Abstract
The prefrontal cortex (PFC) plays an important role in several cognitive functions, such as planning, decision making, and social behavior. We previously reported that periodontal sensory input significantly increases PFC activity during the motor task of maintaining occlusal (biting) force. However, the relationships between periodontal sensation, PFC activity, and the performance of motor tasks have not been evaluated in detail. Therefore, using functional near-infrared spectroscopy, we investigated PFC activity by monitoring changes in cerebral blood flow (CBF) to specific areas of the PFC that corresponded to changes in occlusal force generated during four different biting tasks: (1) occlusion with the central incisor with an interocclusal distance of 5 mm (BI-5 mm); or (2) 10 mm (BI-10 mm); (3) occlusion with the first molars with an interocclusal distance of 5 mm (BM-5 mm), or (4) 10 mm (BM-10 mm). Occlusion of molars generated increased PFC regional CBF as the interocclusal distance decreased (BM-10 mm vs BM-5 mm). No significant differences in CBF during occlusion of incisors were found when comparing 5 mm and 10 mm intercostal distances (BI-5 mm vs BI-10 mm). The mean occlusal force generated by BM-5 mm occlusion was significantly lower than that generated by BM-10 mm occlusion. Taken together, our results suggest that the PFC decreases efferent signaling to motor units, to reduce occlusal force generated when periodontal sensation, which is greater when the interocclusal distance is reduced, is primarily responsible for maintaining occlusal force in the absence of sensations from the temporomandibular joint and muscle spindles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prefrontal cortex (PFC) is located in the anterior portion of the cerebral cortex and integrates sensory information from association areas located in the parietal, temporal, and occipital lobes, and within the limbic system (Miller and Choen 2001; Corbetta and Shulman 2002; Kimoto et al. 2011). The PFC plays an important role in motivational and cognitive control processes, such as planning, decision making, and social behavior, and plays a central role in executive functions (Pinti et al. 2015). The relationship between modulations of PFC activity and motivational and cognitive control processes has been reported by several clinical researchers. Damasio et al. (1994) reported the famous case of Phineas Gage who, after an industrial accident, had PFC lesions and become irreverent and capricious. He also exemplified deficits in decision making, cognitive, social, and behavioral functions. Sabri et al. (1999) reported that dementia, or other neuropsychological deficits, are reflected exclusively by functional imaging parameters (regional CBF and regional glucose metabolism) in patients with proven cerebral microangiopathy. Hornberger et al. (2014) reported that insight loss, which is a major feature of frontotemporal dementia, correlated with ventromedial and frontopolar prefrontal atrophy across a large cohort of neurodegenerative patients, including frontotemporal dementia and Alzheimer’s disease patients. Matsuo et al. (2003) investigated the relationship between alterations in the hemodynamic response of the PFC during a verbal fluency task, and memory function in a patient with post-traumatic stress disorder. The authors reported a significant correlation between the total hemoglobin in the PFC and the Wechsler Memory Scale score. Moreover, an aging population has led to an exponential increase in the number of patients with dementia. According to the Alzheimer’s Disease International report (2015), this number is estimated to reach 131.5 million by 2050. Thus, it is particularly important to better understand the functions of the PFC, which underlies normal cognitive functioning.
Previous work has also identified an association between cognitive function and physical activity. For example, Weuve et al. (2004) reported that higher levels of physical activity were associated with better cognitive performance and less cognitive decline in women over the age of 70. Similarly, a prospective cohort study from Abbott et al. (2004) reported that physically capable, elderly men, who walk on a regular basis, are less likely to experience an accelerated cognitive decline and have a reduced risk of dementia. Yaffe et al. (2001) also reported that elderly people (aged 65 and above), with greater baseline physical activity, such as increased walking distance per week, were less likely to experience cognitive declines over a 6- to 8-year follow-up. In addition, many epidemiological studies have reported a relationship between cognitive and oral functions. For example, Yamamoto et al. (2012) reported, over a 4-year follow-up, that incomplete mastication, because of poor dental health (missing teeth, etc.), was associated with a higher risk of dementia in populations over 65 years. Kaye et al. (2010) and Batty et al. (2013) reported that the risk of cognitive decline increased as more teeth, which are essential for mastication, were lost. However, it is not yet clear which aspect of mastication, or tooth loss, underlies the significant association with cognitive function, or whether these factors are also associated with PFC activity.
In our previous work, we focused on the relationship between PFC activity and sensory integration during a motor task; we found that periodontal afferent input significantly affected PFC activity during maintenance of occlusal force, with or without local anesthesia, which can eliminate sensory information from the periodontal ligament (Higaki et al. 2016). However, the relationship between PFC activity and sensory information from the periodontal ligament, or resulting performance of a motor task, has not yet been evaluated in detail. In the present study, we focused on the differences in distribution density of periodontal mechanoreceptors in the incisors and molars, and differences of significant sensory receptors according to the interocclusal distance. The hypothesis of this study was that PFC activity during a biting task would be different when biting with incisors and molars based on the interocclusal distance. The relationship between cognitive and oral functions has been suggested by many epidemiological studies. Our work here provides proof-of-concept of this relationship and serves to clarify this point raised in brain research.
The purpose of the present study was to investigate the effect of different interocclusal distances, during occlusion of incisors and molars, on PFC activity, and performance of a motor task requiring maintenance of occlusal force. We additionally sought to clarify the effect of PFC activity on this motor task.
Materials and methods
Participants
Eleven young, healthy Japanese participants (7 males and 4 females with a mean age of 28.0 ± 3.7 years) attending the Tokushima University of Japan, volunteered to participate in this study. All participants had normal dentitions without any stomatognathic, or central nervous system complications. This study was conducted with the approval of the Ethics Committee of the Tokushima University Hospital (No. 1780) and all experiments were carried out in accordance with the approved guidelines.
PFC activation
PFC activation was evaluated using a wearable functional near-infrared spectroscopy (fNIRS) device that measured cerebral blood flow (CBF). The fNIRS device measures relative changes in the concentration of oxy-hemoglobin using light attenuation at two wavelengths of 705 nm and 830 nm, which easily pass through skin, tissue, and bone. A total of 16 measuring probes were used in the study, out of which 10 were spaced at intervals of 30 mm and covered three measurement areas: Brodmann’s area 10, and the right and left side of Brodmann’s area 46. Brodmann’s area 10 is located across the frontal pole within the frontal lobe, and Brodmann’s area 46 is divided into right and left sides, dorsolateral to the PFC.
Experimental task and protocol
The experimental task involved maintaining occlusal force within an instructed range under three different external feedback conditions and was based on a study by Higaki et al. (2016). Figure 1 shows the experimental apparatus used. We tested either the left side central incisors, or first molars. Participants were seated on a chair, in a relaxed position, and asked to bite down on an occlusal force transducer with both upper and lower target teeth engaged and to maintain an instructed occlusal strength value. The occlusal force transducer was modified using an auto-curing acrylic resin: a commercial load cell was fitted to the occlusal surface to accommodate the bite of the upper and lower target teeth. Signals from the occlusal force transducer were digitized using a digital data acquisition device with an indicator and transferred to a computer.
Measurements were performed in a sound-proof room. Any external disturbances were minimized, and room lighting and temperature were kept constant. Participants were instructed to continuously bite the occlusal force transducer with a force of 27.5 ± 2.5 N (instructed range) for 30 s. Before the experimental task, participants practiced maintaining occlusal force for 2 min, while watching the occlusal force value displayed on the indicator.
All participants completed this same task under three different external feedback conditions (participants were allowed to rest for 1 min between each task, and the order of conditions was randomized). External feedback conditions provided information to guide participants in maintaining occlusal force. Three feedback conditions were used: (1) visual feedback using green and red LED lights. A green light was illuminated to indicate if the measured occlusal force value was within the instructed range, and a red light when it was not; (2) auditory feedback that consisted of sounding a buzzer when occlusal force left the instructed range; and (3) no external feedback information was provided. A series of measurements related to CBF in Brodmann’s areas were taken as these tasks were performed.
In addition, we modified the occlusal force transducer to measure four biting conditions defined as follows: (1) occlusion with the central incisors with an interocclusal distance of 5 mm (BI-5 mm); or (2) 10 mm (BI-10 mm); (3) occlusion with the first molars with an interocclusal distance of 5 mm (BM-5 mm), or (4) 10 mm (BM-10 mm). Tasks under a single biting condition were repeated 4 times under each external feedback condition (12 tasks total). Additionally, each biting condition was conducted on a separate day.
Data analysis
Data from the fNIRS and occlusal force transducer were synchronized and analyzed. The time resolution of both signals was 200 ms. The mean CBFs for 10 s immediately after measurement were defined as the baseline and used to correct data obtained during each task. The changes in CBF at task completion were calculated by subtracting the CBF at task onset. CBF values were analyzed in accordance with our previous study method (Higaki et al. 2016). That is, regional CBF values were calculated using average probe data from each area, as follows: probes 7, 8, and 9 for the left side of Brodmann’s area 46; probes 11, 12, 13, and 14 for Brodmann’s area 10; and probes 15, 16, and 17 for the right side of Brodmann’s area 46. The mean occlusal force from 10 to 30 s after task onset was calculated and compared between biting conditions and feedback conditions to eliminate early adjustments, such as rapid force rising, that occurred before reaching a constant occlusal force. The Wilcoxon signed-rank test was used to compare CBF in the PFC that occurred under different biting conditions. All statistical analyses were conducted using SPSS® version 24.0 software (IBM Corp. Armonk, NY, USA), and significance was defined as p < 0.05.
Results
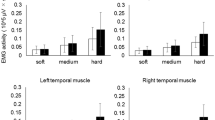
Figure 2 shows typical topographical patterns of CBF in the PFC just after the completion of each task. In the BM-5 mm task, a strong increase in CBF was observed in almost all oral regions, regardless of the external feedback condition. In the BI-5 mm, BI-10 mm, and BM-10 mm tasks, an increase in CBF in almost all regions or partly decrease in CBF was observed.
Table 1 shows regional CBF changes upon completion of each task. The highest regional CBF values were observed during the BM-5 mm task; these were significantly higher than those recorded for the BM-10 mm task, and this trend was maintained in all three areas of the PFC measured. No significant differences were found between the BI-5 mm, BI-10 mm, and BM-10 mm biting conditions, regardless of the measurement area and the presence, or absence, of feedback information.
Table 2 shows the mean occlusal force values during each task. The mean occlusal force in the BM-5 mm task was lower than the instructed range, regardless of feedback information, and resulted in significantly lower values than those recorded during the BM-10 mm task (Visual feedback: p = 0.021, Auditory feedback: p = 0.05, No feedback: p = 0.026). In the biting task with incisors, no significant difference in occlusal force was found between the BI-5 mm and BI-10 mm biting conditions, regardless of the external feedback method used. However, the mean occlusal force in the tasks performed without feedback information did not reach the instructed range and showed significantly lower values than those tasks performed with feedback information.
Discussion
Several studies concerning periodontal sensation and brain activity, including in the PFC, have been reported. Tamura et al. (2002) reported that brain activity in the motor and premotor cortexes increased during teeth clenching. Takada and Miyamoto (2004) reported that activity in the dorsolateral and ventral PFC increased during a gum-chewing task, but not during a sham chewing task (i.e., just opening-closing movements of the jaw). In addition, Iida et al. (2010) reported that PFC activity, during a teeth-clenching task, was greater than that during a fist-clenching task. These reports were based on functional magnetic resonance imaging data, with which it is difficult to assess the quantitative relationship between task performance and brain activity, or to clarify how PFC activity is related to the task. In the present study, we tried to clarify how the PFC is related to occlusal force control by comparing PFC activity between various motor tasks in a more physiological state using fNIRS.
In the present study, regional CBF was increased in all three PFC areas during the BM-5 mm tasks, both with and without feedback information. However, the regional CBFs in the PFC were not increased in continuous biting with BM-5 mm when periodontal sensation around the molars was inhibited by local anesthetic, as found in our previous report. This present study also found that regional CBF in the PFC did not increase in the BM-10 mm, BI-5 mm, or BI-10 mm biting conditions. This suggests that afferent sensory input affects PFC activity. Compared to periodontal sensations, somatic sensations in the temporomandibular joint and muscle spindles of jaw-closing muscles are primarily responsible for maintaining occlusal force. Manly et al. (1952) reported that periodontal sensation, however, dominates maintenance of the occlusal force when the interocclusal distance is less than 5 mm. Conversely, Christensen and Morimoto (1977) reported that somatic sensations in the temporomandibular joint and muscle spindles are primarily responsible for maintaining occlusal force when the interocclusal distance is greater than 10 mm. Regarding the significance of sensations from the temporomandibular joint and muscle spindles during mastication, Hidaka et al. (1999) and Inoue et al. (1981) reported that the timing of the facilitatory masseteric response is primarily controlled by muscle spindles, while the magnitude of the facilitatory masseteric response is primarily controlled by muscle spindles, and partly by periodontal receptors. At times when there is weak jaw-closing muscle activity, in the early stages of occlusion, periodontal sensation ensures that the occlusal force is executed to firmly catch hold of food with the teeth (Goldberg 1971; Trulsson and Johansson 1994). Once the occlusal force reaches a certain threshold, the periodontal sensation inhibits the excessive contraction of jaw-closing muscles to prevent tissue destruction due to overstressing (Brodin et al. 1993; Türker et al. 1994). Basically, the main role of molars is to grind food and, in contrast, incisors play a more prominent role in cutting food. Thus, form reflects function, and the molars can exert a much greater occlusal force than the incisors due to the anatomical structure of gnathological region (Gosen 1974). Moreover, it has been reported that incisors have a greater distribution of mechanoreceptors in the periodontal ligament than molars (Maeda 1994). Therefore, the periodontal sensation in the molar might ensure that excessive occlusal force is not executed through PFC activity.
During a sensory-motor task, such as eating, mastication and occlusal force control are mainly regulated by the chewing central pattern generator (CPG) located in the medulla oblongata, downstream of the PFC (Lund et al. 1998; Lund and Kolta 2006). It has recently been suggested that the CPG is influenced by higher brain centers (Morquette et al. 2012). Peripheral sensory information is transmitted to the primary somatosensory cortex and subsequently processed in multiple association areas (Johansson et al. 1988; Morissette and Bower 1996). The processed information can then be transmitted to the motor cortex directly, or via the PFC. Here, we present a hypothesis that the PFC contributes to inhibition of excessive occlusal force executed to molars. The motor cortex might receive the processed information directly, and be able to control occlusal force based on the CPG, without being actively assisted or supervised by the PFC. This could occur when the periodontal sensation in the molar region is not primarily responsible for maintaining occlusal force and there is sufficient afferent sensation from the temporomandibular joint and muscle spindles, as in the BI-5 mm, BI-10 mm, and BM-10 mm conditions. In the BM-5 mm condition, the periodontal sensation in the molar region was primarily responsible for maintaining occlusal force, and there were fewer inputs from the temporomandibular joint and muscle spindles. In this condition, the PFC might act as a supervisory attentional system to ensure that excessive occlusal force is not executed to the teeth. The supervisory attentional system, proposed by Shallice (1982), is an executive monitoring system that controls contention scheduling, i.e., the process by which routine actions are selected and others are inhibited. Our finding of an average occlusal force level in the BM-5 mm condition, that was lower than the instructed range, supports our hypothesis.
Visual and auditory feedback information significantly affected CBF in the PFC in the BM-5 mm condition, compared with CBF in the task without feedback information. In our previous study, CBF in the PFC was higher when sensory integration was required to complete the task, i.e., between the periodontal sensation and feedback information (Higaki et al. 2016). Both of the findings above indicate that the PFC plays a significant role in sensory integration. However, we found no difference in CBF in the PFC, regardless of whether feedback information was used, when the periodontal sensation in the molar region was not primarily responsible for maintaining occlusal force in the task.
We used a task which required the participant to maintain constant occlusal force consciously in this study. Regarding the influence of a jaw motor task on changes in the central nervous system, several reports have been published that illustrate a motor task can affect central nervous system changes irrespective of the level of consciousness. For example, studies utilizing conscious motor tasks, such as by Iida et al. (2014), reported in a study using transcranial magnetic stimulation that a consciously repeated tooth-clenching task could trigger neuroplastic changes in the central nervous system. Moreover, Zhang et al. (2016), who also using transcranial magnetic stimulation, reported that a “Hold-and-split” task (participants hold and spit out a test tablet with their incisors) induced signs of neuroplastic changes in the corticomotor pathways related to the masseter muscle. Unconscious tasks, such as bruxism (teeth clenching/grinding) during sleep, have also been reported to trigger central nervous system responses, specifically PFC activity. Ikuta et al. (2019) suggested that sleep bruxism might be associated with a significant reduction in the excitability of corticomotor pathways related to the masseter muscle. However, in the above-mentioned reports, the relationship between jaw motor tasks and the PFC were not discussed. Further research on the PFC activity that occurs during repetitive jaw motor tasks, in addition to continuous jaw motor tasks, will be needed.
To conclude, our results suggest that the PFC contributes to inhibition of excessive occlusal force executed by the teeth when the occlusal force is maintained at the molars with an interocclusal distance of 5 mm, in which the periodontal sensation is primarily responsible for maintaining occlusal force and there are few afferent inputs from the temporomandibular joint and muscle spindles.
References
Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petrovitch H (2004) Walking and dementia in physically capable elderly men. JAMA 292(12):1447–1453
Alzheimer’s Disease International (2015) World Alzheimer report 2015 https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf. Accessed 19 Jan 2019
Batty GD, Li Q, Huxley R et al (2013) Oral disease in relation to future risk of dementia and cognitive decline: prospective cohort study based on the action in diabetes and vascular disease: preterax and diamicron modified-release controlled evaluation (ADVANCE) trial. Eur Psychiatry 28(1):49–52
Brodin P, Türker KS, Miles TS (1993) Mechanoreceptors around the tooth evoke inhibitory and excitatory reflexes in the human masseter muscle. J Physiol 464:711–723
Christensen J, Morimoto T (1977) Dimension discrimination at two different degrees of mouth opening and effect of an anesthesia applied to the periodontal ligaments. J Oral Rehabil 4(2):157–164
Corbetta M, Shulman GL (2002) Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3(3):201–215
Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR (1994) The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science 264(5162):1102–1105
Goldberg LJ (1971) Masseter muscle excitation induced by stimulation of periodontal and gingival receptors in man. Brain Res 32(2):369–381
Gosen AJ (1974) Mandibular leverage and occlusion. J Prosthet Dent 31(4):369–376
Hidaka O, Morimoto T, Kato T, Masuda Y, Inoue T, Takada K (1999) Behavior of jaw muscle spindle afferents during cortically induced rhythmic jaw movements in the anesthetized rabbit. J Neurophysiol 82(5):2633–2640
Higaki N, Goto T, Ichikawa T (2016) Periodontal tactile input activates the prefrontal cortex. Sci Rep 6:36893
Hornberger M, Yew B, Gilardoni S et al (2014) Ventromedial-frontopolar prefrontal cortex atrophy correlates with insight loss in frontotemporal dementia and Alzheimer’s disease. Hum Brain Mapp 35(2):616–626
Iida T, Kato M, Komiyama O et al (2010) Comparison of cerebral activity during teeth clenching and fist clenching: a functional magnetic resonance imaging study. Eur J Oral Sci 118(6):635–641
Iida T, Komiyama O, Obara R, Baad-Hansen L, Kawara M, Svensson P (2014) Repeated clenching causes plasticity in corticomotor control of jaw muscles. Eur J Oral Sci 122(1):42–48
Ikuta M, Iida T, Kothari M, Shimada A, Komiyama O, Svensson P (2019) Impact of sleep bruxism on training-induced cortical plasticity. J Prosthodont Res 63(3):277–282
Inoue H, Morimoto T, Kawamura Y (1981) Response characteristics an classification of muscle spindles of the masseter muscle in the cat. Exp Neurol 74(2):548–560
Johansson RS, Trulsson M, Olsson KA, Abbs JH (1988) Mechanoreceptive afferent activity in the infraorbital nerve in man during speech and chewing movements. Exp Brain Res 72(1):209–214
Kaye EK, Valencia A, Baba N, Spiro A 3rd, Dietrich T, Garcia RI (2010) Tooth loss and periodontal disease predict poor cognitive function in older men. J Am Geriatr Soc 58(4):713–718
Kimoto K, Ono Y, Tachibana A et al (2011) Chewing-induced regional brain activity in edentulous patients who received mandibular implant-supported overdentures: a preliminary report. J Prosthodont Res 55(2):89–97
Lund JP, Kolta A (2006) Generation of the central masticatory pattern and its modification by sensory feedback. Dysphagia 21(3):167–174
Lund JP, Kolta A, Westberg KG, Scott G (1998) Brainstem mechanisms underlying feeding behaviors. Curr Opin Neurobiol 8(6):718–724
Maeda T (1994) Sensory innervation of the periodontal ligament in the incisor and molar of the monkey, Macaca fuscata. An immunohistochemical study for neurofilament protein and glia-specific S-100 protein. Arch Histol Jpn 50(4):437–454
Manly RS, Pfaffman C, Lathrop DD, Keyser J (1952) Oral sensory thresholds of persons with natural and artificial dentition. J Dent Res 31(3):305–312
Matsuo K, Taneichi K, Matsumoto A et al (2003) Hypoactivation of the prefrontal cortex during verbal fluency test in PTSD: a near-infrared spectroscopy study. Psychiatry Res 124(1):1–10
Miller EK, Choen JD (2001) An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24:167–202
Morissette J, Bower JM (1996) Contribution of somatosensory cortex to responses in the rat cerebellar granule cell layer following peripheral tactile stimulation. Exp Brain Res 109(2):240–250
Morquette P, Lavoie R, Fhima MD, Lamoureux X, Verdier D, Kolta A (2012) Generation of the masticatory central pattern and its modulation by sensory feedback. Prog Neurobiol 96(3):340–355
Pinti P, Cardone D, Merla A (2015) Simultaneous fNIRS and thermal infrared imaging during cognitive task reveal autonomic correlates of prefrontal cortex activity. Sci Rep 5:17471
Sabri O, Ringelstein EB, Hellwig D et al (1999) Neuropsychological impairment correlates with hypoperfusion and hypometabolism but not with severity of white matter lesions on MRI in patients with cerebral microangiopathy. Stroke 30(3):556–566
Shallice T (1982) Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci 298(1089):199–209
Takada T, Miyamoto T (2004) A fronto-parietal network for chewing of gum: a study on human subjects with functional magnetic resonance imaging. Neurosci Lett 360(3):137–140
Tamura T, Kanayama T, Yoshida S, Kawasaki T (2002) Analysis of brain activity during clenching by fMRI. J Oral Rehabil 29:467–472
Trulsson M, Johansson RS (1994) Encoding of amplitude and rate of forces applied to the teeth by human periodontal mechanoreceptive afferents. J Neurophysiol 72(4):1734–1744
Türker KS, Brodin P, Miles TS (1994) Reflex responses of motor units in human masseter muscle to mechanical stimulation of a tooth. Exp Brain Res 100(2):307–315
Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F (2004) Physical activity, including walking, and cognitive function in older women. JAMA 292(12):1454–1461
Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K (2001) A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med 16:1703–1708
Yamamoto T, Kondo K, Hirai H, Nakade M, Aida J, Hirata Y (2012) Association between self-reported dental health status and onset of dementia: a 4-year prospective cohort study of older Japanese adults from the Aichi Gerontological Evaluation Study (AGES) Project. Psychosom Med 74(3):241–248
Zhang H, Kumar A, Kothari M et al (2016) Can short-term oral fine motor training affect precision of task performance and induce cortical plasticity of the jaw muscles? Exp Brain Res 234(7):1935–1943
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kishimoto, T., Goto, T. & Ichikawa, T. Prefrontal cortex activity induced by periodontal afferent inputs downregulates occlusal force. Exp Brain Res 237, 2767–2774 (2019). https://doi.org/10.1007/s00221-019-05630-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-019-05630-y