Abstract
Although patients with type 2 diabetes show no bone mineral density (BMD) reduction, fracture risks are known to increase. It is unclear why the patients have an increased risk of fracture despite sufficient BMD. We investigated the relationships of body mass index (BMI), HbA1c, and urinary C-peptide (uC-peptide) versus BMD, bone metabolic markers, serum adiponectin, and prevalent vertebral fracture (VF). A total of 163 Japanese type 2 diabetic men were consecutively recruited, and radiographic and biochemical data were collected. BMI was positively correlated with BMD at the whole body, lumbar spine, and femoral neck (P < 0.05) and negatively correlated with osteocalcin and urinary N-terminal cross-linked telopeptide of type-I collagen (uNTX) (P < 0.01). HbA1c was negatively correlated with osteocalcin (P < 0.01) but not BMD at any site. Subjects were classified into four groups based on BMI and HbA1c (group LL BMI < 24 and HbA1c < 9, group LH BMI < 24 and HbA1c ≧ 9, group HL BMI ≧ 24 and HbA1c < 9, group HH BMI ≧ 24 and HbA1c ≧ 9). Serum adiponectin, osteocalcin, and uNTX were lower and the incidence of VF was higher despite sufficient BMD in the HH group. Multivariate logistic regression analysis adjusted for age, duration of diabetes, uC-peptide, and estimated glomerular filtration rate showed that the HH group was associated with the presence of a VF and multiple VFs (odds ratio [OR] = 3.056, 95% confidence interval [CI] 1.031–9.056, P = 0.0439, and OR = 5.415, 95% CI 1.126–26.040, P = 0.0350, respectively). Combination of obesity with hyperglycemia was a risk factor for VF despite sufficient BMD in diabetic men.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The number of patients with diabetes mellitus and osteoporosis is rapidly increasing in industrialized countries where Western-style aging societies are prevalent. The relationship between diabetes and osteoporotic fractures is becoming increasingly recognized [1]. Both vertebral and hip fractures are most important osteoporotic fractures because they frequently occur and enhance the mortality of elderly people as high as six- to ninefold [2, 3]. The mortality increase is more prominent in men than in women [2], and absolute risk of subsequent fracture after an initial one in men is higher than or similar to that in women [4]. Therefore, it is no less important to predict the risk of vertebral and hip fractures in diabetic subjects than in their nondiabetic counterparts, especially in men.
Previous studies have shown that type 1 diabetes is associated with a decrease in bone mineral density (BMD) and an increased risk of osteoporotic hip and other fractures [5, 6]. In contrast, although patients with type 2 diabetes show no BMD reduction, fracture risks are known to increase approximately up to 1.5-fold at the hip, proximal humerus, forearm, and foot not only in women [6–8] but also in men [6, 7]. However, it is unclear why patients with type 2 diabetes have an increased risk of fracture despite normal BMD. Hyperglycemia in type 2 diabetes might be associated with factors that influence bone strength and quality independent of BMD. Several experiments using cultured osteoblasts showed that high glucose and/or increased advanced glycemic end products (AGEs) impaired mineralization or osteocalcin production in the cells [9, 10], suggesting that hyperglycemia may cause diminished bone formation.
Body weight is known to impact bone turnover and to be positively correlated with BMD, and higher weight/body mass index (BMI) therefore reduce risks for vertebral and hip fractures in nondiabetic subjects [11, 12]. Previous studies have shown that BMD is closely related to body weight [13] and that change in spinal BMD is related to baseline BMI [14]. On the other hand, type 2 diabetes is caused by obesity-induced insulin resistance and is therefore strongly associated with obesity, and some studies have indicated that patients with the disease also have high BMD equivalent to nondiabetic obese counterparts [1]. However, it is unclear whether or not obese patients with type 2 diabetes would also be at reduced risk for fractures as seen in nondiabetics.
Insulin stimulates proliferation of osteoblasts [15] and increases indices of bone formation such as insulin-like growth factor-I [16] and bone morphogenetic protein [17] when administered locally over bone [18]. Previous studies have shown that circulating insulin concentration is the principal determinant of BMD at the femoral neck and lumbar spine [19]. Although obese diabetic patients have insulin resistance that blunts the hypoglycemic effect of the hormone and causes its compensatory oversecretion, other hormonal actions are kept intact, and increased circulating insulin may exert anabolic actions on bone [20]. A previous study has shown that insulin resistance, which was estimated from an intravenous glucose tolerance test, was significantly and positively correlated with BMD [21]. Thus, high BMD is a very consistent finding across a wide range of hyperinsulinemic states, including obesity and type 2 diabetes [22]. However, it is unclear whether or not this beneficial effect of hyperinsulinemia on BMD also prevents fractures in patients with type 2 diabetes.
In this study, to examine these issues, we selected serum HbA1c, BMI, and urinary C-peptide (uC-peptide) as markers for hyperglycemia, obesity, and residual insulin secretion, respectively, in Japanese men with type 2 diabetes. We investigated the relationship between each of these parameters and BMD, bone metabolic markers, serum adiponectin levels, and the presence of vertebral fractures. We found that combination of obesity with hyperglycemia predisposes type 2 diabetic men to vertebral fractures in spite of normal BMD, possibly through low bone turnover and resultant bone fragility.
Subjects and Methods
Subjects
The subjects in this study were 163 Japanese men with type 2 diabetes aged 25–83 years (mean 57.7). We consecutively recruited subjects who visited Shimane University Hospital for education, evaluation, or treatment of diabetes. Subjects agreed to participate in the study and gave informed consent. This study was approved by the institutional review board of our institution. Nobody had hepatic or renal dysfunction or nutritional derangements that might cause changes in bone metabolism. We excluded patients with anemia (hemoglobin < 12.0 g/dL) because HbA1c values might be influenced by anemia. Twenty-six patients had received insulin treatment and 61 patients had taken oral hypoglycemic agents (sulfonylurea, 55; metformin, 23; alpha-glucosidase inhibitor, 18). All subjects were free of drugs known to influence bone and calcium metabolism like vitamin D and bisphosphonate as well as thiazolidinedione until the time of the present study.
Radiography
Lateral X-ray films of the thoracic and lumbar spine were taken at the same week as serum collection. The anterior, central, and posterior heights of each of the 13 vertebral bodies from Th4 to L4 were measured. A vertebral fracture was diagnosed if at least one of three height measurements along the length of the same vertebrae had decreased by >20% compared to the height of the nearest uncompressed vertebral body [23]. Multiple vertebral fractures were identified as two or more vertebral fractures. None of the subjects had a history of serious trauma.
BMD and Biochemical Measurements
BMD values of the whole body (W), lumbar spine (L), femoral neck (F), and one-third of the radius (1/3R) were measured by dual-energy X-ray absorptiometry (QDR-4500; Hologic, Waltham, MA). The same operator tested all of the subjects during the study to eliminate operator discrepancies. The coefficients of variation (precision) of measurements of the lumbar spine, femoral neck, and mid-radius by our methods were 0.9%, 1.7%, and 1.9%, respectively. Z score indicates deviation from the normal age- and sex-matched mean in standard deviation (SD).
After overnight fasting, serum was collected. Biochemical markers were measured by standard biochemical methods. Estimated glomerular filtration rate (eGFR) was calculated using the equation reported in the clinical practice guidelines for diagnosis and treatment of chronic kidney disease [24] as follows: 0.741 × 175 × age−0.203 × serum creatinine−1.154. HbA1c (normal range 4.3–5.8%) was determined by high-performance liquid chromatography (HPLC), as previously described [25–27]. Bone-specific alkaline phosphatase (BAP) in serum and C-peptide in urine pooled for 24 hours (uC-peptide) were measured by enzyme immunoassay (EIA) and chemiluminescent EIA (CLEIA), respectively. Serum osteocalcin and urinary N-terminal cross-linked telopeptide of type-I collagen (uNTX) were measured by radioimmunoassay (RIA) and enzyme-linked immunosorbent assay (ELISA). Total adiponectin and high–molecular weight (HMW) adiponectin were measured by ELISA kits (Otsuka Pharmaceuticals, Tokyo, Japan, and Fujirebio, Tokyo, Japan, respectively) as indicated by the manufacturers. The coefficients of variation of measurements of total adiponectin and HMW adiponectin by each ELISA kit were 3.1% and 2.0%, respectively.
Statistical Analysis
Data were expressed as mean ± SD. Because serum total and HMW adiponectin showed a markedly skewed distribution, logarithmic (log) transformation of these values was carried out before performing correlation and regression analysis. Statistical significance between two groups was determined using the Mann–Whitney U-test. Simple, multiple, and logistic regression analyses were performed using the statistical computer program StatView (Abacus Concepts, Berkely, CA). P < 0.05 was considered significant.
Results
Baseline Characteristics of Subjects and Comparison of Parameters Among Those with and without Vertebral Fractures
Baseline characteristics of subjects are shown in Table 1. We compared these parameters among subjects with a vertebral fracture, with multiple vertebral fractures, and without vertebral fractures and found no significant differences among them (data not shown).
Relationship of BMI, HbA1c, and uC-Peptide Versus BMD, Bone Metabolic Markers, and Serum Adiponectin Levels
We selected BMI, HbA1c, and uC-peptide as markers for obesity, hyperglycemia, and endogenous insulin secretion, respectively. Since our simple regression analysis showed that BMI, HbA1c, and uC-peptide were affected by age and renal function (data not shown), multiple regression analyses were performed with BMI, HbA1c, and uC-peptide adjusted for age, duration of diabetes, and eGFR as independent variables versus BMD at each skeletal site, bone metabolic markers, or serum adiponectin levels as dependent variables (Table 2). BMI was significantly and positively correlated with W-, L-, and F-BMD (P = 0.0070, P = 0.0128, and P < 0.0001, respectively) but not with 1/3R-BMD. BMI was also significantly and negatively correlated with osteocalcin, uNTX, and log(total adiponectin) (P = 0.0070, P = 0.0002, and P = 0.0359, respectively) and tended to be negatively correlated with log(HMW adiponectin). HbA1c was significantly and negatively correlated with osteocalcin (P = 0.0022) but not with BMD at any site, any other bone metabolic markers, or serum adiponectin levels. On the other hand, uC-peptide was not correlated with BMD at any site or any bone metabolic markers, except for a negative correlation with log(total adiponectin).
Comparison of Various Variables Among Four Groups Classified by BMI and HbA1c
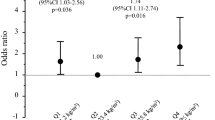
Next, since these results showed that BMI and HbA1c, but not uC-peptide, were significantly and independently associated with serum osteocalcin levels, we compared demographic and biochemical parameters including bone metabolic markers and serum adiponectin concentrations as well as BMD values at each skeletal site among four groups classified by BMI and HbA1c (group LL BMI < 24 and HbA1c < 9, group LH BMI < 24 and HbA1c ≧ 9, group HL BMI ≧ 24 and HbA1c < 9, group HH BMI ≧ 24 and HbA1c ≧ 9) (Table 3). There were no significant differences in age or duration of diabetes among the groups. Absolute values and Z scores of L-BMD in group HL and F-BMD in groups HL and HH were significantly higher than those in group LH (at least P < 0.05). Absolute values of L-BMD in group HL and absolute values and Z score of F BMD in group HH were significantly higher than those in group LL (at least P < 0.05). Serum osteocalcin level in group HH was significantly lower than that in group LL (P < 0.01). Moreover, uNTX in groups HL and HH were significantly lower than those in groups LL and LH (at least P < 0.05). Serum levels of total and HMW adiponectins in group HH were lower than those in group LH (P < 0.05), and HMW adiponectin in group HL was lower than that in group LH (P < 0.05). The prevalence of vertebral fractures was highest in group HH despite sufficient BMD.
Multivariate logistic regression analysis was performed with the presence of a vertebral fracture or multiple vertebral fractures as a dependent variable and each group adjusted for age, duration of diabetes, uC-peptide, and eGFR as independent variables (Table 4). Group HH was significantly associated with the presence of a vertebral fracture and multiple vertebral fractures (P = 0.0440 and P = 0.0354, respectively).
Discussion
In this study, combination of obesity with poor glycemic control in men with type 2 diabetes was significantly associated with the presence of vertebral fractures in spite of sufficient BMD. Multiple regression analysis showed that BMI was negatively correlated with serum osteocalcin and uNTX levels and that HbA1c was negatively correlated with serum osteocalcin level. Moreover, these bone metabolic markers were lower in the group with higher BMI and higher HbA1c (HH group), suggesting that low bone turnover, especially suppression of bone formation, may cause bone fragility that is not defined by BMD and may be linked to vertebral fractures in the obese and hyperglycemic group.
The present study showed that HbA1c level was not correlated with BMD and was not different between patients with or without vertebral fractures. In contrast, HbA1c level was negatively correlated with serum osteocalcin level but not with BAP. Although a few studies demonstrated that glycemic control estimated by HbA1c was associated with BMD or bone fractures [5, 8], meta-analysis of accumulating studies has shown that HbA1c level was not significantly associated with BMD or fracture incidence [6]. In previous studies, we have also shown that HbA1c levels in postmenopausal women with type 2 diabetes were not significantly different between those with and without vertebral fractures [25–27]. On the other hand, several studies have indicated that hyperglycemia induced a low turnover bone with osteoblast dysfunction and caused suppression of the serum osteocalcin level [28, 29]. Gerdhem et al. [29] showed that serum osteocalcin level, but not BAP, was lower in diabetic women after correction for covariance of body weight and serum creatinine. Okazaki et al. [30] showed that serum osteocalcin level was low before treatment and elevated after treatment of diabetes, while BAP was reduced. Previous in vitro studies have shown that chronic hyperglycemia increased the activity and expression of alkaline phosphatase while it decreased osteocalcin expression and cellular calcium uptake [31], explaining the discrepancy in serum levels of osteocalcin and BAP in the clinical studies.
Obesity is known to suppress both bone formation and resorption and to be positively associated with BMD. A large clinical study on Danish postmenopausal women showed that subjects in the highest tertiles of percentage of body fat or BMI had up to 12% higher BMD at baseline and a more than twofold lower 2-year bone loss compared with women in the lowest tertiles [32]. Women with a higher percentage of body fat or BMI had lower baseline levels of uNTX and serum osteocalcin [32]. On the other hand, weight loss over 6–8 months resulted in increases in indices of bone resorption marker expression, deoxypyridinoline and hydroxyproline, of up to 50% [33], suggesting that body weight directly influenced bone resorption and bone turnover. The present findings also showed that BMI was strongly and negatively correlated with serum osteocalcin and uNTX and strongly and positively correlated with BMD. These findings suggest that obesity induces low bone turnover through suppression of both osteoblastic and osteoclastic activities. Combination of hyperglycemia with obesity could enhance the suppression of serum osteocalcin level and may accelerate reduction in bone formation rate.
Adipocytokines, which are secreted from adipocytes, are known to influence bone metabolism. Adiponectin is one of the adipocytokines specifically and highly expressed in visceral, subcutaneous, and bone marrow fat depots [34]. Several studies have demonstrated that circulating adiponectin concentrations are decreased in patients with obesity, diabetes, and cardiovascular disease [35] and inversely associated with obesity [35, 36] as well as insulin-resistance parameters [36]. Recently, we and other researchers have shown that cultured osteoblasts have an adiponectin receptor and that their proliferation, differentiation, and mineralization are enhanced by adiponectin, showing that adiponectin could stimulate bone formation [37]. In animal studies, Oshima et al. [38] showed that adiponectin adenovirus–treated mice increased trabecular bone mass in vivo and that administration of adiponectin enhanced bone formation and suppressed bone resorption in vitro. Moreover, in a clinical study, Richards et al. [39] showed that serum adiponectin was positively correlated with serum osteocalcin in a large population of women. Thus, adiponectin appears to enhance osteoblastogenesis and bone formation, and reduction in its plasma concentration induced by combination of obesity with hyperglycemia may cause suppressed bone formation. We actually observed that serum adiponectin concentrations were lowest in the group with higher BMI and higher HbA1c (HH group), suggesting that serum hypoadiponectinemia might be involved in the present findings of low turnover bone in patients with obesity and hyperglycemia.
Although circulating insulin is considered to stimulate osteoblastogenesis and enhance bone formation, the present study showed that uC-peptide, as a surrogate marker for residual insulin secretion, was not significantly associated with BMD, bone metabolic markers, or serum adiponectin levels in men with type 2 diabetes. We also found that its level was not different between patients with or without vertebral fractures. These findings are consistent with our previous ones in women with type 2 diabetes, in which there were no associations between serum fasting C-peptide and BMD, bone metabolic markers, or vertebral fractures [24–26]. However, subjects in these studies had received several treatments including insulin administration. Therefore, we should be careful when making conclusions about the relationship between capacity of residual insulin secretion and bone metabolism.
Previous studies showed that hyperglycemia and AGEs could impair parathyroid hormone (PTH) secretion [40–42] and that vitamin D metabolism may also be disturbed by hyperglycemia and insulin deficiency [43–45]. Thus, impaired PTH and vitamin D metabolism might be involved in low turnover bone in diabetes, although we did not measure serum PTH and vitamin D levels in this study. Further studies are needed to investigate whether these hormone levels are associated with bone fractures in type 2 diabetes.
This study has some limitations. First, the sample size was not large enough to make definite conclusions. Second, we analyzed only subjects who visited Shimane University Hospital, a tertiary center, for evaluation or treatment of diabetes mellitus and osteoporosis. Therefore, the patients enrolled in this study might have relatively severe states of the disorders and might not be representative of Japanese men with the disorders. Consequently, assessment of larger numbers of patients is necessary to determine the usefulness of BMI and HbA1c for predicting the risk of vertebral fractures. Third, the subjects in this study were only Japanese. The capacity of insulin secretion and the degree of obesity in Asians are known to be different from those of Western people [46]. Therefore, we need to investigate whether or not our findings are universal. Finally, longitudinal studies are needed to investigate whether the treatments of both obesity and diabetes can improve bone fragility in type 2 diabetes.
In conclusion, we found that BMI was negatively associated with serum osteocalcin and uNTX and positively associated with BMD, while HbA1c level was negatively correlated with serum osteocalcin but not with BMD in diabetic men. Although BMI or HbA1c per se are not associated with the presence of vertebral fractures, the combination of higher values of both parameters was a risk factor for vertebral fractures in spite of sufficient BMD. Reduced bone turnover and resultant impairment in bone quality in patients with obesity and hyperglycemia may be involved in this observation.
References
Barrett-Connor E, Holbrook TL (1992) Sex differences in osteoporosis in older adults with non-insulin-dependent diabetes mellitus. JAMA 268:3333–3337
Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA (1999) Mortality after all major types of osteoporotic fracture in men and women an observational study. Lancet 353:878–882
Cauley JA, Thompson DE, Ensrud KC, Scott JC, Black D (2000) Risk of mortality following clinical fractures. Osteoporos Int 11:556–561
Center JR, Bliuc D, Nguyen TV, Eisman JA (2007) Risk of subsequent fracture after low-trauma fracture in men and women. JAMA 297:387–394
Ivers RQ, Cumming RG, Mitchell P, Peduto AJ (2001) Diabetes and risk of fracture: the Blue Mountains Eye Study. Diabetes Care 24:1198–1203
Vestergaard P (2007) Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes-a meta-analysis. Osteoporos Int 18:427–444
Lipscombe LL, Jamal SA, Booth GL, Hawker GA (2007) The risk of hip fractures in older individuals with diabetes: a population-based study. Diabetes Care 30:835–841
Strotmeyer ES, Cauley JA, Schwartz AV, Nevitt MC, Resnick HE, Bauer DC, Tylavsky FA, de Rekeneire N, Harris TB, Newman AB (2005) Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: the health, aging, and body composition study. Arch Intern Med 165:1612–1617
Ogawa N, Yamaguchi T, Yano S, Yamauchi M, Yamamoto M, Sugimoto T (2007) The combination of high glucose and advanced glycation end-products (AGEs) inhibits the mineralization of osteoblastic MC3T3-E1 cells through glucose-induced increase in the receptor for AGEs. Horm Metab Res 39:871–875
Inaba M, Terada M, Koyama H, Yoshida O, Ishimura E, Kawagishi T, Okuno Y, Nishizawa Y, Otani S, Morii H (1995) Influence of high glucose on 1,25-dihydroxyvitamin D3-induced effect on human osteoblast-like MG–63 cells. J Bone Miner Res 10:1050–1056
Ensrud KE, Lipschutz RC, Cauley JA, Seeley D, Nevitt MC, Scott J, Orwoll ES, Genant HK, Cummings SR (1997) Body size and hip fracture risk in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Am J Med 103:274–280
Johnell O, O’Neill T, Felsenberg D, Kanis J, Cooper C, Silman AJ (1997) Anthropometric measurements and vertebral deformities. European Vertebral Osteoporosis Study (EVOS) Group. Am J Epidemiol 146:287–293
Felson DT, Zhang Y, Hannan MT, Anderson JJ (1993) Effects of weight and body mass index on bone mineral density in men and women: the Framingham Study. J Bone Miner Res 8:567–573
Tremollieres FA, Pouilles JM, Ribot C (1993) Vertebral postmenopausal bone loss is reduced in overweight women: a longitudinal study in 155 early postmenopausal women. J Clin Endocrinol Metab 77:683–686
Hickman J, McElduff A (1989) Insulin promotes growth of the cultured rat osteosarcoma cell line UMR-106–01: an osteoblast-like cell. Endocrinology 124:701–706
McCarthy TL, Centrella M, Canalis E (1989) Insulin-like growth factor (IGF) and bone. Connect Tissue Res 20:277–282
Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, Boyce B, Zhao M, Gutierrez G (1999) Stimulation of bone formation in vitro and in rodents by statins. Science 286:1946–1949
Cornish J, Callon KE, Reid IR (1996) Insulin increases histomorphometric indices of bone formation in vivo. Calcif Tissue Int 59:492–495
Reid IR, Evans MC, Cooper GJ, Ames RW, Stapleton J (1993) Circulating insulin levels are related to bone density in normal postmenopausal women. Am J Physiol Endocrinol Metab 265:E655–E659
Reid IR (2002) Relationships among body mass, its components, and bone. Bone 31:547–555
Abrahamsen B, Rohold A, Henriksen JE, Beck-Nielsen H (2000) Correlations between insulin sensitivity and bone mineral density in nondiabetic men. Diabet Med 17:124–129
Leidig-Bruckner G, Ziegler R (2001) Diabetes mellitus a risk for osteoporosis? Exp Clin Endocrinol Diabetes 109:S493–S514
Genant HK, Jergas M, Palermo L, Nevitt M, Valentin RS, Black D, Cummings SR (1996) Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res 11:984–996
Japanese Society of Nephrology (2007) Clinical practice guidelines for diagnosis and treatment of chronic kidney disease [in Japanese]. Jpn J Nephrol 49:755–870
Yamamoto M, Yamaguchi T, Yamauchi M, Kaji H, Sugimoto T (2007) Bone mineral density is not sensitive enough to assess the risk of vertebral fractures in type 2 diabetic women. Calcif Tissue Int 80:353–358
Kanazawa I, Yamaguchi T, Yamamoto M, Yamauchi M, Yano S, Sugimoto T (2007) Serum insulin-like growth factor-I is associated with the presence of vertebral fractures in postmenopausal women with type 2 diabetes mellitus. Osteoporos Int 18:1675–1681
Yamamoto M, Yamaguchi T, Yamauchi M, Yano S, Sugimoto T (2008) Serum pentosidine levels are positively associated with the presence of vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab 93:1013–1019
Verhaeghe J, Suiker AM, Nyomba BL, Visser WJ, Einhorn TA, Dequeker J, Bouillon R (1989) Bone mineral homeostasis in spontaneously diabetic BB rats. II. Impaired bone turnover and decreased osteocalcin synthesis. Endocrinology 124:573–582
Gerdhem P, Isaksson A, Akesson K, Obrant KJ (2005) Increased bone density and decreased bone turnover, but no evident alteration of fracture susceptibility in elderly women with diabetes mellitus. Osteoporos Int 16:1506–1512
Okazaki R, Totsuka Y, Hamano K, Ajima M, Miura M, Hirota Y, Hata K, Fukumoto S, Matsumoto T (1997) Metabolic improvement of poorly controlled noninsulin-dependent diabetes mellitus decreases bone turnover. J Clin Endocrinol Metab 82:2915–2920
Botolin S, McCabe LR (2006) Chronic hyperglycemia modulates osteoblast gene expression through osmotic and non-osmotic pathways. J Cell Biochem 99:411–424
Ravn P, Cizza G, Bjarnason NH, Thompson D, Daley M, Wasnich RD, McClung M, Hosking D, Yates AJ, Christiansen C (1999) Low body mass index is an important risk factor for low bone mass and increased bone loss in early postmenopausal women. Early Postmenopausal Intervention Cohort (EPIC) study group. J Bone Miner Res 14:1622–1627
Ricci TA, Heymsfield SB, Pierson RN, Stahl T, Chowdhury HA, Shapses SA (2001) Moderate energy restriction increases bone resorption in obese postmenopausal women. Am J Clin Nutr 73:347–352
Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA (2001) Hypoadiponectemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 86:1930–1935
Matsubara M, Maruoka S, Katayose S (2002) Inverse relationship between plasma adiponectin and leptin concentrations in normal-weight and obese women. Eur J Endocrinol 147:173–180
Ryan AS, Berman DM, Nicklas BJ, Shiha M, Gingerich RL, Meneilly GS, Egan JM, Elahi D (2003) Plasma adiponectin and leptin levels, body composition, and glucose utilization in adult women with wide ranges of age and obesity. Diabetes Care 26:2383–2388
Kanazawa I, Yamaguchi T, Yano S, Yamauchi M, Yamamoto M, Sugimoto T (2008) Adiponectin and AMP kinase activator stimulate proliferation, differentiation, and mineralization of osteoblastic MC3T3–E1 cells. BMC Cell Biol 8:51–62
Oshima K, Nampei A, Matsuda M, Iwaki M, Fukuhara A, Hashimoto J, Yoshikawa H, Shimomura I (2005) Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem Biophys Res Commun 331:520–526
Richards JB, Valdes AM, Burling K, Perks UC, Spector TD (2007) Serum adiponectin and bone mineral density in women. J Clin Endocrinol Metab 92:1517–1523
Sugimoto T, Ritter C, Morrissey J, Hayes C, Slatopolsky E (1990) Effects of high concentrations of glucose on PTH secretion in parathyroid cells. Kidney Int 37:1522–1527
Kawagishi T, Morii H, Nakatsuka K, Sasao K, Kawasaki K, Miki T, Nishizawa Y (1991) Parathyroid hormone secretion in diabetes mellitus. Contrib Nephrol 90:217–222
Yamamoto T, Ozono K, Miyauchi A, Kasayama S, Kojima Y, Shima M, Okada S (2001) Role of advanced glycation end products in adynamic bone disease in patients with diabetic nephropathy. Am J Kidney Dis 38:S161–S164
Ikeda K, Matsumoto T, Morita K, Yamato H, Takahashi H, Ezawa I, Ogata E (1987) The role of insulin in the stimulation of renal 1,25-dihydroxyvitamin D synthesis by parathyroid hormone in rats. Endocrinology 121:1721–1726
Pietschmann P, Schernthaner G, Woloszczuk W (1988) Serum osteocalcin levels in diabetes mellitus: analysis of the type of diabetes and microvascular complications. Diabetologia 31:892–895
Scragg R, Holdaway I, Singh V, Metcalf P, Baker J, Dryson E (1995) Serum 25-hydroxyvitamin D3 levels decreased in impaired glucose tolerance and diabetes mellitus. Diabetes Res Clin Pract 27:181–188
Fujimoto WY (1996) Overview of non-insulin-dependent diabetes mellitus (NIDDM) in different population groups. Diabet Med 13:S7–S10
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanazawa, I., Yamaguchi, T., Yamamoto, M. et al. Combination of Obesity with Hyperglycemia is a Risk Factor for the Presence of Vertebral Fractures in Type 2 Diabetic Men. Calcif Tissue Int 83, 324–331 (2008). https://doi.org/10.1007/s00223-008-9178-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-008-9178-6