Abstract
Olfaction is an important mechanism of orientation in amphibians toward the breeding site. It is known that anurans can memorize the odor of the native pond during larval development and prefer this odor prior to the beginning of dispersion. However, such a mechanism in urodeles has not been studied yet. We conducted experiments on recognition of the odor of a native water body in juveniles of the smooth newt Lissotriton vulgaris. One group of larvae were reared in pure water (control), the other group in water with morpholine (10–7 mol/L). A few days after metamorphosis, the newts were tested under paired-choice conditions in a T-maze. A total of 73 newts from the experimental group and 47 newts from the control group were tested. The results of the experiment show that the newts in the experimental group preferred the morpholine solution, whereas the individuals of the control group made the choice randomly. We conclude that newts can learn the odor of the environment in which they developed and use this memory for orientation in later stages of life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animals need to recognize and find a suitable environment to meet species´ basic needs, and they develop various orientation and navigation mechanisms to fulfill this need (Davis and Stamps 2004). Among vertebrates, amphibians are of particular interest because many of them have a complex biphasic life cycle with a change of habitat during metamorphosis and seasonal migrations. Since breeding of most species is associated with a suitable breeding pond, such amphibians need reliable mechanisms to memorize it and find a path to it later. In addition, many species consistently demonstrate philopatry, i.e., the use of the same water bodies for breeding (Wells 2007).
Olfaction is the sensory base of one such orientation mechanism that has been confirmed by numerous studies (Sinsch 2006). For navigation, amphibians can potentially utilize a variety of odor sources. For example, a study on metamorphs of the great crested newt Triturus cristatus showed that in an olfactometer, these animals preferred the odor of their own previously used substrates over the substrates used by other metamorphs. The metamorphs also showed preference for substrates previously used by an adult over the clean substrates. Authors of the study suggest that juvenile newts favored familiar stimuli and used them during the dispersal (Hayward et al. 2000). However, this ability to recognize substrate odor is not universal: in a similar study, adult northern spectacled salamanders Salamandrina perspicillata could not recognize the odor of soil from the vicinity of their breeding pond in a Y-maze; nevertheless, the salamanders still showed a preference for their own smell (Vignoli et al. 2012). One of the most important odor sources is a breeding pond. For example, in the field experiments where alpine newts Triturus alpestris were released into circular arenas at a distance of 10 m from their breeding pond, the newts oriented toward the pond. After the pond surface was covered, thus preventing the odor spread, the newts changed direction and moved toward another nearby body of water (Joly and Miaud 1993). In another study, anosmic red-spotted newts Notophthalmus viridescens exhibited random orientation in a circular arena at a distance of 20 m from the breeding pond, whereas the sham-operated control group oriented toward the pond (Hershey and Forester 1980). In similar experiments with toads Bufo japonicus and Epidalea calamita, individuals with impaired sense of smell were unable to orient themselves toward the breeding pond (Sinsch 1992; Ishii et al. 1995).
The ability to distinguish the odor of water from the breeding pond from any other water body has also been shown in laboratory experiments, when tests in a T-maze used as an olfactometer were performed on a number of species of anurans (Grubb 1973a, b). In Urodela, similar results were obtained on Ambystoma maculatum, which preferred the odor of its breeding pond to that of other water bodies in substrate preference boxes (Mcgregor and Teska 1989).
Odor learning can occur either during the breeding or during larval development. The latter is probably providing an evolutionary benefit, since if an individual developed in this specific body of water, it may also be suitable for the survival of its offspring. Odor learning during larval development has been demonstrated in experiments with Anura species. Larvae of several species were reared in a chemically marked environment in laboratory conditions, pool frog Pelophylax lessonae (Ogurtsov and Bastakov 2001; Ogurtsov 2005), common frog Rana temporaria (Ogurtsov 2007), green toad Bufotes viridis (Shakhparonov and Ogurtsov 2005; Ogurtsov 2007), and common toad Bufo bufo (Bastakov 1992). After metamorphosis, the juveniles of this species showed a preference for familiar odors in the test chambers. Anuran amphibians presumably use olfaction to orient themselves to their native pond to successfully complete development, to move away from the pond during dispersal (during this period, they reject the native pond odor), and to return to their native pond as adult individuals for breeding (Ogurtsov 2004; Shakhparonov and Ogurtsov 2005). The kin recognition in young amphibians, which has been demonstrated in a number of studies (Blaustein and Waldman 1992; Hepper and Waldman 1992), can be another case of odor learning during larval development since, under laboratory conditions, the kin odor would be the main environmental odor available (Pfennig 1990; Orgutsov 2004). Importantly, the olfactory organs undergo changes during the metamorphosis, since the mechanisms of odor detection in the water and in the air are different (Arzt et al. 1986; Różański and Żuwała 2019). The Bowman’s and vomeronasal glands are formed to lubricate the nasal and vomeronasal cavities (Getchell and Getchell 1992; Kovtun and Stepanyuk 2015; Weiss et al. 2021). In the olfactory epithelium, microvillous receptor neurons, which are used for the odor perception in the water, disappear or degrade (Freitag et al. 1995; Hansen et al. 1998; Weiss et al. 2021). Additionally, odorant-binding proteins begin to form in the mucus of olfactory epithelium for the purpose of capturing volatile compounds (Millery et al. 2005). For the aforementioned reasons, compounds recognizable in either the air or water can be used for orientation.
However, to date, there have been no studies devoted to the memorization of a chemical stimulus in urodeles and it is unclear whether metamorphosed individuals can use the odor of such stimulus as an orientation cue. Therefore, the aim of our work was to study the memorization and subsequent recognition of the native pond odor by Urodela. We chose the smooth newt Lissotriton vulgaris (Linnaeus, 1758) as a model species. Its biology is well studied, and the species itself is abundant enough to allow long-term experiments to be conducted.
Materials and methods
Embryos and larvae of the smooth newt, obtained from eggs in the laboratory, were used in this study. Adults (10 males and 20 females) were collected during the breeding season on April 27, 2022 in a natural pond on the territory of the Biosphere nursery (town of Bronnitsy, Moscow Oblast). The newts were kept in aquariums in groups of ten, following the method developed for a closely related species, Lissotriton lantzi (Kidov 2018). Spawning occurred without hormonal stimulation. The freshly laid eggs were transferred to 28 × 19 × 14 cm plastic containers (with a volume of water of 3 L). Subsequently, the density of four larvae per liter of water was maintained during rearing.
The eggs obtained from adults were divided into 2 experimental groups.
Group 1: “marker”, embryos and larvae developed in chemically marked dechlorinated tap water.
Group 2: “control”, embryos and larvae developed in pure dechlorinated tap water.
Morpholine (C4H9NO) at a concentration of 10–7 mol/L was used as a marker (artificial source of odor of the native water body). This chemical was chosen because it is well soluble in water, chemically stable, is not present in the pure form in the natural environment, and has been successfully tested on anurans (Ogurtsov 2005).
The water in the containers was completely replaced three times a week with fresh water of the same temperature and chemical marker concentration. From the beginning of feeding, the newt larvae were fed live Artemia salina (Linnaeus, 1758) nauplii, and as the newts grew, they were gradually transferred to a diet of Chironomidae larvae.
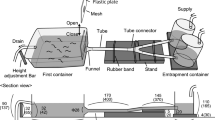
The experiments were conducted in T-mazes designed to test the odor preference of the chemical marker 0–2 days after metamorphosis (Fig. 1). Petri dishes with dechlorinated tap water or morpholine solution (10–7 mol/L) were placed at the ends of the T-maze arms. Each newt was tested individually and only once. Newt was transferred from the terrarium to the maze in an opaque release container, and the container with the animal inside was rotated in different directions before the beginning of the experiment to disrupt the kinesthetic orientation. Then the release container was covered with a starter box (4 cm in diameter, 1 cm high walls) and placed upside down in the starting chamber. The animals were given two minutes to calm down, after which the release container was lifted, and the newts were left in the starter box. The starter box prevented the newts from instantaneous movement, which could be related to the direction of the animal’s head. The testing time was 40 min. The chambers were covered with transparent plastic to avoid any external odors. The testing took place under artificial diffused lamp light (13.45 lx). The mazes were horizontally aligned using a construction level. In the experiments, two mazes were used simultaneously in the positions mirroring each other. The arrangement of the stimuli was crosswise; after each test, the position of the Petri dishes was reversed. Movements of the newts were recorded on a Panasonic CH-V770 video camera for further analysis.
Scheme of the T-maze used for the experiments. The starting zone is indicated in white color; the dark gray indicates the arms of the maze with a chemical stimulus; the light gray indicates the stimulus area; dotted circle indicates the position of a starter box; arrows indicate the movement of newts to either arm of the maze; A and B indicate Petri dishes with chemical stimulus (morpholine solution or water, respectively)
A total of 73 individuals from the “marker” group and 47 individuals from the "control" group were tested.
To analyze the behavior of newts, the maze was divided into the starting zone (from the anterior wall of the maze to the posterior wall) and the right and left arms. Additionally, a chemical stimulus area was distinguished, defined as the space near the petri dish (Fig. 1).
We recorded the following parameters: (1) direction of first choice, i.e., the first exit of a newt from the starting zone into one of the maze arms; (2) number of the newts that reached a stimulus area; (3) total time spent in one of the three sections of the maze.
Statistical analysis of the direction of first choice and the number of individuals that reached a stimulus area was performed using the binomial test. The time spent in the different maze sections was analyzed using the Wilcoxon test (Lehner 1996). The calculations were performed in Statistica 8.0 (Statsoft Inc. 1984–2007).
Results
In our experiments, 9 out of 73 newts in the “marker” group and 6 out of 47 newts of the “control” group did not leave the starting chamber (i.e., these newts did not completely cross the borders of the maze arms) until the end of the experiment.
In the “marker” group, the newts were significantly more likely to make the first choice of direction toward the maze arm containing a Petri dish with morpholine solution (40 vs. 24 individuals, P = 0.03), whereas the result of this choice was random in the “control” group (20 vs. 21 individuals, P = 0.5).
Analysis of the number of newts that reached a stimulus area showed no statistically significant differences in the choice in either the “marker” group (38 vs. 26 individuals, P = 0.12) and the “control” group (23 vs. 18 individuals, P = 0.26).
Analysis of the time that newts spent in each of the maze arms during the entirety of the experiment revealed no significant differences in any of the groups (Fig. 2).
Direction of first choice in the T-maze (a, b) and total time spent in one of three sections of the maze (c, d). a, c—“Marker” group; b, d—“Control” group; Morpholine: maze arm with Petri dish containing morpholine solution (10–7 mol/L); Water: maze arm with Petri dish containing tap water. Unresponsive: individuals that have not left the starting zone (these individuals were not included in the analysis of time spent in the parts of the maze). Significant differences are bolded
To test whether the movement of newts was associated with some other cues (such as the Earth magnetic field or other uncontrolled cues), we analyzed the obtained results on the orientation of the newts factoring in the geographical position of the maze arms (“geographical” bearings). During the study, the arms of the maze were oriented in four directions: geographical Northeast and Southwest, or geographical Northwest and Southeast. We found no statistically significant preferences of a certain compass direction (Table 1). Analysis of the possible preference for turning left or right did not reveal any preferences in either group (Table 1).
Discussion
The results of our experiment showed that the juvenile smooth newts prefer to make the first turn toward the odor that had been present during their larval development, while no such preference was observed in the control group. Thus, newts are able to learn the odor of the environment in which they developed, similarly to juvenile fishes (Cooper and Hasler 1973, 1976) and anurans (Pfennig 1990; Bastakov 1992; Ogurtsov 2005). This may also indicate the possibility of olfactory orientation near the native pond, which has been found in Anura species (Orgutsov 2004; Popescu et al. 2012; Shakhparonov and Ogurtsov 2005).
It is important to understand the cause underlying the preference for the odor of the chemical marker in our experiments. It is known that dispersal from the native pond after the metamorphosis occurs among the juvenile urodeles and anurans (Bell 1977; Kusano and Miyashita 1984; Sinsch 1997; Gamble et al. 2007; Pittman and Semlitsch 2013). There are two variants of this process: (1) immediate dispersal after emerging onto land without a stay of any length in the vicinity of the native pond; in this case, lack of reaction to the chemical stimulus or its avoidance are to be expected, since there is no need in remaining near the native pond; (2) juveniles remain near the native pond for some time after their metamorphosis, and in this case, a reaction of preference toward the odor of the pond can be expected during the post-metamorphic period. This second variant of development is known for several species of anurans (Bastakov 1992; Ogurtsov 2005).
Smooth newts are known to disperse from their native ponds (Bell 1977), but there is no precise data (that we were able to find) describing whether the smooth newt leaves its native pond immediately or after a delay. However, if we consider urodeles in general, the Mitsjama salamander Hynobius nebulosus is known to initially remain near the native pond. Juveniles of this species that recently underwent metamorphosis stayed in the vicinity of their native pond, moving farther away as the salamanders grew in size (Kusano and Miyashita 1984). Another indirect example of delayed dispersal of the juvenile urodeles is the dispersal from the pond of metamorphs of the Eastern newt Notophthalmus viridescens being associated with rains (Healy 1975), i.e., the metamorphs can remain near the native pond until the conditions are suitable for the migration, similarly to anurans (Ogurtsov 2012). Therefore, it is possible that the odor preference we observed in the smooth newt indicates a yet undescribed period in the life cycle, where the newt remains near the native pond until the completion of the metamorphosis, or until the conditions become suitable for the migration.
There is another possible explanation of the preference for the chemical marker, where a specimen in the experiment attempts to move toward a familiar cue. This behavior is an appropriate reaction to an unfamiliar environment, which an animal encounters after being released into a maze. The juvenile individuals of the great crested newt acted in this manner, choosing the more familiar odor in the tests (Hayward et al. 2000). Additional studies are required to determine which explanation is more accurate.
Interestingly, the analysis of the number of individuals reaching a stimulus area and of the time spent in a certain zone did not reveal statistically significant differences. The newts do not linger in the maze arm of their first choice, but actively move through the maze. This is possibly due to the exploratory activity of the animals in the new environment, which masks their initial choice. A similar effect of exploratory behavior masking the preference in a T-maze has been noted in mice (Habedank et al. 2001). In urodeles, it has also been shown that higher exploratory activity is characteristic of juveniles that were raised at higher densities (Ousterhout and Semlitsch 2018). This is due to the more active dispersal of the juvenile urodeles seeking a more favorable habitat compared to unfavorable conditions of the native pond (high concentration of the larvae, low water level, strong competition, etc. (Ousterhout and Semlitsch 2018). In our experiments, rearing densities were higher than in nature. In nature, the rearing density reaches up to 7 larvae per m2 of the bottom of the pond (Hagstrom 1979)), in our case it was 4 larvae per L, equivalent to approximately 226 larvae per m2 of the aquarium bottom. This higher density should further stimulate exploratory behavior. Additional analysis of newt movement may shed light not only on the mechanisms of newt orientation, but also on their behavior in unfamiliar environments.
In the Introduction, we noted that the olfactory system changes during the metamorphosis. Thus, our results indicate that the newts can perceive morpholine as both water- and airborne odorant, which is detected by the larval and adult olfactory systems. The ability to detect odor of a compound in the water and air environments using has been shown electrophysiologically on the tiger salamander Ambystoma tigrinum. The tiger salamander larvae reacted only to the chemical stimuli dissolved in water (from a wide variety of odorants used in the study). Only one single larva reacted to an odor of the airborne water vapor. The adult specimens reacted to both water- and airborne chemical stimuli of the same odorants (Arzt et al. 1986). Similarly to our case, anurans reared in a chemically marked environment (the markers also included morpholine) preferred the odor of the chemical marker, therefore, were able to detect it after the transition to the terrestrial olfaction (Hepper and Waldman 1992; Ogurtsov and Bastakov 2001; Ogurtsov 2007). We can therefore conclude that the change of the receptor does not affect the memory of an odor of a chemical stimulus in either urodeles or anurans. Notably, this phenomenon is not unique to these animals. Studies on insects have also shown the ability to memorize chemical stimuli on the larval stage of development and retain this memory after the metamorphosis (Ray 1999; Rietdorf and Steidle 2002; Blackiston et al. 2008).
An odor learned during larval development can also be used by adult newts to return to the native pond for breeding. The olfactory orientation of the adults has been demonstrated in several studies (Twitty 1959; Hershey and Forester 1980). The results of our study expand our understanding of homing in amphibians, as well as the role of olfaction as a mechanism of spatial orientation. In addition, our findings can be used to improve conservation techniques of Urodela amphibians. For population recruitment of newts, it is more advantageous to transfer the eggs in lieu of the adult specimens, since the growing larvae will form associations with the new habitat. The preferences of larvae should also be taken into account during the anthropogenic transformation of their habitat in order to preserve the population.
Future studies should determine whether odor learning occurs uniformly throughout development, or there are specific sensitive periods when the odor learning occurs actively and the periods when the learning does not does not occur, as characteristic for the imprinting. Such sensitive periods have been noted in anurans (Ogurtsov 2004). Another problem for future studies is how the reaction changes over a longer time after metamorphosis.
Data availability
The data collected in this study is stored by the author and can be provided to everyone on request.
References
Arzt AH, Silver WL, Mason JR, Clark L (1986) Olfactory responses of aquatic and terrestrial tiger salamanders to airborne and waterborne stimuli. J Comp Physiol A 158:479–487. https://doi.org/10.1007/BF00603794
ASAB Ethical Committee/ABS Animal Care (2012) Guidelines for the treatment of animals in behavioural research and teaching. Anim Behav 83:301–309. https://doi.org/10.1016/j.anbehav.2011.10.031
Bastakov VA (1992) Experimental study of the memorizing of pond odor during larval development of two anuran species. Zool Zhurnal 71:123–127
Bell G (1977) The Life of the smooth newt (Triturus vulgaris) after metamorphosis. Ecol Monogr 47:279–299. https://doi.org/10.2307/1942518
Blackiston DJ, Casey ES, Weiss MR (2008) Retention of memory through metamorphosis: can a moth remember what it learned as a caterpillar? PLoS One. https://doi.org/10.1371/journal.pone.0001736
Blaustein AR, Waldman B (1992) Kin recognition in anuran amphibians. Anim Behav 44:207–221. https://doi.org/10.1016/0003-3472(92)90027-7
Cooper JC, Hasler AD (1973) An electrophysiological approach to salmon homing. Fisheries research board of canada technical report. Pacific Biological Station, Nanaimo
Cooper JC, Hasler AD (1976) Electrophysiological studies of morpholine-imprinted coho salmon (Oncorhynchus kisutch ) and rainbow trout (Salmo gairdneri ). J Fish Res Board Canada 33:688–694. https://doi.org/10.1139/f76-085
Davis JM, Stamps JA (2004) The effect of natal experience on habitat preferences. Trends Ecol Evol 19:411–416. https://doi.org/10.1016/j.tree.2004.04.006
Freitag J, Krieger J, Strotmann J, Breer H (1995) Two classes of olfactory receptors in Xenopus laevis. Neuron 15:1383–1392. https://doi.org/10.1016/0896-6273(95)90016-0
Gamble LR, McGarigal K, Compton BW (2007) Fidelity and dispersal in the pond-breeding amphibian, Ambystoma opacum: Implications for spatio-temporal population dynamics and conservation. Biol Conserv 139:247–257. https://doi.org/10.1016/j.biocon.2007.07.001
Getchell ML, Getchell TV (1992) Fine structural aspects of secretion and extrinsic innervation in the olfactory mucosa. Microsc Res Tech 23:111–127. https://doi.org/10.1002/jemt.1070230203
Grubb JC (1973a) Olfactory orientation in Bufo woodhousei fowleri, Pseudacris clarki and Pseudacris streckeri. Anim Behav 21:726–732. https://doi.org/10.1016/S0003-3472(73)80098-3
Grubb JC (1973b) Orientation in newly metamorphosed Mexican toads, Bufo valliceps. Herpetologica 29:95–100
Habedank A, Kahnau P, Lewejohann L (2001) Alternate without alternative: neither preference nor learning explains behaviour of C57BL/6J mice in the T-maze. Behaviour 158:625–662. https://doi.org/10.1163/1568539X-bja10085
Hagstrom T (1979) Population ecology of Trituras cristatus and T. vulgaris (Urodela) in SW Sweden. Ecography (cop) 2:108–114. https://doi.org/10.1111/j.1600-0587.1979.tb00688.x
Hansen A, Reiss JO, Gentry CL, Burd GD (1998) Ultrastructure of the olfactory organ in the clawed frog, Xenopus laevis, during larval development and metamorphosis. J Comp Neurol 398:273–288. https://doi.org/10.1002/(SICI)1096-9861(19980824)398:2%3c273::AID-CNE8%3e3.0.CO;2-Y
Hayward R, Oldham RS, Watt PJ, Head SM (2000) Dispersion patterns of young great crested newts (Triturus cristatus). Herpetol J 10:129–136
Healy WR (1975) Breeding and postlarval migrations of the redspotted newt, Notophthalmus Viridescens, in Massachusetts. Ecology 56:673–680. https://doi.org/10.2307/1935501
Hepper PG, Waldman B (1992) Embryonic olfactory learning in frogs. Q J Exp Psychol Sect B Comp Physiol Psychol 44:179–197. https://doi.org/10.1080/02724999208250611
Hershey JL, Forester DC (1980) Sensory orientation in Notophthalmus v. viridescens (Amphibia: Salamandridae). Can J Zool 58:266–276. https://doi.org/10.1139/z80-032
Ishii S, Kubokawa K, Kikuchi M, Nishio H (1995) Orientation of the toad, Bufo japonicus, toward the breeding pond. Zool Sci 12:475–484
Joly P, Miaud C (1993) How does a newt find its pond? The role of chemical cues in migrating newts (Triturus alpestris). Ethol Ecol Evol 5:447–455
Kidov AA (2018) Captive breeding of the Caucasian smooth newt, Lissotriton lantzi (Wolterstorff, 1914) (Salamandridae, Amphibia). Curr Stud Herpetol 2:1–23. https://doi.org/10.2418/ncr.2017.002
Kovtun MF, Stepanyuk YV (2015) The development of olfactory organ of Lissotriton vulgaris (Amphibia, Caudata). Vestn Zool 49:559–566. https://doi.org/10.1515/vzoo-2015-0066
Kusano T, Miyashita K (1984) Dispersal of the Salamander, Hynobius nebulosus tokyoensis. J Herpetol 18:349. https://doi.org/10.2307/1564094
Lehner PN (1996) Handbook of ethological methods. Cambridge Univ Press, Cambridge
McGregor JH, Teska WR (1989) Olfaction as an orientation mechanism in migrating Ambystoma maculatum. Copeia 1989:779–781. https://doi.org/10.2307/1445516
Millery J, Briand L, Bézirard V et al (2005) Specific expression of olfactory binding protein in the aerial olfactory cavity of adult and developing Xenopus. Eur J Neurosci 22:1389–1399. https://doi.org/10.1111/j.1460-9568.2005.04337.x
Ogurtsov SV (2004) Olfactory orientation in anuran amphibians. Russ J Herpetol 11:35–40
Ogurtsov SV (2005) Basis of native pond fidelity in anuran amphibians: the case of chemical learning. Herpetol petropolitana. Citeseer, Princeton, pp 198–200
Ogurtsov SV (2007) Influence of chemical exposition during larval development on postmetamorphic behaviour of juveniles of three anuran species. Curr Stud Herpetol MSU 7:88–97
Ogurtsov SV (2012) A problem of multicomplex interaction in studying the chemical orientation of juveniles of terrestrial amphibian species. Zool Zhurnal 91:1330–1339
Ogurtsov SV, Bastakov VA (2001) Imprinting on native pond odour in the pool frog, Rana lessonae Cam. In: Marchlewska-Koj A, Lepri JJ, Müller-Schwarze D (eds) Chemical signals in vertebrates 9. Springer, Boston, pp 433–438
Ousterhout BH, Semlitsch RD (2018) Effects of conditionally expressed phenotypes and environment on amphibian dispersal in nature. Oikos 127:1142–1151. https://doi.org/10.1111/oik.05276
Pfennig DW (1990) “Kin recognition” among spadefoot toad tadpoles: a side-effect of habitat selection? Evolution (n y) 44:785–798
Pittman SE, Semlitsch RD (2013) Habitat type and distance to edge affect movement behavior of juvenile pond-breeding salamanders. J Zool 291:154–162. https://doi.org/10.1111/jzo.12055
Popescu VD, Brodie BS, Hunter ML, Zydlewski JD (2012) Use of olfactory cues by newly metamorphosed wood frogs (Lithobates sylvaticus) during emigration. Copeia 2012:424–431. https://doi.org/10.1643/CE-11-062
Ray S (1999) Survival of olfactory memory through metamorphosis in the fly Musca domestica. Neurosci Lett 259:37–40. https://doi.org/10.1016/S0304-3940(98)00892-1
Rietdorf K, Steidle JLM (2002) Was Hopkins right? Influence of larval and early adult experience on the olfactory response in the granary weevil Sitophilus granarius (Coleoptera, Curculionidae). Physiol Entomol 27:223–227. https://doi.org/10.1046/j.1365-3032.2002.00289.x
Różański JJ, Żuwała KD (2019) The influence of habitat on olfactory organ structure in selected species of salamanders (Salamandridae, Caudata). Zool Anz 281:1–10. https://doi.org/10.1016/j.jcz.2019.05.003
Shakhparonov V V, Ogurtsov S V (2005) The role of the native pond odor in orientation of the green toad (Bufo viridis Laur.) youngs-of-the-year. In: Ananjeva N, Tsinenko O (eds) Herpetologica Petropolitana. Proc. of the 12th Ord. Gen.Meeting Soc. Eur. Herpetol., August 12–16, 2003, St. Petersburg. Russ. J. Herpetol. St. Petersburg, pp 209–212
Sinsch U (1992) Sex-biassed site fidelity and orientation behaviour in reproductive natterjack toads (Bufo calamita). Ethol Ecol Evol 4:15–32. https://doi.org/10.1080/08927014.1992.9525347
Sinsch U (1997) Postmetamorphic dispersal and recruitment of first breeders in a Bufo calamita metapopulation. Oecologia 112:42–47. https://doi.org/10.1007/s004420050281
Sinsch U (2006) Orientation and navigation in Amphibia. Mar Freshw Behav Physiol 39:65–71. https://doi.org/10.1080/10236240600562794
Twitty VC (1959) Migration and speciation in newts. Science 130:1735–1743. https://doi.org/10.1126/science.130.3391.1735
Vignoli L, Silici R, Bissattini AM, Bologna MA (2012) Aspects of olfactory mediated orientation and communication in Salamandrina perspicillata (Amphibia Caudata): an experimental approach. Ethol Ecol Evol 24:165–173. https://doi.org/10.1080/03949370.2011.591437
Weiss L, Manzini I, Hassenklöver T (2021) Olfaction across the water–air interface in anuran amphibians. Cell Tissue Res 383:301–325. https://doi.org/10.1007/s00441-020-03377-5
Wells KD (2007) The ecology and behavior of amphibians. The University of Chicago Press, Chicago
Acknowledgements
We are grateful to Sergey Viktorovich Ogurtsov for consulting us during the preparation of this study, as well as Evgeny Igorevich Sarychev for providing access to the breeding pond located in the “Biosphere” nursery, where the adult newt specimens were collected. We are also thankful to Alexandra Lisenkova and Varvara Bogatyreva for invaluable help in translating the article. We are grateful to two anonymous reviewers for very useful comments that greatly improved the presentation of the material.
Author information
Authors and Affiliations
Contributions
YAV collected the material, as well as collected and processed the basic data. He conducted all the experiments. He also wrote the main part of the text and created illustrative material. VVS helped with writing and editing the text of the article and illustrative material. He also made a significant contribution to the correction of the study and the formulation of its tasks.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
The study was conducted in accordance with: Legislation of the Russian Federation and the requirements of the Bioethics Committee of Lomonosov Moscow State University (GOST 33219-2014); “Guidelines for accommodation and care of animals” of the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (ETS No. 123); "Guidelines for the treatment of animals in mental research and teaching’ by the ASAB Ethical Committee/ABS Animal Care Committee (2012).
Additional information
Handling Editor: Wolfgang Rössler.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vyatkin, Y.A., Shakhparonov, V.V. Learning the native pond odor as one of the mechanisms of olfactory orientation in juvenile smooth newt Lissotriton vulgaris. J Comp Physiol A 210, 57–63 (2024). https://doi.org/10.1007/s00359-023-01640-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-023-01640-y