Abstract
In contrast to the modern cephalopods, the nautiloids use primarily the chemosensory sense to explore their environment. So far there have been few studies on sexual-selection processes in solitary-living nautiloid cephalopods, but it can be posited that conspecifics are also discovered by odour. In order to determine whether a special area of the rectum, the rectal gland, plays a role in the intraspecific communication processes of Nautilus pompilius, Y-maze experiments were performed. We tested the reaction of juvenile, early-adolescent male and female, and adult male N. pompilius to homogenates of the rectum of male and female conspecifics. As negative controls, homogenates of gills or mantle, or seawater were used. To check the set-up of the experiment, carrion was presented as a positive attractant. We demonstrated that the adult (mature) males significantly preferred the rectum homogenate containing the stimulus of females, whereas the homogenates from males and/or females had no influence on the behaviour of immature animals of both sexes. Our behavioural studies provide evidence that sexually mature male N. pompilius are attracted to females by excretions of the rectum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The nautiloid cephalopods, only represented by two genera with six extant species, live outside the coral reefs in the Pacific Ocean (Saunders 1981; Ward and Saunders 1997). These animals are primarily solitary, nocturnal organisms that remain at depths of up to 300 m during the day and migrate vertically up tropical reef slopes at night (Carlson et al. 1984; Ward et al. 1984; Zann 1984; O’Dor et al. 1993). In contrast to modern cephalopods that have a predatory way of life, the nautiloid cephalopods are primarily scavengers (Hamada et al. 1980; Ward and Wicksten 1980). Modern cephalopods show a greatly modified brain development and locomotory system, as well as a highly developed visual sense that enables a fast lifestyle. The nautiloids possess a primitive pinhole eye that lacks a lens, suggesting that vision is not the most essential sensory system for foraging, in contrast to most modern cephalopods (Muntz 1986, 1991; Barber 1987). Nautiloids locate food through a combination of smell and touch, following the stimulus produced by a distant odour source until it touches the item with its tentacles (Bidder 1962; Basil et al. 2000; Ruth et al. 2002). During this process they extend all the digital tentacles, as well as the ocular tentacles, and swim forward to the odour source. In searching, four pairs of digital tentacles, the lateral digital tentacles, are spread out or hang below towards the bottom, respectively (Mikami et al. 1980; Ruth et al. 2002).
Nautiloids are also equipped with a pair of rhinophores, each one a fleshy papilla of 4 mm with a sensory pit, located below the eyes (Barber and Wright 1969; Barber 1987). Previous histological and scanning electron microscopic investigations show characteristic ciliary processes within the epithelium of the ocular tentacles, in the lamellae of four pairs of lateral digital tentacles, and in the olfactory pits of the rhinophores, similar to the ciliary structures found in the chemoperceptive cells of coleoid cephalopods. These data, together with observations on feeding habits, suggest that these cells may serve in a long-distance chemosensory mechanism. Different types of ciliary processes within the epithelium of the medial digital tentacles are considered as mechanosensory structures (Ruth et al. 2002).
Behavioural observations reveal that N. pompilius can detect and follow turbulent odour plumes to the source over distances of up to 10 m. It seems that the paired rhinophores are necessary for orientation. When these organs were blocked, the animals could not track the plume or locate the source (Basil et al. 2000). Further experiments suggest that female N. pompilius are attracted to the odour of males (Basil et al. 2002).
In previous cytological investigations of the digestive tract of N. pompilius we demonstrated a kind of rectal gland within the epithelium of the rectal loop (Fig. 1). Because the secretion of digestive enzymes in this part of the digestive tract is not very likely, it can be supposed that the secreted substances of these cells serve to mark the faeces (Westermann and Schipp 1998b). This marking could probably have some biological significance for intraspecific chemotactic communication on the basis of the biotope and the poorly developed optical apparatus of these animals. Thus, we set out to determine whether N. pompilius is able to detect conspecifics using excretions from the rectum. In Y-maze experiments, we tested the reactions of male and female juvenile, early-adolescent and adult N. pompilius to homogenates of the rectum of both sexes. As a positive control we used carrion and as negative controls we tested homogenates of mantle and gill tissues, as well as seawater.
Nautilus pompilius. The digestive tract of nautiloids (Westermann and Schipp 1998a), with a schematic diagram of the mucosal epithelium of the rectum showing the characteristic rectal gland cells containing numerous (sv) secretory vesicles. (bl basal lamina; c crop; ca caecum; cf collagen fibres; de desmosome; dh ductus hepatopancreas; er endoplasmic reticulum; g golgi apparatus; j jaw; ly lysosome; m midgut; me mesenterium; mi mitochondrion; mv microvilli; n nucleus; nu nucleolus; o oesophagus; r rectum; s stomach; v vestibulum)
Materials and methods
Animals
Animals were captured at a depth of about 200 m in the coastal waters of the South China Sea, northern Philippines, and air shipped to the Institute for Zoology, Germany.
For the behavioural studies, Nautilus pompilius L. of both sexes in three different developmental stages were used. Based on observations of Collins and Ward (1987), we used late juveniles, with a moderate shell length of 10.5 cm; animals in the early-adolescent phase, characterised by a shell length of 13.0 cm and a white venter; and adult male N. pompilius, with a shell length of 15.5 cm. The adult animals had formed the last septum and were sexually mature. Unfortunately, adult female N. pompilius were not available. The sex of the early-adolescent and adult animals was determined by the presence or absence of the spadix, the male secondary organ that is usually located to the right of the mouth (Haven 1977; Saunders and Spinosa 1978). The sex of the juveniles was analysed by histological investigations of the gonads, after the behavioural studies. For the behavioural experiments, 14 N. pompilius served as recipients and 13 as donors (Table 1).
Maintenance
All the animals were kept at a constant water temperature of 18.1±0.4°C in a closed 250-l aquarium (size: 65×60×65 cm) and in a 200-l aquarium (size: 100×40×50 cm) with alternating 12-h periods of faint light and complete darkness. The water was filtered through an exterior filter containing shell and wadding, and ammonia and nitrite were removed with an exterior bacterial gravel filter. The animals were fed with frozen shrimp (Crangon sp.) every second day.
Y-maze experiments
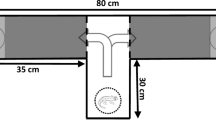
For the behavioural studies, a special aquarium (length 160 cm, width 100 cm, height 35 cm) was designed, which was divided into four courses/arms of 100 cm. The animal could move freely within the area without the divisions (60 cm). At a distance of 20 cm on the opposite side of the courses, a grating was inserted to prevent the animals from moving into the front area (Fig. 2). The artificial seawater used was cleaned by directing its flow over a living sand filter, as well as over an activated-carbon filter. The temperature in this aquarium was adjusted to 17.2±0.5°C. With the help of a pump, the water streamed in via an inlet in the front of each course so that a weak flow existed in the direction of the outlet. Experiments with dyes demonstrated that it took approximately 5–6 min for the water flow to reach the animal. The dye was flushed out after about 30 min. Lighting was placed above the Y-maze, parallel to the longitudinal axis. This arrangement provided a light level of about 150 lux at the water surface. A video camera was mounted at the front of the Y-maze to record the nautiloids’ movements. The Y-maze was covered with non-transparent sheets to minimise evaporation.
Procedure
Each animal was transferred from the holding aquarium to the Y-maze and acclimated for 3 days before the trials started. During the period of the trials, the experimental animal was maintained in the Y-maze to prevent stress by moving. Before each trial started, the animal was restricted behind the grating and the covering was removed. After a habituation time of 1 h, the odour source was placed in the area of the inlet of one course. Three different substances were tested: carrion, homogenates and seawater. Initial experiments with carrion (frozen shrimp) were carried out to examine the exact behaviour pattern on a positive olfactory stimulus. After these positive controls, homogenates of the rectum of female and male N. pompilius were used, and the reaction to males and females were tested. Sea water with 10% protease inhibitor cocktail and homogenates of the mantle and gills served as negative controls. The experiments with carrion were carried out with hungry animals, whereas the homogenates were tested on well-fed nautiloids. The shrimp were put directly in a sieve in the inlet, whereas the homogenates were placed on cotton wool within the sieve. The odours were presented at random in each course, and each recipient was tested at random in three to nine trials with each odour. The courses without the odour contained blank seawater. In each trial, the behaviour of the animal was observed for 30 min and the orientation paths were videotaped.
A trial was considered positive if the nautiloid had chosen the course containing the odour as its first choice and if the animal swam directly to the inlet at the end of each course. If the animal showed no reaction or took one of the courses without the odour, the trial was negative. Between each trial a period of 24 h was allowed to elapse to ensure that the odour had been removed by the filter system.
For the statistical evaluation, recipients were divided into groups: immature males and females (juvenile and early-adolescent animals) and adult (mature) males. Differences in the responses of males and females to the various substances were tested by means of generalised linear model analysis (GLZ). Type III error was applied in the GLZ to account for unequal sample sizes between the data groups. Whether a specific animal group recognised a specific substrate was tested by comparing the related success frequency against the random expectation (binomial test). P-values were corrected for repeated testing by means of the truncated product method (Zaykin et al. 2002). All statistical analyses were performed using the STATISTICA package (Statsoft, Tulsa, USA).
Preparation of the homogenates
The animals were anaesthetised in a 2–3% ethanol–seawater solution, depending on their size, and the rectum, gills and mantle were removed. In sacrificing specimens, the principles of laboratory animal care were followed. The organs were homogenised in seawater with the addition of 10% protease inhibitor cocktail (Sigma) on ice. After the cellular components were removed by centrifugation at 10,000 g for 20 min at 4°C, the cell-free suspensions were directly frozen in liquid nitrogen and stored in a freezer at −80°C. The homogenates were applied in a concentration of 2 ppm (2×10−6). For each trial 1 ml of the different homogenates was used.
Results
Behaviour after the application of carrion
After the acclimation time of 1 h, all the Nautilus pompilius tested were in a resting position, in which only the tips of the ocular tentacles were visible. The digital tentacles were retracted into their tentacle sheaths, except for one or two that were used for attaching the animal to the aquarium. Then, 7.0±4.7 min (mean value of all tested animals) after presentation of the carrion in one course, the animal detached itself from the aquarium wall with swaying movements. In this moment the experimenter removed the grating carefully without disturbing the animal. After this reaction, the animal swam first into the area in front of the courses, before it manoeuvred itself into the course containing the food (Fig. 3). After detaching itself from the aquarium wall, the animal stretched the ocular out widely, as well as the digital tentacles for orientation. Two to four pairs of digital tentacles, the so-called lateral digital tentacles (see Ruth et al. 2002), were also extended and maintained constant contact with the bottom. The odour source was reached after 8.5±3.9 min, and the medial digital tentacles were used to grab the carrion. The experimental assembly described above was carried out in 69 trials with different animals (number of trials for each group: juvenile females=11, juvenile males=20, early-adolescent males=22, early-adolescent females=11, adult males=5). In 61 of the 69 trials the animals chose the course with the carrion, and in eight cases they showed no reaction at all. After the statistical evaluation, the reaction of N. pompilius to this positive olfactory stimulus was significant (P<0.01, Tables 2, 3; Fig. 4).
Nautilus pompilius. Schematic diagram of the behaviour of nautiloids to an attractant stimulus, e.g. carrion, presented in course I. X marks the start position of the animal. After detachment from the outlet, the animal searched within the front area of the aquarium in a backward swimming position and then chose the course with the attractant odour. Within the course N. pompilius changed its swimming position (arrowhead) and moved forward
Nautilus pompilius. Success rate (in %) of the selected substrates: carrion, controls (control homogenates and blank seawater) and rectal homogenates of females and males. All the tested animals chose carrion more frequently (85–100%) than controls (about 25%, random event). A significant relationship was revealed for adult males and the rectal homogenates from females (91%). The rectal homogenates from females and males showed no significant effect on immature animals
Behaviour after application of gill or mantle homogenates or seawater
In 21 trials we tested the reaction of N. pompilius to gill or mantle homogenates or to seawater with 10% protease inhibitor cocktail as negative controls (number of trials for each group: juvenile and early-adolescent females=4, juvenile and early-adolescent males=9, adult males=8). In 19 trials the animals showed a negative reaction (10 trials no reaction at all, 9 trials blank course). In only 2 trials did they choose the course containing the control medium (Tables 2, 3; Fig. 4).
Behaviour after application of rectal homogenates
In 114 trials we tested the reaction of juveniles and early-adolescent males (number of trials: 68) and females (number of trials: 30) and adult males (number of trials: 16) to the rectal homogenates of male and female N. pompilius. No significant relationship was revealed between the immature conspecifics. In contrast, the fully-grown adult male N. pompilius clearly responded positively to the rectal homogenates in 16 trials. We further demonstrated that only the rectal homogenate of females provoked a significant positive reaction, whereas the homogenates of male N. pompilius had no influence on the behaviour of males (Tables 2, 3; Fig. 4). After the presentation of homogenates from male donors, the male recipients did not detach themselves from the aquarium wall or they took the course without the odour. However, 14.6±5 min after the presentation of female rectal homogenates, adult males detached themselves from the aquarium wall with the ocular and digital tentacles, as well as the lateral digital tentacles exposed for orientation. First, the animal moved into the area without partitions before swimming into the course with the homogenate. Animals often changed their swimming position from backward to forward just before they reached the attractant odour (Fig. 3). After detachment, it took 4.3±1.5 min until the animals reached the inlet with the odour.
Discussion
The statistical evaluation of the experiments demonstrates that all the investigated animals significantly prefer the course containing the stimulus carrion. Nautilus pompilius showed a clear positive reaction to the presented carrion, confirming previous observations that nautiloids use odour to locate food (Bidder 1962; Basil et al. 2000; Ruth et al. 2002). In the current study, animals were kept in the Y-maze during the trials to get used to the odour of seawater. Our own previous studies on the chemotactic behaviour of N. pompilius in a Y-maze in which the animals had not been habituated to the seawater were inconclusive (Westermann, personal observations). The animals were probably too stressed by moving, or they were irritated by the odour of the seawater. Here, the animals chose the course with carrion after an acclimation time of 1–24 h only one of four times, but, after 3 days of acclimation, they reacted positively to the odour carrion (5:1), indicating that the animals need this length of time for acclimation. To ensure that the odour was eliminated by the filter system, the trials were carried out at 24-h intervals.
The present studies on the chemotactic behaviour of N. pompilius indicate that adult males are attracted to the secretions of the rectum of females. In contrast, the males and females that have not reached sexual maturity showed no clearly positive reaction to the homogenates of their conspecifics. These findings provide evidence that the faeces of the rectum contain pheromones, probably from secretions of the rectal gland. The faeces were possibly used as scent marks within the reef to ensure that the solitary-living males and females find each other during the breeding season. Previous investigations showed that the faeces of N. pompilius consist of red-brown threads, with a length of about 2 cm and no solid components. They disintegrate 36 h, at the earliest, after excretion (Westermann et al. 2002).
Furthermore, our present results suggest that the males’ chemosensory sense is used for detection of female conspecifics of N. pompilius. Unfortunately, we were not able to test the reactions of sexually mature females to the rectum homogenates, because it was not possible to obtain such animals. Previous behavioural studies of Basil et al. (2002) on N. pompilius support that female nautiloids were attracted to male conspecific odour. In these experiments, the seawater in which the males spent 4 h was presented in a Y-maze. The females preferred the arm containing the odour of the males. These observations of Basil et al. (2002) also indicate that secretions of the rectum are probably attractive. Unfortunately, they did not mentioned in their study at exactly which developmental stage the animals were and whether they were sexually mature. Further studies with mature females must be performed to investigate whether male or female rectum homogenates influence their behaviour also.
Investigations on the behaviour of Sepia officinalis also suggest the use of chemical cues in sexual-selection processes. Boal and Marsh (1998) found that male cuttlefish could not recognise their own mated female from other mated females, but that they could discriminate between mated and unmated females in general. The authors also determined that sexually receptive females preferred the more recently mated of two males in the absence of visual information, suggesting strongly that chemical cues were being used for mate choice (Boal 1997).
For sepioids and octopods, it has also been demonstrated that they use chemical cues for the detection of their environment in addition to their visual sense. Experiments on S. officinalis provide strong evidence that cuttlefish detect ecologically salient chemical cues, like predators and prey, in general water chemistry (Boal and Golden 1999). In Octopus maya, amino acids, nucleotides and crab extracts stimulate chemotaxis (Lee 1992). Furthermore, the breeding behaviour of cephalopods is also influenced by pheromones. Contact with squid egg capsules increases aggressive behaviour in male Loligo peali. This behaviour is elicited by a heat-labile factor embedded within the egg capsules that appears to be a contact pheromone (King et al. 1999, Buresch et al. 2003). Similarly, several peptides with pheromonal characteristics were isolated from the egg masses of S. officinalis; these may be regulating peptides in oocyte transport and in the successive steps of egg laying (Zatylny et al. 2000a, 2000b; Bernay et al. 2004; Susswein and Nagle 2004). Among these peptides is a hexapeptide with sperm-attracting activity (Zatylny et al. 2002).
Also, in the marine mollusc Aplysia, pheromones are involved in coordinating breeding behaviour. Egg cordons contain pheromones that establish and maintain breeding aggregations (Painter et al. 1991; Painter 1992; Susswein and Nagle 2004). The peptide “Aplysia attractin” was isolated from the albumen gland, a large exocrine organ that packages the eggs into the cordons and stimulates mature animals to approach egg cordons (Fan et al. 1997; Painter et al. 1998). Attractin sequences from five different Aplysia species are about 40% identical (Schein et al. 2001). Our own previous investigations on the ultrastructure of the rectal gland cells and secreted substances (Westermann and Schipp 1998b) suggest that the content of the gland cells probably consists of peptide-like compounds. Future investigations will target isolation of the content of rectal gland cells and characterisation of the secreted substances.
The results of our behavioural studies indicate that in N. pompilius excretions of the rectum are probably involved in intraspecific communication processes. In view of the fact that nautiloid cephalopods have a low population density (Saunders and Ward 1987) and a low reproductive rate, as well as a long egg development times of up to 400 days (Carlson 1991; Uchiyama and Tanabe 1999), it is unlikely the animals find each other by pure coincidence.
References
Barber VC (1987) The sense organs of Nautilus. In: Saunders WB, Landman NW (eds) Nautilus. The biology and paleobiology of a living fossil. Plenum, New York, pp 223–230
Barber VC, Wright DE (1969) The fine structure of the sense organs of the cephalopod mollusc Nautilus. Z Zellforsch Mikrosk Anat 77:147–174
Basil JA, Hanlon RT, Sheikh SI, Atema J (2000) Three-dimensional odor tracking by Nautilus pompilius. J Exp Biol 203:1409–1414
Basil JA, Lazenby GB, Nakanuku L, Hanlon RT (2002) Female Nautilus are attracted to male conspecific odor. Bull Mar Sci 701:217–225
Bernay B, Gagnon J, Henry J (2004) Egg capsule secretion in invertebrates: a new ovarian regulatory peptide identified by mass spectrometry comparative screening in Sepia officinalis. Biochem Biophys Res Commun 3141:215–222
Bidder AM (1962) Use of the tentacles, swimming and buoyancy control in the pearly Nautilus. Nature 4853:451–454
Boal JG (1997) Female choice of males in cuttlefish (Mollusca: Cephalopoda). Behaviour 134:975–988
Boal JG, Golden DK (1999) Distance chemoreception in the common cuttlefish, Sepia officinalis (Mollusca, Cephalopoda). J Exp Mar Biol Ecol 235:307–317
Boal JG, Marsh SE (1998) Social recognition using chemical cues in cuttlefish (Sepia officinalis Linnaeus, 1758). J Exp Mar Biol Ecol 230:183–192
Buresch KC, Boal, JG, Knowles J, Debrose J, Nichols A, Erwin A, Painter SD, Nagle GT, Hanlon RT (2003) Contact chemosensory cues in egg bundles elicit male–male agonistic conflicts in the squid Loligo pealeii. J Chem Ecol 293:547–560
Carlson BA (1991) Nautilus hatches at the Waikiki aquarium. Chambered Nautilus Newsl 63:2–3
Carlson BA, McKibben JN, de Gruy MV (1984) Telemetric investigation of vertical migration of Nautilus belauensis in Palau. Pac Sci 383:183–188
Collins D, Ward PD (1987) Adolescent growth and maturity in Nautilus. In: Saunders BW, Landman N (eds) Nautilus. The biology and paleobiology of a living fossil. Plenum, New York, pp 421–432
Fan X, Wu B, Nagle GT, Painter SG (1997) Molecular cloning of a c-DNA encoding a potential water-borne pheromonal attractant released during Aplysia egg laying. Mol Brain Res 48:167–170
Hamada T, Obata I, Okutani T (1980) Behavior in captivity. In: Nautilus macromphalus in captivity. Tokai University Press, Tokyo, pp 11–20
Haven N (1977) The reproductive behaviour of Nautilus pompilius in the Philippines. Mar Biol 42:177–184
King AJ, Adamo SA, Hanlon RT (1999) Contact with squid egg capsules increases agonistic behaviour in male squid (Loligo pealei). Biol Bull (Woods Hole) 197:256
Lee GP (1992) Chemotaxis by Octopus maya Voss et Solis in a Y-maze. J Exp Mar Biol Ecol 153:53–67
Mikami S, Okutani, T, Hirano, H, Kanie Y, Hamada T (1980) Behaviour in captivity. In: Nautilus macromphalus in captivity. Tokai University Press, Tokyo, pp 11–22
Muntz WRA (1986) The spectral sensivity of Nautilus pompilius. J Exp Biol 126:513–517
Muntz WAR (1991) Anatomical and behavioural studies on vision in Nautilus and Octopus. Am Malacol Bull 91:69–74
O’Dor RK, Forsythe J, Webber DM, Wells J, Wells MJ (1993) Activity levels of Nautilus in the wild. Nature 362:626–628
Painter SD (1992) Coordination of reproductive activity in Aplysia: peptides neurohormones, neurotransmitters, and pheromones encoded by the egg-laying hormone family of genes. Biol Bull (Woods Hole) 183:165–172
Painter SD, Chong MG, Wong MA, Gray A, Cormier JG (1991) Relative contribution of the egg layer and egg cordon to pheromonal attraction and the induction of mating and egg-laying behaviour in Aplysia. Biol Bull (Woods Hole) 181:81–94
Painter SD, Clough B, Garden RW, Sweedler JV, Nagle GT (1998) Characterization of Aplysia attractin, the first water-borne peptide pheromone in invertebrates. Biol Bull (Woods Hole) 194:120–131
Ruth P, Schmidtberg H, Westermann B, Schipp R (2002) The sensory epithelium of the tentacles and the rhinophore of Nautilus pompilius L. (Cephalopoda, Nautiloidea). J Morphol 251:239–255
Saunders WB (1981) The species of Nautilus and their distribution. Veliger 24:8–17
Saunders WB, Spinosa C (1978) Sexual dimorphism in Nautilus from Palau. Paleabiology 4:349–358
Saunders WB, Ward PD (1987) Ecology, distribution and population characteristics of Nautilus. In: Saunders WB, Landman NH (eds) Nautilus. The biology and paleobiology of a living fossil. Plenum, New York, pp 137–162
Schein CH, Nagle GT, Page JS, Sweedler JV, Xu Y (2001) Aplysia attractin: biophysical characterization and modeling of a water-borne pheromone. Biophys J 81:463–472
Susswein AJ, Nagle GT (2004) Peptide and protein pheromones in molluscs. Peptides (Tarryt) 25:1523–1530
Uchiyama K, Tanabe K (1999) Hatching of Nautilus macromphalus in the Toba aquarium, Japan. In: Olóriz, Rodriques-Tovar (eds) Advancing research on living and fossil cephalopods. Kluwer, New York, pp 13–16
Ward PD, Saunders WB (1997) Allonautilus: a new genus of nautiloid cephalopod and its bearing on phylogeny of the nautiloida. J Paleont 716:1054–1064
Ward PD, Wicksten MK (1980) Food sources and feeding behavior of Nautilus macromphalus. Veliger 232:119–124
Ward PD, Carlson B, Weekly M, Brumbaugh B (1984) Remote telemetry of daily vertical and horizontal movement of Nautilus in Palau. Nature 309:248–250
Westermann B, Schipp R (1998a) Morphology and histology of the digestive tract of Nautilus pompilius and Nautilus macromphalus (Cephalopoda, Tetrabranchiata). Zoomorphology 117:237–245
Westermann B, Schipp R (1998b) Cytological and enzyme-histochemical investigations on the digestive organs of Nautilus pompilius (Cephalopoda, Tetrabranchiata). Cell Tissue Res 293:327–336
Westermann B, Ruth P, Litzlbauer HD, Beck I, Beuerlein K, Schmidtberg H, Kaleta EF, Schipp R (2002) The digestive tract of Nautilus pompilius (Cephalopoda, Tetrabranchiata): an X-ray analytical and computational study on the living animal. J Exp Biol 205:1617–1624
Zann LP (1984) The rhythmic activity of Nautilus pompilius, with notes on its ecology and behavior in Fiji. Veliger 271:19–28
Zatylny C, Gagnon J, Boucaud-Camou E, Henry J (2000a) ILME: a waterborne pheromonal peptide released by the eggs of Sepia officinalis. Biochem Biophys Res Commun 275:217–222
Zatylny C, Gagnon J, Boucaud-Camou E, Henry J (2000b) The SepOvotropin: a new ovarian peptide regulating oocyte transport in Sepia officinalis. Biochem Biophys Res Commun 276:1013–1018
Zatylny C, Marvin L, Gagnon J, Henry J (2002) Fertilization in Sepia officinalis: the first mollusk sperm-attracting peptide. Biochem Biophys Res Commun 2965:1186–1193
Zaykin DV, Zhivotovsky LA, Westfall PH, Weir BS (2002) Truncated product method for combining p-values. Gent Epidemiol 22:170–185
Acknowledgements
The authors would like to thank Dr. K. Eckschmitt for statistical evaluation, Dr. H. Schmidtberg for critical discussion of the manuscript and H. Schmidt for her valuable technical assistance. The investigations were supported by grants from the German Science Foundation (WE 2358) and “Land Hessen” (HWP).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Kinne, Oldendorf/Luhe
Rights and permissions
About this article
Cite this article
Westermann, B., Beuerlein, K. Y-maze experiments on the chemotactic behaviour of the tetrabranchiate cephalopod Nautilus pompilius (Mollusca). Marine Biology 147, 145–151 (2005). https://doi.org/10.1007/s00227-005-1555-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-1555-3