum and Scott 2011; Yager 2013). These changes are related to the presence of well-characterized gradients from the entrance to the deepest sections, involving a progressive reduction of light as well as availability of allochtonous organic matter (Gili et al. 1986; Fichez 1990, 1991), with conspicuous effects on the fauna dwelling in the different sections (Iliffe 1986). Several studies have focused on characterizing these changes using benthic sessile communities as well as macrofaunal assemblages of crustaceans and foraminifera (Iliffe 1986, 1992; Benedetti-Cecchi et al. 1997, 1998; Gili and Coma 1998; Oertel and Patzner 2007; Bamber et al. 2008; van Hengstum and Scott 2011; Navarro-Barranco et al. 2012, 2014), collectively showing how the abundance of individuals and taxonomic richness is reduced progressively from the entrance. However, studies on similar processes using interstitial meiobenthic communities are scarcer or focus more in single taxonomic groups rather than in overall communities (Palacín and Masalles 1986; Palacín et al. 1992; Núñez et al. 1997; Todaro et al. 2006; Brito et al. 2009). Moreover, scarce studies have dealt with meiofauna responses to salinity variations, especially from stochastic events such as run-offs (Riera et al. 2012). To our knowledge, there are no studies concerning the effects of freshwater filtration on meiofaunal communities inhabiting in caves.
Marine interstitial meiofauna are integrated by a heterogeneous assemblage of metazoans with different phylogenetic affinities that dwell among the sand grains (Giere 2009; Rundell and Leander 2010). Interstitial meiofauna are a very important component of marine diversity due to their taxonomic diversity and high species richness (Curini-Galletti et al. 2012). Despite their different phylogenetic origins, interstitial animals present a set of common adaptations to survive amongst the sand grains, including small and elongated bodies, a specialized pharyngeal apparatus, direct development, presence of adhesive structures and epidermal ciliation (Giere 2009). These adaptations have evolved convergently in several independent lineages (Di Domenico et al. 2014; Andrade et al. 2015; Martínez et al. 2015), although some cases might represent plesiomorphies that are important to understand the evolution of particular metazoan lineages (Rieger 1980; Worsaae and Rouse 2009; Mwinyi et al. 2010; Laumer et al. 2015). Due to their high abundances, taxonomical diversity and short life cycles, interstitial meiobenthic assemblages are very sensitive to environmental conditions at microscales and exhibit fast time responses to punctual environmental conditions (Schratzberger and Jennings 2002). Therefore, they represent a feasible tool for environmental monitoring and assessment.
In the present study, we describe the effect of distance from the entrance on the abundance of major meiofaunal groups in Los Cerebros cave, on the west coast of Tenerife (Canary Islands, NE Atlantic Ocean). Los Cerebros cave is relatively large and harbors a combination of endemic species (Ortea 1995; Pérez Dionis et al. 2011; Martínez et al. 2013) and typical marine assemblages (Martínez et al. 2004; Álvarez et al. 2005; Riera et al. 2007; Schmidt-Rhaesa et al. 2013). The cave is exposed to waves and currents, which produce turbulence and perturbation on the sediments, potentially affecting the distribution of meiofauna along the cave. We explore any pattern of variability (spatial, temporal and environmental) through multivariate analyses, to infer if the variation can be explained by any of the environmental parameters or, instead, if only seasonality or spatial patchiness are responsible for changes.
Materials and methods
Sampling localities, working hypothesis and experimental design
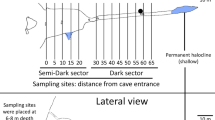
This study was carried out in Los Cerebros cave, a marine cave located on the west coast of Tenerife (maximum age of the island, 12 Ma; coordinates: 28°10´N/16°48´W; Fig. 1). The cave is a lava tube with a complex structure. The entrance opens at 8 m and leads to two galleries extending ca. 80 m inland. The average depth of the passages is 5–8 m. Two terrestrial collapse entrances, locally known as jameos, open at the dead ends of the two main galleries, facilitating the indirect presence of light in these areas. Freshwater flows near one of these dead ends and mixes with the marine waters inside the cave. The water column of the cave is not stratified, as it is affected by waves and currents. These currents carry a significant amount of particulate organic matter, which sustains a rich community of sessile organisms (Álvarez et al. 2005). Marine medium sandy sediments are deposited at the entrance and bottom of the main passages, whereas gravel and boulders occupy certain areas of the middle sections (Martínez et al. 2004, 2013).
We sampled at eight stations situated at increasing distance from the entrance of the cave and at different regions of the cave, namely entrance, twilight zone, dark zone and jameos (i.e. secondary entrances; Fig. 1). Sediment cores (3.6 in inner diameter, 10 cm2 surface) were hammered into the sediment to a depth of 30 cm randomly (1 m apart) for meiofauna analysis. Sampling was conducted throughout five field surveys throughout 3 years [June 2003 (1st), October 2003 (2nd), February 2004 (3rd), June 2004 (4th) and February 2005 (5th)]. The type of sediment (sand or gravel) and the presence of light and freshwater was also noted. Light formed a gradient along the cave, with the entrance being illuminated (stations M1 and M8), the mid-section and the jameos areas being twilight (stations M2–M4), with the stations M5 and M6 being always in darkness. Freshwater was consistently registered at the bottom of the cave (station M7).
Analysis of meiofauna
Samples were fixed in 4 % formalin solution. A 0.5-mm sieve was used to separate macrofauna and the residue collected from a 0.063-mm sieve. The residue was posteriorly separated into taxonomic groups under a binocular microscope, and preserved in 70 % ethanol (Higgins and Thiel 1988). Nematodes and polychaetes were mounted in glycerine for taxonomic identification. These specimens were examined with a Leica DMLB microscope equipped with Nomarski interference contrast.
Statistical analysis
We investigate the changes in the abundance of meiofauna along the different sections of the cave using generalized linear models (GLMs), calculated with the function GLM implemented in R (Zuur et al. 2007). Variable responses were total abundance, and abundance of those groups exhibiting a >5 % dominance, i.e. Nematoda, Copepoda, Polychaeta, Oligochaeta, Ostracoda, Cumacea, Priapulida and Gammaridea. As explanatory variables, we consider five factors: (i) survey (five levels, random: survey 1–5), (ii) section (four levels, fixed: entrance, twilight zone, dark zone and jameos), (iii) light (three levels, fixed: light, twilight and dark), (iv) habitat (two levels, fixed: sand, gravel), and (v) freshwater (two levels, fixed: presence, absent). Our null hypothesis predicts random variation of densities of meiofaunal groups (number of individuals per taxonomic group and core), amongst different sampling surveys, whereas gradients would predict an overall reduction of abundances from the entrance to the bottom. Higher heterogeneity at the jameos zone is expected due to the presence of indirect light and freshwater flows. Polychaeta and Oligochaeta are treated as independent groups, following the standards of other ecological studies, despite them representing, in fact, the same clade. Boxplots used to describe the changes of abundance according to the most important factors were prepared using the function boxplot implemented in R (Murrell 2005; R Team 2008).
Differences in meiofauna community structure with varying distance to the entrance of the cave (entrance, twilight, dark and jameos zones) were tested by means of a permutational multivariate analysis of variance (PERMANOVA) calculated using the function adonis from the R-package vegan (Dixon and Palmer 2003). We included all the factors from the best GLM for total abundances selected using model average (see above), which included the factors “section” (fixed factor) and “survey” (fixed factor), “habitat” (fixed factor) and “freshwater” (fixed factor). To visualize affinities in meiofauna assemblage structure, a nm-MDS (non-metric multidimensional scaling) ordination was carried out on square-rooted transformed abundance data via the Bray–Curtis similarity index (Clarke and Warwick 1994) using the function metaMDS included in the R package vegan (Dixon and Palmer 2003). A similarity percentage analysis calculated with the R function simper (Clarke 1993) was used to compute the percentage contribution of each meiofauna taxonomic group to the dissimilarities between all pairs of sampling sectors (entrance, twilight, dark zone and jameos sections).
Results
A total of 11,224 individuals (ind) belonging to 26 taxonomic groups, plus the larval stage “nauplius”, were collected throughout the 5 field surveys. Nematodes and copepods were the most abundant groups making up the 26.7 % (2995 ind) and 18.7 % (2103 ind) of the overall abundance, respectively. In contrast, 17 taxonomic groups were scarce, with low abundances (<100 ind; Table 1). The percentage of dominance and the frequency of occurrence were globally correlated if all meiofaunal groups are considered (Fig. 2).
Consistent temporal variations were recorded throughout the study period, with highest abundances in the last survey (February 2005; Fig. 3). Meiofaunal densities showed spatial variability among the three sections of the cave, reaching the highest abundances at the entrance (4489 ind), followed by the deepest sector (4207 ind). However, meiofaunal abundances varied greatly among field surveys, with highest densities in inner sections of the cave in the second (June 2003), the fourth (February 2004) and the last (February 2005) survey. The lowest densities were recorded in the middle section (2528 ind) in all surveys (Fig. 3).
Overall abundance of meiofauna considering the variables with higher explanatory powers according to the generalized linear model analyses (see Table 1)
Meiofaunal composition also varied temporally along the study period, especially among the most abundant taxonomic groups, i.e. nematodes, copepods, and polychaetes. Nematodes obtained the highest abundances in June 2003, October 2003 and February 2005. In the remaining surveys, they were the second (February 2004) and the third (June 2004) most abundant group, being overcome by polychaetes and copepods and polychaetes, respectively (Fig. 4). The remaining groups showed little variability, mostly related to the highest abundances recorded in February 2005 (Fig. 4).
Abundance of the dominant meiofaunal groups (dominance > 5 %) according to the explanatory variables with higher explanatory powers according to the generalized linear model analyses (see Table 1)
Comparison of different nested GLMs using Akaike information criteria (AIC) showed that two models predicted equally well the variation of total meiofauna as well as the variation of the abundances of major groups. Model 1, included the variables “survey”, “freshwater”, “light” and, “section”; and model 2, with “survey”, “freshwater” and “section”. The only exceptions to that were found for the total abundance of Cumacea and Gammaridea, better explained by “survey”, “freshwater”, “habitat” and “section”. The model average amongst nested models indicated that the variables with the highest relative importance (RI = 1.00, Table 2) in predicting the variations in abundances are “survey”, “freshwater” and “section”; exceptions were for Copepoda, for which “survey” and “freshwater” have the highest relative importance; and Ostracoda, the variation of which was better explained by “survey”, “freshwater”, ”light” and “section”. A summary of the better models for each group is shown in Table 2.
Total abundance of meiofauna was higher at the cave entrances during the February 2005 survey (Fig. 3), and in the station with freshwater filtrations. Higher abundances at the entrances of the cave were also observed for all dominant meiofaunal groups individually, except for Copepoda, Oligochaeta and Priapulida, which were more abundant near the jameos; and Polychaeta, with maximum abundances at the twilight section (Fig. 4). Regarding survey and freshwater, maximum abundances were also found in February 2005 and in the station with freshwater consistently for all the dominant meiofaunal groups.
Meiofauna assemblage structure varied consistently among the four cave sections (entrance, twilight, dark zone and jameos; pseudo-F = 2.733, p = 0.0005). Temporal variations of meiofauna structure were not significant throughout the study period (Pseudo-F = 1.410, p = 0.120). Differences in seabed composition did not affect significantly to meiofauna assemblage structure (Pseudo F = 1.626, p = 0.001), but freshwater inputs underpinned consistent changes on meiofauna (Pseudo-F = 5.026, p = 0.001; Table 3, Fig. 5).
n-MDS of meiofauna community at “Los Cerebros” cave. Sections “entrance”, “middle”, “bottom” and “jameo” are represented in grey colours from light grey (“entrance”) to black (“jameo”) . Presence of freshwater was represented by polygons. Circles: absence of freshwater; triangles: presence of freshwater
Dissimilarities in meiofauna composition among the sections ranged from 44.75 % (entrance–twilight section) to 50.31 % (entrance–dark zone). Differences are explained by the highest abundances of nematodes at the entrance (10.48 % of overall abundance), cumaceans (6.10 %) and ostracods (5.84 %). The latter two taxonomic groups were scarce at the middle and the inner sections. The twilight section was characterized by harboring the highest polychaete abundances (6.69 %) and the jameos by the highest oligochaete abundances (4.1 %). The lowest abundances were found in the dark zone (bottom) of the cave.
Species composition of Nematoda and Polychaeta
A total of 32 meiofaunal nematode species were identified in Los Cerebros cave, belonging to 7 orders (Araeolaimida, Chromadorida, Desmodorida, Enoplida, Monhysterida, Plectida and Triplonchida) and 18 families (Table 4). Half of the species were determined to a genus level and ca. 9–10 species belonged to previously undescribed species. Most of the species are typical of shallow subtidal sandy seabeds from the Canary Islands, including several putative new species.
A total of 70 polychaetes species, 18 of them determined to the genus level, were identified in Los Cerebros cave, belonging to 35 families (Table 5). About 15 species are recorded for the first time in the Canary Islands, 10 of them possibly undescribed species that are currently under taxonomic study. Except for two species, all annelids discovered during this survey belong to marine species, also found in marine sediments in the Canary Islands (Núñez et al. 2005). The species Leptonerilla diatomeophaga is exclusively found in anchialine and marine caves in the Canary Islands, including La Corona lava tube (Lanzarote) and Aguadulce cave system (Tenerife; Worsaae et al. 2009). It belongs to a genus mostly represented by cave species distributed in the Mediterranean Sea, the Caribbean Sea and Bermuda (Martínez et al. in press). The species Axiokebuita cavernicola, though not recorded through the study period, is endemic from Los Cerebros cave, where it is exclusively found in the gravel beds found in the middle section of the cave, in complete darkness. The species belongs to a genus with other two species, exclusively known from the deep sea (Martínez et al. 2013, 2014).
Discussion
The present study showed consistent spatial differences in meiofauna assemblage structure, with the complex nature of this cave being of utmost importance. The presence of several entrances (jameos) and corridors creates an environment without a pattern from the entrance to the insides of the cave. The jameos and corridors create an environment with conditions (trophic supply, lightness, etc.) that favor the development of benthic fauna. No temporal trends have been found in the studied cave and this may be explained by its shallow nature (8 m at the entrance), affected by hydrodynamics that could suspend the sediment and leave only coarse sedimentary types (e.g. gravels). The temporal patterns of rough seas does not fit with seasons, though they are more frequent in autumn and winter.
A decrease of univariate descriptors, i.e. species richness and individual abundances, of faunal communities has been observed from the entrance to the inward end of marine caves (Cicogna 2003; Martí et al. 2004). This feature is commonly explained by the depletion of trophic supply in inner parts of caves (Zabala et al. 1989), but, surprisingly, no decrease of organic matter has been detected in several caves (Navarro-Barranco et al. 2013). An other plausible explanation may be variations of other environmental variables, such as grain size (Bamber et al. 2008), habitat heterogeneity (Zabala et al. 1989), water turbulence and exposure (Carvalho et al. 2012). In contrast, several studies have revealed that infaunal communities do not respond in the same way (Navarro-Barranco et al. 2013) and community response uniquely depends on the cave characteristics. Thus, soft-bottom communities from caves seem to be context- and scale-dependent, with no well-defined spatio-temporal patterns, as has been reported for hard-bottom communities (Bussotti et al. 2006). In our particular case, the most important parameters explaining the changes in meiofauna abundances were the survey, cave section and freshwater inputs. The importance of survey highlights the temporal variation of abundances of meiofauna in the cave, whereas the section and the presence of freshwater might be related to the presence of organic matter in the sediments dragged inside the cave by waves from the ocean, or introduced by the jameos and the freshwater from surrounding terrestrial areas. Freshwater flowing into the cave might be enriched with organic matter coming from banana plantations.
Meiofauna communities from marine caves have remained overlooked compared to studies based on large-sized biota, e.g. macro- and megafauna (Gerovasileiou and Voultsiadou 2015). Most of the studies are focused on taxonomic aspects (e.g. Núñez et al. 1997, 2009; Worsaae et al. 2009; Curini-Galletti et al. 2012; Schmidt-Rhaesa et al. 2013) or ecology of certain taxonomic groups [e.g. gastrotrichs, (Todaro et al. 2006), tardigrades (Grimaldi De Zio et al. 1982; D’Addabbo et al. 2001; Jørgensen et al. 2014) or nematodes (Ape et al. 2015)]. A limited number of studies regarding overall meiofauna community in caves has been conducted (e.g. Palacín and Masalles 1986; Palacín et al. 1992; Sandulli et al. 1999) and most of them identified meiofaunal specimens to major taxa (e.g. nematodes, copepods, polychaetes, etc.). Thus, scarce information is provided to extract general and consistent patterns of meiofaunal communities within caves; even the high variability among caves makes each cavity a unique ecosystem (Navarro-Barranco et al. 2013). In the present study, a high spatial variability of meiofauna community was observed because of the cave complexity (secondary openings and freshwater inputs) and the influence of hydrodynamics, i.e. tides and rough seas, due to shallow depths (<8 m) and orientation of the entrance. The lack of consistent spatial and temporal trends may be explained by the stochasticity of events (rough seas and freshwater input) that affect directly and indirectly meiofauna abundances.
Most of the ecological studies have been conducted in anchialine caves, with relatively small influence of hydrodynamics (Iliffe et al. 2000) and even in sheltered areas with minor influence of tides, e.g. the Mediterranean Sea (Sandulli et al. 1999; Todaro et al. 2006). To our knowledge, no ecological studies on meiofaunal communities have been focused on caves exposed to hydrodynamics where environmental conditions greatly differ depending on tide height and sea conditions. Moreover, the freshwater input from terrestrial runoffs is periodical throughout the year, with special emphasis during the summer season since most of the runoff comes from the irrigation of plantations on the surrounding land. Freshwater inputs may have sporadic influence in meiofauna assemblages directly through changes in salinity, and indirectly by carrying organic matter to the cave; however, a more exhaustive environment monitoring assessment is necessary to elucidate the importance of this factor structuring the fauna composition of Los Cerebros cave. Above all, the studied cave system may not be considered as anchialine cave since there is continuous connection to the sea, no permanent stratified water column and no oxygen zonation, among other factors that characterize these environments (Gerovasileiou et al. 2016).
In short, communities of polychaetes and nematodes at Los Cerebros cave were characterized by low abundances and species richness. Samples need to be re-examined for in-depth taxonomic identification, but in preliminary tests, the annelid and nematode fractions were dominated by marine species that have been previously recorded in subtidal sandy bottoms of the Canarian Archipelago (e.g. Martínez et al. 2004, 2009, 2013; Riera et al. 2006, 2010, 2011, 2013; Worsaae et al. 2009). The low representation of cave-dwelling species may be explained by the hydrodynamic conditions inside the cave system that affects directly, i.e. erosion and drifting effects of tides and currents, and indirectly, i.e. resuspension of fine-grained sediments and particulate organic matter.
References
Ahyong ST, Andreakis N, Taylor J (2011) Mitochondrial phylogeny of the deep-sea squat lobsters, Munidopsidae (Galatheoidea). Zoologischer Anzeiger 250:367–377
Álvarez F, Martínez A, Núñez L, Núñez J (2005) Sobre la presencia en Canarias de varias especies de braquiópodos (Brachiopoda: Rhynconellata) en cuevas y cornisas submarinas. Vieraea 33:261–279
Andrade SC, Novo M, Kawauchi GY, Worsaae K, Pleijel F, Giribet G, Rouse GW (2015) Articulating “archiannelids”: phylogenomics and annelid relationships, with emphasis on meiofaunal taxa. Mol Biol Evol: msv157
Ape F, Arigo C, Gristina M, Genovese L, Di Franco A, Di Lorenzo M, Baiata P, Aglieri G, Milisenda G, Mirto S (2015) Meiofaunal diversity and nematode assemblages in two submarine caves of a Mediterranean protected area. Mediterranean Marine Science 17:202–215
Bamber RN, Evans N, Robbins RS (2008) The marine soft‐sediment benthic communities of Hong Kong: a comparison of submarine cave and open habitats. Journal of Natural History 42:953–965
Bauzà-Ribot MM, Juan C, Nardi F, Oromí P, Pons J, Jaume D (2012) Mitogenomic phylogenetic analysis supports continental-scale vicariance in subterranean thalassoid crustaceans. Current Biology 22:2069–2074
Benedetti-Cecchi L, Airoldi L, Abbiati M, Cinelli F (1997) Exploring the causes of spatial variation in an assemblage of benthic invertebrates from a submarine cave with sulphur springs. Journal of Experimental Marine Biology and Ecology 208:153–168
Benedetti-Cecchi L, Airoldi L, Abbiati M, Cinelli F (1998) Spatial variability in the distribution of sponges and cnidarians in a sublittoral marine cave with sulphur-water springs. Journal of Marine Biological Association of United of Kingdom 78:43–58
Bishop RE, Humphreys WF, Cukrov N, Zic V, Boxhall GA, Cukrov M, Iliffe TM, Krsinic F, Moore WS, Pohlman JW, Sket B (2015) “Anchihaline” redefined as a subterranean estuary in crevicular or cavernous gelogical setting. J Crustac Biol 35:511–504
Brito MC, Martínez A, Núñez J (2009) Changes in the stygobiont polychaete community of the Jameos del Agua, Lanzarote, as a result of bioturbation by the echiurid Bonellia viridis. Marine Biodiversity 39:183–188
Bussotti S, Terlizzi A, Fraschetti S, Belmonte G, Boero F (2006) Spatial and temporal variability of sessile benthos in shallow Mediterranean marine caves. Marine Ecology Progress Series 325:109–119
Carvalho S, Cunha MR, Pereira F, Pousão-Ferreira P, Santos M, Gaspar M (2012) The effect of depth and sediment type on the spatial distribution of shallow soft-bottom amphipods along the southern Portuguese coast. Helgoland Marine Research 66:489–501
Cicogna F (2003) Grotte marine: cinquant’anni di ricerca in Italia. Ministero dell’ambiente e della tutela del territorio
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology 18:117
Clarke K, Warwick R (1994) Similarity-based testing for community pattern: the two-way layout with no replication. Marine Biology 118:167–176
Curini-Galletti M, Artois T, Delogu V, De Smet WH, Fontaneto D, Jondelius U, Leasi F, Martínez A, Meyer-Wachsmuth I, Nilsson KS, Tongiorgi P, Worsaae K, Todaro MA (2012) Patterns of diversity in soft-bodied meiofauna: dispersal ability and body size matter. PLoS ONE 7:e33801
D’Addabbo MG, de Zio Grimaldi S, Sandulli R (2001) Heterotardigrada of two submarine Cavesin S. Domino Island (Tremiti Islands) in the Mediterranean Sea with the description of two new species of stygarctidae. Zoologischer Anzeiger-A Journal of Comparative Zoology 240:361–369
Di Domenico M, Martínez A, Lana P, Worsaae K (2014) Molecular and morphological phylogeny of Saccocirridae (Annelida) reveals two cosmopolitan clades with specific habitat preferences. Molecular Phylogenetics and Evolution 75:202–218
Dixon P, Palmer M (2003) VEGAN, a package of R functions for community ecology. Journal of Vegetation Science 14:927–930
Fichez R (1990) Decrease in allochthonous organic inputs in dark submarine caves, connection with lowering in benthic community richness. Hydrobiologia 207:61–69
Fichez R (1991) Suspended particulate organic matter in a Mediterranean submarine cave. Marine Biology 108:167–174
Fosshagen A, Boxshall GA, Iliffe TM (2001) The Epacteriscidae, a cave-living family of calanoid copepods. Sarsia 86:245–318
Gerovasileiou V, Voultsiadou E (2015) Mediterranean marine caves as biodiversity reservoirs: a preliminary overview. Symposium on the conservation of dark habitats. p. 45
Gerovasileiou V, Martínez A, Álvarez F, Boxshall G, Humphreys WF, Jaume D, Becking LE, Muricy G, van Hengstrum PJ, Dekeyzer S, Decock W, Vanhoorne B, Vandepitte L, Bailly N, Iliffe TM (2016) World Register of Marine Cave Species (WoRCS): a new thematic species database for marine cave biodiversity. Research Ideas and Outcomes 2:e10451. doi:10.3897/rio.2.e10451
Giere O (2009) Meiobenthology. The microscopic motile fauna of aquatic sediments. Springer, Berlin
Gili JM, Coma R (1998) Benthic suspension feeders: their paramount role in littoral marine food webs. Trends in Ecology and Evolution 13:316–321
Gili J, Riera T, Zabala M (1986) Physical and biological gradients in a submarine cave on the Western Mediterranean coast (north-east Spain). Marine Biology 90:291–297
Gonzalez B, Petersen H, Martínez A, Worsaae K (2015) Colonization and adaptation of scale worms to interstitial and anchialine habitats (Aphroditiformia, Annelida). Oxford University Press, Cary, p E68
Grimaldi De Zio S, D’Addabbo Gallo M, Morone De Lucia R, Vaccarella R, Grimaldi P (1982) Quattro nuove specie di Halechiniscidae rinvenute in due grotto sottomarine dell’Italia meridionale (Tardigrada: Heterotardigrada) = four new species of marine Tardigrada found in two submarine caves of South Italy (Tardigrada: Heterotardigrada). Cahiers de Biologie Marine
Higgins RP, Thiel H (1988) Introduction to the study of meiofauna. Smithsonian Institution Press, Washington
Hoenemann M, Neiber MT, Humphreys WF, Iliffe TM, Li D, Schram FR, Koenemann S (2013) Phylogenetic aalysis and systematic revision of Remipedia (Nectiopoda) from Bayesia analysis of molecular data. Journal of Crustacean Biology 33:603–619
Hou Z, Sket B, Li S (2014) Phylogenetic analyses of Gammaridae crustacean reveal different diversification patterns among sister lineages in the Tethyan region. Cladistics 30:352–365
Humphreys WF (2000) Relict faunas and their derivation. In: Wilkens H, Culver DC, Humphreys WF (eds) Ecosystems of the world, 30 subterranean ecosystems. Elsevier, Amsterdam, pp 417–432
Iglikowska A, Boxshall GA (2013) Danielopolina revised: phylogenetic relationships of the extant genera of the family Thaumatocyprididae (Ostracoda: Myodocopa). Zoologischer Anzeiger 252:469–485
Iliffe TM (1986) The zonation model for the evolution of aquatic faunas in anchialine caves. Stygologia 2:2–9
Iliffe TM (1992) Anchialine cave biology. In: Camacho A (ed) The natural history of biospeleology. Monografías Museo Natural de Ciencias Naturales, CSIC, Madrid
Iliffe TM, Botosaneanu L (2006) The remarkable diversity of subterranean Cirolanidae (Crustacea, Isopoda) in the peri-Caribbean and Mexican Realm. Bulletin l’Institut Royal des Sciences Naturelles de Belgique 76:5–26
Iliffe TM, Kornicker L (2009) Worldwide diving discoveries of living fossil animals from the depths of anchialine and marine caves. In: Macintyre IG, Rützler K, Lang MA (eds) Proc Biol Soc Wash. Smithsonian Institution Scholary Press, Washington DC, pp 269–280
Iliffe TM, Wilkens H, Parzefall J, Williams D (1984) Marine lava cave fauna: composition, biogeography and origins. Science 225:309–311
Iliffe TM, Parzefall J, Wilkens H (2000) Ecology and species distribution of the Monte Corona lava tunnel on Lanzarote (Canary Islands). In: Wilkens H, Culver DC, Humphreys WF (eds) Subterranean ecosystems ecosystems of the world. Elsevier, Amsterdam
Jørgensen A, Boesgaard TM, Møbjerg N, Kristensen RM (2014) The tardigrade fauna of Australian marine caves: with descriptions of nine new species of Arthrotardigrada. Zootaxa 3802:401–443
Juan C, Guzik MT, Jaume D, Coopers SJB (2010) Evolution in caves: Darwin’s ‘wrecks of ancient life’ in the molecular era. Molecular Ecology 19:3865–3880
Koenemann S, Holsinger JR (1999) Phylogenetic analysis of the Amphipod Family Bogidiellidae S. Lat., and revision of taxa above the species level. Crustacean Research 72:781–816
Koenemann S, Bloechl A, Martínez A, Iliffe TM, Hoenemann M, Oromí P (2009) A new, disjunct species of Speleonectes (Remipedia, Crustacea) from the Canary Islands. Marine Biodiversity 39:215–225
Laumer CE, Bekkouche N, Kerbl A, Goetz F, Neves RC, Sørensen MV, Kristensen RM, Hejnol A, Dunn CW, Giribet G (2015) Spiralian phylogeny informs the evolution of microscopic lineages. Current Biology 25:1–7
Martí R, Uriz MJ, Ballesteros E, Turon X (2004) Benthic assemblages in two Mediterranean caves: species diversity and coverage as a function of abiotic parameters and geographic distance. Journal of Marine Biological Association of United of Kingdom 84:557–572
Martínez A, Núñez L, Monterroso Ó, Núñez J (2004) Tanatocenosis de moluscos gasterópodos en sedimentos de una cueva submarina de la costa oeste de Tenerife (Islas Canarias). Revista de la Academia Canaria de Ciencias 16:161–177
Martínez A, Palmero AM, Brito MC, Núñez J, Worsaae K (2009) Anchialine fauna of the Corona lava tube (Lanzarote, Canary Islands): diversity, endemism and distribution. Marine Biodiversity 39:169–187
Martínez A, Di Domenico M, Worsaae K (2013) Evolution of cave Axiokebuita and Speleobregma (Scalibregmatidae, Annelida). Zoologica Scripta 51:623–636
Martínez A, Di Domenico M, Worsaae K (2014) Gain of palps within a lineage of ancestrally burrowing annelids (Scalibregmatidae). Acta Zoologica 95:421–429
Martínez A, Di Domenico M, Rouse G, Worsaae K (2015) Phylogeny of Protodrilidae (Annelida) inferred by total evidence analyses. Cladistics 31:250–276
Martínez A, Kvindebjerg K, Iliffe TM, Worsaae K (2016) Evolution of cave suspension feeding in Protodrilidae (Annelida). Zoologica Scripta. doi:10.1111/zsc.12198
Martínez A, Gonzalez BC, Worsaae K, Wilkens H, Núñez J, Oromí P, Iliffe TM (in press) Guide to the anchialine ecosystems of Jameos del Agua and Túnel de la Atlántida. Medio Ambiente, Cabildo de Lanzarote, Arrecife, Lanzarote
Meland K, Willassen E (2007) The disunity of “Mysidacea”(Crustacea). Molecular Phylogenetics and Evolution 44:1083–1104
Murrell P (2005) R graphics, CRC computer science & data analysis. Chapman & Hall, Boca Raton
Mwinyi A, Bailly X, Bourlat SJ, Jondelius U, Littlewood DTJ, Podsiadlowski L (2010) The phylogenetic position of Acoela as revealed by the complete mitochondrial genome of Symsagittifera roscoffensis. BMC Evolutionary Biology 10:309–312
Navarro-Barranco C, Guerra-García J, Sánchez-Tocino L, García-Gómez J (2012) Soft-bottom crustacean assemblages in Mediterranean marine caves: the cave of Cerro Gordo (Granada, Spain) as case study. Helgoland Marine Research 66:567–576
Navarro-Barranco C, Guerra-García JM, Sánchez-Tocino L, Jiménez-Prada P, Cea S, García-Gómez JC (2013) Soft-bottom diversity patterns in marine caves; lessons from crustacean community. Journal of Experimental Marine Biology and Ecology 446:22–28
Navarro-Barranco C, Guerra-García JM, Sánchez-Tocino L, García-Gómez JC (2014) Amphipods from marine cave sediments of the southern Iberian Peninsula: diversity and ecological distribution. Scientia Marina 78:415–424
Neiber MT, Hartke TR, Stemme T, Bergmann A, Rust J, Iliffe TM, Koenemann S (2011) Global biodiversity and phylogenetic evaluation of Remipedia (Crustacea). PLoS ONE 6:e19627
Núñez J, Ocaña O, Brito MC (1997) Two new species (Polychaeta: Fauveliopsidae and Nerillidae) and other polychaetes from the marine lagoon cave of Jameos del Agua, Lanzarote (Canary Islands). Bulletin of Marine Science 60:252–260
Núñez J, Brito MC, Docoito JR (2005) Anélidos poliquetos de Canarias: Catálogo de especies, distribución y hábitats. Vieraea 33:297–321
Núñez J, Martínez A, Brito MC (2009) A new species of Sphaerosyllis Claparède, 1863 (Polychaeta: Syllidae: Exogoninae) from the Atlantida Tunnel, Lanzarote, Canary Islands. Marine Biodiversity 39:209–214
Oertel A, Patzner RA (2007) The biology and ecology of a submarine cave: the Grotta del Bel Torrente (Central‐East Sardegna, Italy). Marine Ecology 28:60–65
Ortea J (1995) Estudio de las especies atlánticas de Paradoris Bergh, 1884 (Mollusca: Nudibranchia: Discodorididae) recolectadas en las Islas Canarias. Avicennia 3:5–27
Palacin C, Masalles D (1986) Some data on the meiofauna of an underwater cave of the island of Majorca, Spain. Publ Dep Zool, Barcelona 12:15–26
Palacín C, Gili JM, Martin D (1992) Evidence for coincidence of meiofauna spatial heterogeneity with eutrophication processes in a shallow-water Mediterranean bay. Estuarine Coastal and Shelf Science 35:1–16
Pérez Dionis G, Espinosa Sáez J, Ortea Rato JA (2011) Una nueva especie del género Neritilia Martens, 1879 (Mollusca: Gastropoda: Neritiliidae) de las islas Canarias. Vieraea 38:117–121
Pettibone MH (1985) Polychaete worms from a cave in the Bahamas and from experimental wood panels in deep water of the North Atlantic (Polynoidae, Macellicephalinae, Harmothoinae). Proceedings of the Biological Society of Washington 98:127–149
Riedl R, Ozretić B (1969) Hydrobiology of marginal caves. Part I. General problems and introduction. International Review of Hydrobiology 54:661–683
Rieger RM (1980) A new group of interstitial worms, Lobatocerebridae nov. fam. (Annelida) and its significance for metazoan phylogeny. Zoomorphologie 95:41–84
Riera R, Núñez J, Brito MC (2006) Two new species of Comesomatidae Filipjev, 1922 (Nematoda: Chromadorida) from sandy bottoms of Tenerife, Canary Islands. Zootaxa 1126:53–61
Riera R, Núñez J, Brito MC (2007) A new species of the interstitial genus Neopetitia (Polychaeta, Syllidae, Eusyllinae) from Tenerife, with modified acicular chaetae in males. Helgoland Marine Research 61:221–223
Riera R, Núñez J, Brito MC (2010) Check-list of interstitial polychaetes from intertidal and shallow subtidal soft bottoms of Tenerife, Canary Islands. Archipelago 27:21–39
Riera R, Núñez J, Brito MC, Tuya F (2011) Temporal variability of a subtropical meiofaunal assemblage: contrasting effects at the species and assemblage-level. Vie et Milieu 61:129–137
Riera R, Núñez J, Brito MC (2012) Influence of a freshwater runoff on temporal variations of an intertidal meiofauna assemblage. Vie et Milieu 62:105–114
Riera R, Núñez J, Brito MC (2013) Temporal dynamics of shallow subtidal meiobenthos from a beach in Tenerife (Canary Islands, northeast Atlantic Ocean). Acta Oceanologica Sinica 32:44–54
Rundell RJ, Leander BS (2010) Masters of miniaturization: convergent evolution among interstitial eukaryotes. Bioessays 32:430–437
Sandulli R, D’addabbo MG, De Lucia MM, D’addabbo R, Pietanza R, de Zio Grimaldi S (1999) Preliminary investigations on meiofauna of two caves in San Domino Island (Tremiti Archipelago, Adriatic Sea). Biologia Marina Mediterranea 6:437–440
Schmidt-Rhaesa A, Rothe BH, Martínez AG (2013) Tubiluchus lemburgi, a new species of meiobenthic Priapulida. Zoologischer Anzeiger 253:158–163
Schratzberger M, Jennings S (2002) Impacts of chronic trawling disturbance on meiofaunal communities. Marine Biology 141:991–1000
Sket B (1996) The ecology of anchihaline caves. Trends in Ecology and Evolution 11:221–225
Stock JH, Iliffe TM, Williams WD (1986) The concept of “anchialine” reconsidered. Stygologia 2:90–92
Team RDC (2008) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Tilzer M (1970) Hydrobiology of marginal caves. Part III. Nerilla marginalis n.sp. (Polychaeta Archiannelida) a recent immigrant into a marginal cave in Istra (Yugoslavia). Internationale Revue der gesammten Hydrobiologie 55:221–226
Todaro MA, Leasi F, Bizzarri N, Tongiorgi P (2006) Meiofauna densities and gastrotrich community composition in a Mediterranean sea cave. Marine Biology 149:1079–1091
Vacelet J (2006) New carnivorous sponges (Porifera, Poecilosclerida) collected from manned submersibles in the deep Pacific. Zoological Journal of the Linnean Society 148:553–584
Vacelet J, Boury-Esnault N (1995) Carnivorous sponges. Nature 373:26–28
Vacelet J, Boury-Esnault N, Harmelin J-G (1994) Hexactinellid cave, a unique deep-sea habitat in the scuba zone. Deep Sea Research I 41:965–973
van Hengstum PJ, Scott DB (2011) Ecology of foraminifera and habitat variability in an underwater cave: distinguishing anchialine versus submarine cave environments. Journal of Foraminiferan Research 41:201–229
Wägele J-W (1985) On the Tethyan origin of the stygobiont Anthuridea Curassanthura and Cyathura (Stygocyathura), with description of Curassanthura canariensis n. sp. from Lanzarote (Crustacea, Isopoda). Stygologia 1:258–269
Wagner HP (1994) A monographic review of the Thermosbaenacea (Crustacea: Peracarida). A study on their morphology, taxonomy, phylogeny and biogeography. Zoologische Verhandelingen Leiden 291:1–338
Wilkens H, Parzefall J, Iliffe TM (1986) Origin and age of the marine stygofauna of Lanzarote, Canary Islands. Mitteilungen aus den Hamburgischen Zoologische Museum und Institut 83:223–230
Wilkens H, Parzefall J, Ribowski A (1990) Population biology and larvae of the Anchialine Crab Munidopsis polymorpha (Galatheidae) from Lanzarote (Canary Islands). Journal of Crustacean Biology 10:667–675
Wilkens H, Iliffe TM, Oromí P, Martínez A, Tysall TN, Koenemann S (2009) The Corona lava tube, Lanzarote: geology, habitat diversity and biogeography. Marine Biodiversity 39:155–167
Worsaae K, Rouse GW (2009) Is Diurodrilus an annelid? Journal of Morphology 269:1426–1455
Worsaae K, Martínez A, Núñez J (2009) Nerillidae (Annelida) from the Corona lava tube, Lanzarote with description of Meganerilla cesari n. sp. Marine Biodiversity 39:195–207
Yager J (2013) Speleonectes cokei, new species of Remipedia (Crustacea: Speleonectidae) from a submerged ocean cave near Caye Chapel, Belize. Zootaxa 3710:354–362
Zabala M, Riera T, Gili JM, Barange M, Lobo A, Peñuelas J (1989) Water flow, trophic depletion, and benthic macrofauna impoverishment in a submarine cave from the Western Mediterranean. Marine Ecology 10:271–287
Zuur A, Ieno EN, Smith GM (2007) Analysing ecological data. Springer Science & Business Media, New York
Acknowledgments
The staff of the Benthos Lab (University of La Laguna) are acknowledged for their help sorting samples and interchange of ideas throughout the study. We are grateful to Diego Fontaneto (National Research Council, Rome) for constructive comments on an earlier draft of this manuscript, and to Aguirre Servicios Topográficos SLL for financial support and logistic help during the field surveys.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Schratzberger
Rights and permissions
About this article
Cite this article
Riera, R., Monterroso, Ó., Núñez, J. et al. Distribution of meiofaunal abundances in a marine cave complex with secondary openings and freshwater filtrations. Mar Biodiv 48, 203–215 (2018). https://doi.org/10.1007/s12526-016-0586-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-016-0586-y