Abstract
Bone geometry is an important determinant of bone strength and is influenced by muscle pull and weight-bearing. Muscle mass and exposure to weight-bearing decrease with age and thus the purpose of the study was to compare bone geometry of the weight-bearing (tibia) and non-weight-bearing (fibula) bones of the leg in different age groups. Magnetic resonance images of the right leg were acquired in 13 young (26 yr), 13 old (66 yr), and 13 very old men (83 yr). Cortical, medullary and total cross-sectional areas (CSA) of the bones were measured at approximately one-third and two-thirds the length of the leg. Muscle CSA of the anterior, lateral and posterior compartments was measured at the proximal site. Cortical CSA was ~14 to 22% smaller in the elderly in the tibia but similar across age in the fibula. Medullary CSA was larger with age (~5 to 65%) in both bones but ~15 to 440% greater in the tibia than fibula. Total CSA was similar across age in both bones. Muscle mass was similar between young and old but ~25% less in the very old and as a consequence, the magnitude of differences in bone geometry at proximal and distal sites varied in the two elderly groups. These findings indicate that there is a complex age-dependent interaction between muscle pull and weight-bearing. The greater age-related differences in bone geometry in the tibia suggest the weight-bearing role of the tibia makes it more susceptible than the fibula to the reduced activity typically associated with aging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging is associated with osteopenia, osteoporosis and an increased risk of fracture. There is an increasing level of importance attributed to these conditions due to the global demographic shift toward a more elderly population and the escalating health care costs associated with treatment. Particularly troubling from the perspectives of patient recovery and financial cost are fractures to the hip joint or bones of the lower limb. These fractures are associated with long periods of hospitalization, immobilization, or inactivity which can lead to a permanent loss of independence and increased morbidity in the elderly [1, 2].
As many more women than men suffer the effects of osteoporosis, the majority of research on bone health has been done with women. Nevertheless, men can experience significant bone loss and the importance of bone health in men has begun to garner attention. Consequently, it has been suggested that further research is needed in men [3–5]. Regardless of sex, the majority of studies have examined age-related changes in bone mineral density using dual energy X-ray absorptiometry (DXA), and relatively few [6, 7] have examined age-related changes in cross-sectional area or bone geometry using magnetic resonance imaging (MRI). However, bone geometry is known to play an important role in bone strength [8–10] and has been suggested to be a better indicator of bone fragility and fracture risk than bone mineral density [11]. The use of MRI is advantageous for an investigation of age-related changes in bone geometry because it does not expose subjects to ionizing radiation, bone geometry assessment is highly accurate and reproducible with the technique [12], and reliable muscle mass data [13, 14] can be obtained simultaneously thereby allowing one to explore the relationship between muscle and bone [15]. Because muscle contraction induces the largest voluntary load on bone [8, 16], this relationship should be explored.
Weight-bearing is also known to be an important determinant of bone strength [17–19] and thus it is of interest to consider the combined influence of age and weight-bearing on bone. Surprisingly, few such studies exist, particularly in men. Although the bones were not directly compared, a few studies have assessed age-related changes in the non-weight-bearing humerus and the weight-bearing femur [20, 21]. Cortical cross-sectional area (CSA) appeared to be similarly reduced in the study by Lindahl and Lindgren [20], but in contrast, the results of Martin and colleagues [21] suggest a greater decrease in cortical area and a greater increase in porosity of the humerus compared to the femur. Similarly, McMillan and Marsh [7] reported greater age-related differences in humeral rather than femoral measures of bone geometry. A model that, to our knowledge, has not yet been considered is a comparison between the tibia and fibula. These bones represent a unique comparison because they are in the same segment of the lower limb and thus possess many common muscle attachments, but they experience dramatically different stresses with respect to weight-bearing activity (~94 vs. 6% of the load applied to the knee joint for the tibia and fibula, respectively) [22].
Thus, the purpose of the current study was to examine the geometry of the tibia and fibula across different age groups. In an effort to provide some insight into the possible rate of change, a control group of young men was compared to two groups of elderly men; i.e., those in their 7th decade (old) and those in their 9th or 10th decades (very old). We hypothesized that an assumed progressive age-related decrease in exposure to weight-bearing would result in greater differences in the tibia than the fibula in old men, and that these differences would be exacerbated in the very old men.
Materials and Methods
Subjects
Thirteen young men (aged 23–31), 13 old men (aged 61–69), and 13 very old men (aged 80–91), volunteered for this study. Young subjects were recruited from the university environment and considered to be recreationally active. Subjects in the two elderly groups were healthy, active men recruited from a local exercise program designed to maintain cardiovascular fitness, flexibility and muscular endurance. All elderly men were ambulatory and living independently in the community. The mean age, height, fibular length, and body mass of the three groups are reported in Table 1. The study was conducted in accordance with the guidelines for experimentation on human subjects established by the local university’s ethics review board and conformed to the Declaration of Helsinki. Subjects completed an MRI screening questionnaire concerning metallic objects in the body and informed, written consent was obtained from each of the 39 participants. Data were collected during a single visit to the imaging unit.
Experimental Set-Up
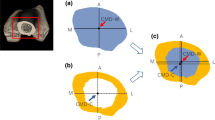
Using a flexible tape measure, right fibula length was determined as the distance between the head of the fibula and the lateral malleolus. Plastic capsules filled with vegetable oil were taped to the right leg at one-third (proximal) and two-thirds (distal) of this distance to serve as external markers during positioning of the subject and image analysis. In a supine position, subjects were inserted feet-first into the bore (64 cm) of the 3.0T superconducting magnet (IMRIS, Winnipeg, Canada) to approximately waist level. In order to position the right leg in the middle of the coil, and thereby maximize the image quality, the right foot was crossed over top of the left foot. MR images of the tibia and fibula were acquired via serial axial plane scans; five slices were obtained at both the proximal and distal sites (centered about the plastic capsules). Two distinct sections were imaged because marked heterogeneity exists within the same bone [23, 24] and thus data from a single section could be misleading. T1-weighted images were acquired using the following parameters: repetition time, 850 ms; echo time, 21 ms; matrix, 512 × 512; field of view, 250 mm; slice thickness, 7 mm; and slice separation, 3 mm. A representative scan for each age group is displayed in Fig. 1.
Data Reduction and Statistics
Analyze software (version 7.0, Mayo Clinic, Rochester, USA) was used to determine cortical, medullary, and total (cortical + medullary) cross-sectional areas (CSAs) of the tibia and fibula in all scans. Group coefficients of variation (CVs) ranged between 0.09–0.22 for cortical CSA, between 0.21–0.37 for medullary CSA, and between 0.10–0.22 for total CSA. Although there were no significant differences among the groups for subject height, the mean of the young was 5 cm taller than that of the very old. To eliminate any possible influence of height, the absolute cortical, medullary and total CSAs were normalized to individual subject height. Statistical analyses were performed on the normalized values but the absolute values are presented in the results and tables. Cortical CSA was divided by total CSA to determine the relative percentage of cortical bone in the tibia and fibula (CV range of 0.06–0.14). In the proximal five scans, muscle, non-contractile and total (muscle + non-contractile) CSA were determined for the anterior, lateral and posterior muscle compartments as well as the leg as a whole (anterior + lateral + posterior compartments). Group CVs ranged between 0.12–0.24 for muscle CSA, between 0.14–0.39 for non-contractile CSA, and between 0.10–0.22 for total CSA. Non-contractile CSA was divided by total CSA to determine the relative percentage of non-contractile tissue in each compartment (CV range of 0.16–0.33). A single investigator performed all analyses by visual inspection. Bone and muscle analyses were performed using the auto-trace and manual trace functions, respectively.
All statistical analyses were performed with SPSS software (version 17). To interpret the complex analyses of variance (ANOVAs) employed, significant interactions were decomposed and lower-order ANOVAs were performed as described below. Absolute cortical and medullary bone were compared among age groups using a four-way repeated measures ANOVA, with location (proximal and distal), bone (tibia and fibula) and type (cortical and medullary) as within-subjects factors. Due to the presence of a significant location * bone * type interaction, three-way repeated measures ANOVAs were then performed separately for the proximal and distal sections. These analyses each contained a significant bone * type interaction and so two-way repeated measures ANOVAs were used separately for the tibia and fibula at proximal and distal sites. Each of these four analyses had a significant type * group interaction and so univariate ANOVAs were then performed separately for cortical and medullary bone in both bones and at both locations. Total CSA (cortical + medullary) and the relative percentage of cortical bone in the tibia and fibula were compared among age groups by separate three-way repeated measures ANOVAs, with location and bone as within-subjects factors. For muscle data, absolute muscle and non-contractile tissue were compared among age groups using a three-way repeated measures ANOVA, with compartment (anterior, lateral, posterior and all) and type (muscle and non-contractile) as within-subjects factors. There was a significant compartment * type interaction and thus two-way repeated measures ANOVAs were used separately for each compartment. Each of these four analyses had a significant type * group interaction and so univariate ANOVAs were performed separately for muscle and non-contractile tissue in each of the four compartments. Total compartment CSA (contractile + non-contractile) and the relative percentage of non-contractile tissue in each compartment were compared among age groups by separate two-way repeated measures ANOVAs. There was a significant compartment * group interaction for relative non-contractile tissue and so univariate ANOVAs were performed separately for each compartment.
For all statistical analyses, when the lowest-order ANOVA had a significant between-subjects (group) effect, Tukey post hoc tests were performed to determine which age groups differed from one another. Correlations were determined between cortical CSA and subject age, height, body mass and leg muscle CSA. A stepwise linear regression was used to determine which variables of age, height, body mass and leg muscle CSA provided the best prediction of cortical CSA in the tibia and fibula. All data are reported in the text as group means, and the level of significance was P < 0.05.
Results
Despite similar height and body mass (Table 1), there were significant differences in bone geometry among age groups. In the proximal section, cortical CSA of the tibia was smaller in both the old and very old compared to young but similar between old and very old (Table 2). In the fibula, cortical CSA was similar among all groups (Table 2). Medullary CSA was greater in the very old than young in the tibia but greater in the very old compared to both young and old in the fibula (Table 2). Total CSA (cortical + medullary) was similar across age groups in both bones (Table 2). In both the tibia and fibula, cortical CSA expressed as a percentage of the total CSA was similar in the young and old and smaller in the very old compared to either the young or old (Table 2). In the distal section, cortical CSA of the tibia was smaller in the old and very old compared to young, with no difference between the latter two groups (Table 2). As in the proximal section, cortical CSA was similar among all groups in the fibula (Table 2). Medullary CSA of the tibia was greater in old and very old than young with no difference between the two elderly groups (Table 2). In the fibula, medullary CSA was greater in the very old compared to both young and old with no difference between these latter two groups (Table 2). Total CSA was similar across age groups in both bones (Table 2). Cortical CSA expressed as a percentage of the total CSA was less in the old and very old relative to young in the tibia, and in the fibula was smaller in the very old compared to young and old (Table 2).
The extent of difference in muscle composition across age groups varied among the three compartments, with the smallest and greatest differences recorded in the anterior and posterior compartments, respectively. Total compartment CSA (muscle + non-contractile) was similar across age groups however this reflected the conflicting effects of less muscle tissue and more non-contractile tissue (Table 3). Muscle CSA was similar between young and old but generally significantly less in the very old compared to both groups for each compartment individually and the leg as a whole (Table 3). Exceptions were that muscle CSA was similar between the very old and young for the lateral compartment and between very old and old for the posterior compartment (Table 3). Old men had significantly greater non-contractile CSA than the young only in the lateral compartment but there were strong trends in the posterior compartment (P = 0.059) and the leg (P = 0.055) (Table 3). Very old men had significantly greater non-contractile tissue than both young and old for all comparisons with the exception of the lateral compartment for which the greater value was different only from young (Table 3). As a result of the greater levels of non-contractile CSA but the equivalent total compartmental CSA, non-contractile tissue expressed as a percentage of the total CSA was substantially higher with age. In the posterior compartment and the leg, the relative percentage of non-contractile tissue was ~50% greater in old than young (Table 3). The differences were most dramatic in the very old as the percentage of non-contractile tissue was 2–3 fold greater than that of young and 1.5–2 fold greater than that of old in all compartments and hence the leg (Table 3).
Correlations for cortical CSA in the tibia and fibula are reported in Table 4. In both bones, cortical CSA was negatively associated with age but positively related to body mass and leg muscle CSA. In contrast, subject height was positively associated to cortical CSA of the tibia but not significantly related to cortical CSA of the fibula. Using a stepwise linear regression, the model of age and body mass explained 64% of the variance in cortical CSA of the tibia (R = 0.80). In the fibula, leg muscle CSA and body mass provided the best model but explained only 36% of the variance in cortical CSA (R = 0.60).
Discussion
In the comparison across age groups, cortical CSA was smaller in the tibia alone, whereas medullary CSA was larger in both the tibia and fibula of the elderly. These findings generally support the hypothesis that age-related differences in bone geometry are greater in the weight-bearing tibia compared to the largely unloaded fibula. Muscle CSA, like bone, tended to be smaller with age; however, the two measures were correlated only moderately.
The loss of cortical CSA in the tibia exceeded that in the fibula at both proximal and distal sections in old and very old men, but the pattern of decline was not uniform between these sections in the two bones. In the tibia, there was a tendency toward a progressive decline in cortical CSA with age at the proximal but not the distal section. Cortical CSA in the distal section of the tibia was an equivalent ~16% less than the young in both the old and the very old. Unlike the tibia, the loss of cortical CSA in the fibula was non-significant and was relatively uniform for both sections in the old and very old. Although these results generally follow the hypothesized pattern, their interpretation is complex because weight-bearing and muscle pull appear to influence the two bones and the two sections of each bone differently in young and middle-age adulthood versus old adulthood. Moreover, the interaction between weight-bearing and muscle pull on bone geometry is complicated by numerous other factors such as genetics, physical activity, circulating hormones and nutrition [18]. It was recently reported that the osteogenic response of cortical bone to weight-bearing exercise increases as one moves distally along the bone [23]. In the weight-bearing tibia, the loss of cortical CSA compared to young was greater in the proximal (22%) than distal (16%) section in the very old but slightly greater in the distal (17%) than proximal (14%) section in the old. Muscle CSA (muscle mass) was equivalent in the old and young so it is plausible that the high loads induced by muscle pull at the proximal section compensated for the smaller osteogenic response at this level of the tibia. However, muscle CSA was markedly less in the very old and therefore perhaps was less able to compensate for the diminished osteogenic response to weight-bearing at the proximal tibia.
In contrast to cortical CSA, the difference (i.e., increase) in medullary CSA was exacerbated in very old age and appeared to occur at substantially different rates for the tibia and fibula. In the comparison between the 3rd and 7th decades, the increase in medullary CSA was ~3.5 to 4.5-fold greater in the tibia than the fibula, whereas in the comparison between the 7th and 9th decades, the increase in the medullary cavity was ~1.5 to 2.5-fold greater in the fibula than the tibia. Thus, more convincingly than the cortical CSA data, these results suggest that weight-bearing does impact some aspects of bone geometry. The medullary data also support the importance of muscle pull. In the old, when muscle mass was similar to that of the young, the medullary CSA was 50–80% greater in the distal than proximal section for both bones. In the very old, when muscle mass was 25% smaller compared to young, the greater increase in medullary CSA at the distal than proximal section was a more modest 30–40% for each bone.
We are unaware of any published data that examine CSA of the fibula across age groups so are unable to draw any comparisons to the literature. In the tibia, the data are generally in agreement with a recent population-based study [9], and in partial agreement with one other population-based study [25] and several reports from one group who studied archaeological and modern-day cadaveric tibiae [10, 24, 26]. The present finding of smaller cortical CSA but maintained total CSA in elderly males is in accordance with the recent study by Riggs and colleagues [9]. The magnitude of the decrease in cortical bone in the present study exceeded that of the previous study [9], but is in keeping with a diminishing osteogenic response as one moves proximally along a weight-bearing bone [23]. That is, our data were collected ~12 and 24 cm proximal to the lateral malleolus as compared to ~2 and 7 cm proximal to the tibial-fibular junction [9]. This discrepancy could also explain why the increase of medullary CSA in the previous study (reported only at the site ~2 cm proximal to ankle) [9] was considerably smaller than the increase noted in the present study. Moreover, using nearly identical sites of measurement, we note an age-related increase of medullary CSA of a magnitude similar to those reported in previous studies [10, 24–26]. Despite the similar degree of medullary expansion, these previous studies did not find an age-related decrease in cortical CSA. However, two of these studies [24, 26] compared archaeological samples in which the oldest age category was only the 6th decade, and another [10] contained data from only nine subjects equal in age to our very old category, and compared with only four young adult subjects. In the remaining study [25], the youngest age category included individuals between 20 and 49 years, a range considerably wider than in the present study and which could contribute to the discrepancy. Because cortical CSA was maintained in these studies [10, 24–26], the medullary expansion resulted in an increase in total CSA that was not found in our results or in those of Riggs and colleagues [9].
The muscle mass data are consistent with previously published values in the anterior [27–29] and posterior compartments [30, 31]. However, as was the case with fibular CSA, muscle mass data of the lateral compartment could not be compared to published data as we were unable to locate any studies in the literature which investigated the presence of age-related changes in this compartment. The age-related decrease in muscle tissue and infiltration of non-contractile tissue were greatest in the posterior compartment, which is in keeping with the finding that age-related changes are greater in magnitude and more rapid in onset for the plantar flexors than dorsiflexors [32, 33]. With age, there is a preferential atrophy or loss of type II muscle fibers [34] and thus the greater percentage of these fibers in the gastrocnemius compared to the tibialis anterior (~50 vs. ~25%; [35]) has been suggested as an explanation for the discrepancy between compartments [36]. Like the anterior compartment, muscle CSA of the lateral compartment was preserved in the old but was smaller in the very old men. The magnitude of increase in non-contractile tissue was also more similar to that noted in the anterior than posterior compartment. These findings indicate that the lateral compartment also undergoes lesser and slower age-related changes than the posterior compartment.
It is possible that the assessment of muscle strength or power rather than muscle mass would have provided better correlations to cortical CSA. For example, it was recently reported that tibial strength is related to maximal neuromuscular performance in young men and women [37]. Although there are conflicting results in the literature, there are numerous studies that report strength per muscle CSA or volume is reduced with age; including examples in the anterior [29] and posterior [30, 31] compartments. If this were true, muscle mass data alone would overestimate the maximum strain that could be applied to a bone via muscle contraction. However, this error would be secondary to the fact that the pattern of physical activity would dictate the magnitude and frequency of the typical strains imposed and thereby have a greater influence on bone strength [16]. It is well established that there is a decrease in physical activity with advancing age, even in healthy active elderly, and this is especially pronounced for strenuous physical activity [30]; i.e., the intensity which is most likely to involve muscle contractions of the strength necessary to induce the strains required to maintain bone or promote modeling [16]. Thus, a decrease in activity would negatively impact bone health by less frequent exposure to weight-bearing and, perhaps more importantly, by a reduction in strain induced by muscle pull [16, 38]. In the case of the latter, the effect would be attributed to fewer muscle contractions as well as a smaller strain per contraction due to muscle atrophy. We attempted to minimize the influence of an age-related decrease in physical activity by selecting healthy elderly participants who continued to lead active lifestyles even beyond 90 years of age in some instances. However, because physical activity was not quantified in our subjects, it is uncertain how similar the activity profiles were among the different age groups.
Longitudinal studies in humans that span 60 years of ageing are challenging, and although cross-sectional studies have inherent limitations, comparing more than one group allows some extrapolation concerning rates of change and is superior to a simple comparison of one young to one older group. Although not performed in this study it is clear that physical activity and nutritional factors should be included to better understand the affect of age on bone geometry.
In conclusion, although the distribution of weight-bearing is dramatically skewed in the bones of the leg, the extent to which cortical CSA was smaller in elderly men was greater in the tibia than fibula but only modestly in the very old compared to old men. In contrast, medullary cavity expansion appeared to occur at a much greater rate in the tibia between the 3rd and 7th decades of life but to a greater extent in the fibula between the 7th and 9th decades. Muscle mass was similar between young and old but smaller in the very old compared to both young and old. There was a positive, moderate association between muscle mass and cortical CSA in both bones. These data suggest that assumed age-related decreases in exposure to weight-bearing, in connection with observed muscle atrophy are likely important but other factors not accounted for also will impact various aspects of bone geometry in the leg differently with age.
References
Jiang HX, Majumdar SR, Dick DA, Moreau M, Raso J, Otto DD, Johnston DW (2005) Development and initial validation of a risk score for predicting in-hospital and 1-year mortality in patients with hip fractures. J Bone Miner Res 20:494–500
Smith EL, Tommerup L (1995) Exercise: a prevention and treatment for osteoporosis and injurious falls in the older adult. J Aging Phys Act 3:178–192
Gennari C, Seeman E (2001) The first international conference on osteoporosis in men. Siena, Italy, February 23–25, 2001. Calcif Tissue Int 69:177–178
Seeman E, Bianchi G, Adami S, Kanis J, Khosla S, Orwoll E (2004) Osteoporosis in men—consensus is premature. Calcif Tissue Int 75:120–122
Seeman E, Bianchi G, Khosla S, Kanis JA, Orwoll E (2006) Bone fragility in men—where are we? Osteoporos Int 17:1577–1583
Klein CS, Allman BL, Marsh GD, Rice CL (2002) Muscle size, strength, and bone geometry in the upper limbs of young and old men. J Gerontol A 57:M455–M459
McMillan SJ, Marsh GD (2002) Humeral and femoral shaft geometry in young and old men. Med Sci Sports Exerc 34:S59
Frost HM (1997) On our age-related bone loss: insights from a new paradigm. J Bone Miner Res 12:1539–1546
Riggs BL, Melton LJIII, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, Rouleau PA, McCollough CH, Bouxsein ML, Khosla S (2004) Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res 19:1945–1954
Ruff CB, Hayes WC (1988) Sex differences in age-related remodeling of the femur and tibia. J Orthop Res 6:886–896
Myers ER, Hecker AT, Rooks DS, Hipp JA, Hayes WC (1993) Geometric variables from DXA of the radius predict forearm fracture load in vitro. Calcif Tissue Int 52:199–204
Woodhead HJ, Kemp AF, Blimkie CJR, Briody JN, Duncan CS, Thompson M, Lam A, Howman-Giles R, Cowell CT (2001) Measurement of midfemoral shaft geometry: repeatability and accuracy using magnetic resonance imaging and dual-energy X-ray absorptiometry. J Bone Miner Res 16:2251–2259
Klein CS, Rice CL, Marsh GD (2001) Normalized force, activation, and coactivation in the arm muscles of young and old men. J Appl Physiol 91:1341–1349
Holmback AM, Askaner K, Holtas S, Downham D, Lexell J (2002) Assessment of contractile and noncontractile components in human skeletal muscle by magnetic resonance imaging. Muscle Nerve 25:251–258
Heinonen A, McKay HA, Whittall KP, Forster BB, Khan KM (2001) Muscle cross-sectional area is associated with specific site of bone in prepubertal girls: a quantitative magnetic resonance imaging study. Bone 29:388–392
Frost HM (2003) Bone’s mechanostat: a 2003 update. Anat Rec 275A:1081–1101
Daly RM, Bass SL (2006) Lifetime sport and leisure activity participation is associated with greater bone size, quality and strength in older men. Osteoporos Int 17:1258–1267
Mikkola TM, Sipila S, Rantanen T, Sievanen H, Suominen H, Kaprio J, Koskenvuo M, Kauppinen M, Heinonen A (2008) Genetic and environmental influence on structural strength of weight-bearing and non-weight-bearing bone: a twin study. J Bone Miner Res 23:492–498
Wang Q, Alen M, Nicholson P, Suominen H, Koistinen A, Kroger H, Cheng S (2007) Weight-bearing, muscle loading and bone mineral accrual in pubertal girls—a 2-year longitudinal study. Bone 40:1196–1202
Lindahl O, Lindgren AG (1967) Cortical bone in man. 1. Variation of the amount and density with age and sex. Acta Orthop Scand 38:133–140
Martin RB, Pickett JC, Zinaich S (1980) Studies of skeletal remodeling in aging men. Clin Orthop Relat Res 149:268–282
Takebe K, Nakagawa A, Minami H, Kanazawa H, Hirohata K (1984) Role of the fibula in weight-bearing. Clin Orthop Relat Res 184:289–292
Hamrick MW, Skedros JG, Pennington C, McNeil PL (2006) Increased osteogenic response to exercise in metaphyseal versus diaphyseal cortical bone. J Musculoskelet Neuronal Interact 6:258–263
Ruff CB, Hayes WC (1982) Subperiosteal expansion and cortical remodeling of the human femur and tibia with aging. Science 217:945–948
Russo CR, Lauretani F, Seeman E, Bartali B, Bandinelli S, Di Iorio A, Guralnik J, Ferrucci L (2006) Structural adaptations to bone loss in aging men and women. Bone 38:112–118
Ruff CB, Hayes WC (1984) Age changes in geometry and mineral content of the lower limb bones. Ann Biomed Eng 12:573–584
Kent-Braun JA, Ng AV (1999) Specific strength and voluntary muscle activation in young and elderly women and men. J Appl Physiol 87:22–29
Kent-Braun JA, Ng AV, Young K (2000) Skeletal muscle contractile and noncontractile components in young and older women and men. J Appl Physiol 88:662–668
McNeil CJ, Vandervoort AA, Rice CL (2007) Peripheral impairments cause a progressive age-related loss of strength and velocity-dependent power in the dorsiflexors. J Appl Physiol 102:1962–1968
Morse CI, Thom JM, Davis MG, Fox KR, Birch KM, Narici MV (2004) Reduced plantarflexor specific torque in the elderly is associated with a lower activation capacity. Eur J Appl Physiol 92:219–226
Morse CI, Thom JM, Reeves ND, Birch KM, Narici MV (2005) In vivo physiological cross-sectional area and specific force are reduced in the gastrocnemius of elderly men. J Appl Physiol 99:1050–1055
Simoneau E, Martin A, Van Hoecke J (2005) Muscular performances at the ankle joint in young and elderly men. J Gerontol A 60:439–447
Winegard KJ, Hicks AL, Sale DG, Vandervoort AA (1996) A 12-year follow-up study of ankle muscle function in older adults. J Gerontol A 51:B202–B207
Lexell J, Taylor CC, Sjostrom M (1988) What is the cause of ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84:275–294
Johnson MA, Polgar J, Weightman D, Appleton D (1973) Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci 18:111–129
Christ CB, Boileau RA, Slaughter MH, Stillman RJ, Cameron JA, Massey BH (1992) Maximal voluntary isometric force production characteristics of six muscle groups in women aged 25 to 74 years. Am J Hum Biol 4:537–545
Rantalainen T, Heinonen A, Komi PV, Linnamo V (2008) Neuromuscular performance and bone structural characteristics in young healthy men and women. Eur J Appl Physiol 102:215–222
Melton LJIII, Riggs BL, Achenbach SJ, Amin S, Camp JJ, Rouleau PA, Robb RA, Oberg AL, Khosla S (2006) Does reduced skeletal loading account for age-related bone loss? J Bone Miner Res 21:1847–1855
Acknowledgements
This work is supported in part by the National Sciences and Engineering Research Council of Canada (NSERC) and the Canadian Institutes of Health Research (CIHR).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McNeil, C.J., Raymer, G.H., Doherty, T.J. et al. Geometry of a Weight-Bearing and Non-Weight-Bearing Bone in the Legs of Young, Old, and Very Old Men. Calcif Tissue Int 85, 22–30 (2009). https://doi.org/10.1007/s00223-009-9261-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-009-9261-7