Abstract
When cognitive load is elevated during a motor task, cortical inhibition and reaction time are increased; yet, standing balance control is often unchanged. This disconnect is likely explained by compensatory mechanisms within the balance system such as increased sensitivity of the vestibulomotor pathway. This study aimed to determine the effects of increased cognitive load on the vestibular control of standing balance. Participants stood blindfolded on a force plate with their head facing left and arms relaxed at their sides for two trials while exposed to continuous electrical vestibular stimulation (EVS). Participants either stood quietly or executed a cognitive task (double-digit arithmetic). Surface electromyography (EMG) and anterior-posterior ground-body forces (APF) were measured in order to evaluate vestibular-evoked balance responses in the frequency (coherence and gain) and time (cumulant density) domains. Total distance traveled for anterior–posterior center of pressure (COP) was assessed as a metric of balance variability. Despite similar distances traveled for COP, EVS–medial gastrocnemius (MG) EMG and EVS–APF coherence and EVS–TA EMG and EVS–MG EMG gain were elevated for multiple frequencies when standing with increased cognitive load. For the time domain, medium-latency peak amplitudes increased by 13–54% for EVS–APF and EVS–EMG relationships with the cognitive task compared to without. Peak short-latency amplitudes were unchanged. These results indicate that reliance on vestibular control of balance is enhanced when cognitive load is elevated. This augmented neural strategy may act to supplement divided cortical processing resources within the balance system and compensate for the acute neuromuscular modifications associated with increased cognitive demand.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Standing balance relies on information stemming from vestibular, visual, proprioceptive, and cutaneous cues (Massion 1998; van der Kooij et al. 1999). These signals, along with cortical and cerebellar inputs, are integrated at the level of the vestibular nuclei in the brainstem in order to optimize a motor response for maintenance of upright balance (Cullen 2012). The vestibular system plays an especially crucial role in this integrated response, as it encodes for angular and linear accelerations of the head. This signaling not only evokes a whole-body balance response (Lund and Broberg 1983; Fitzpatrick et al. 1994), but also helps stabilize vision and head position (Wilson et al. 1995; Jahn et al. 2003), further augmenting postural control.
Considerable research has assessed the effects of sensory feedback on balance control (Day and Cole 2002; Day and Fitzpatrick 2005; Wardman et al. 2003). However, previous reports suggest that increased cognitive processing can also affect neuromuscular function and possibly standing balance (Kerr et al. 1985; Teasdale et al. 1993; Brown et al. 1999; McIlroy et al. 1999; Yardley et al. 2001; Holste et al. 2015). Serial processing limitations within the cortex are likely the reason that increased cognitive loads impair motor task performance (Pashler 1994; Bourke et al. 1996; Sigman and Dehaene 2008). Once the processing requirements elevated by a secondary cognitive task exceed this systemic limitation, the central nervous system must reallocate cortical resources in favor of one task, resulting in decreased performance of one or both tasks (Pashler 1994). Event-related potentials measured under dual-task conditions show that afferent somatosensory and efferent motor processing likely occur in parallel. However, coordination and integration of these signals, such as the complex sensorimotor processing required for balance control, are delayed (Pashler 1994; Sigman and Dehaene 2008). These previous findings support the use of a dual-task paradigm to assess the sensorimotor control of standing balance.
Previous research has determined that increased cognitive load induces neuromuscular alterations such as increased intra-cortical inhibition (Holste et al. 2015) and delayed plantar flexor electromyography (EMG) onset during reactive balance control (Teasdale et al. 1993; McIlroy et al. 1999). However, during quiet standing balance, increased cognitive load does not alter variability in center of pressure (COP) or center of mass (COM) displacement when normalized to anxiety and arousal (Kerr et al. 1985; Teasdale et al. 1993; Maki and McIlroy 1996). This disconnect may be explained by one or more compensatory mechanisms involved in the regulation of quiet standing balance. One such possibility is enhanced vestibular control of balance with increased cognitive load or divided attention.
Functionality of the vestibulomotor pathway can be probed noninvasively using electrical vestibular stimulation (EVS) (Nashner and Wolfson 1974; Lund and Broberg 1983; Fitzpatrick and Day 2004; Dakin et al. 2007). By stimulating over the mastoid processes, a low-amplitude current bypasses the vestibular end organs and directly modulates the firing of peripheral vestibular afferents (Goldberg et al. 1984; Kim and Curthoys 2004). In humans, this vestibular error signal (i.e., EVS) is interpreted by the balance system as a true destabilization of the head (Fitzpatrick and Day 2004; Day and Fitzpatrick 2005), and thus a counteractive whole-body balance response is generated in the direction of the anode (Lund and Broberg 1983; Mian and Day 2009; Dalton et al. 2017).
Electrical vestibular-evoked balance responses exhibit a biphasic pattern, consisting of two distinct peaks of short (~55 ms) and medium (~110 ms) latencies within the muscles of the lower limbs (Britton et al. 1993; Dakin et al. 2007; Luu et al. 2012). Britton et al. (1993) suggested that the short-latency response is representative of a vestibulospinal-propagated motor response, whereas the prolonged delay associated with the medium latency may be the result of a vestibular-evoked balance response propagated through a corticospinal or reticulospinal pathway (Britton et al. 1993). As such, the modulation of the medium-latency peak amplitude response (corrective balance response) with the addition of a cognitive task may be indicative of changes in the vestibular control of balance resulting from an elevated cognitive demand. When characterized in the frequency domain, there is some contribution from the entire vestibular operational bandwidth to both responses (Dakin et al. 2011). However, it is thought that the medium-latency response is comprised predominantly of lower frequencies (<~10 Hz), whereas the short-latency response is composed of higher frequencies (10–25 Hz) in postural muscles of the lower limbs (Dakin et al. 2007).
Elevating perception of postural threat has been shown to increase vestibular control of quiet balance. Specifically, peak short- and medium-latency EVS-evoked APF responses were significantly greater when perceived postural threat was manipulated by increasing standing height (Horslen et al. 2014). However, in this model, it cannot be discerned if this increase is due to cortical processes or increased sympathetic nervous system outflow. Regardless, the aforementioned results establish a relationship between the vestibular nuclei and higher-order brain structures. Similarly, when vestibular-evoked myogenic potentials (VEMP), evoked by short-duration tone bursts, were measured at height compared to ground level, VEMP amplitude was significantly increased (Naranjo et al. 2015, 2016). The VEMPs are similar to EVS-evoked potentials in that the vestibulomotor pathway is activated via an external stimulus. However, VEMPs activate the vestibular end organs rather than directly stimulating the vestibular afferents as with EVS. The demonstrated increases in VEMPs and EVS-evoked responses are likely not explained by spinal or peripheral modifications, as gain of the vestibulo-ocular reflex (VOR), which is not propagated via the spinal cord, also increases with elevated postural threat (Naranjo et al. 2016). Instead, these data implicate supraspinal alterations of the vestibulomotor pathway, possibly at the level of the vestibular nuclei.
Thus, the objective of the present study was to assess the effects of increased cognitive load on vestibular control of standing balance. We hypothesized that quiet standing with an elevated cognitive load would increase the reliance on the vestibular control of balance, demonstrated by a greater coherence and peak medium-latency amplitude, compared to standing with minimal cognitive load.
Methods
Participants
Five males and nine females (age: 23.7 ± 2.8 years; mass: 69.9 ± 12.8 kg; height: 173.3 ± 9.3 cm) with no known history of neurological disease or injuries participated in this study. Written and oral consent, as approved by the local institutional review board involving human subjects, was obtained from all participants prior to data collection. All procedures conformed to the Declaration of Helsinki.
Experimental overview
Following instrumentation of surface EMG, participants underwent a 30-s familiarization trial of EVS and subsequently three 180-s experimental trials. For one trial, participants stood quietly while completing a cognitive task consisting of double-digit addition and subtraction. This trial served as a control for cognitive task accuracy. For a second trial, participants received EVS while simultaneously completing the cognitive task. During a third trial, participants were exposed to EVS with no cognitive task. The order of the three trials was randomized for each participant and rest was given between trials as per their request.
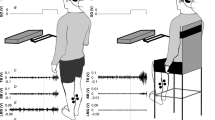
For all trials, participants stood upright on a force plate (OR6-5-2000, Advanced Mechanical Technology Inc., Watertown, MA, USA) with their medial malleoli touching (Fig. 1). In order to control for the effects of vision and environmental auditory cues on balance, participants wore a blindfold and earplugs. Participants stood relaxed with their hands at their sides and head rotated 90° to the left (towards the cathode). The head was tilted upward to orient Reid’s plane to ~19° from horizontal and the position was confirmed using an analog inclinometer. The purpose of this head orientation was to maximize the vestibular-evoked balance response in the sagittal plane about the ankle joints with the primary motor actions generated by the ankle dorsi and plantar flexors (Lund and Broberg 1983; Cathers et al. 2005; Day and Fitzpatrick 2005). Head orientation was maintained via a laser (VLM-635, Quarton Inc., Diamond Bar, CA, USA) affixed to the head and verbal cues from the investigators.
Experimental setup showing a head yaw angle of 90° and pitch angle of 19°. The electrical vestibular stimulation (EVS)-evoked postural response (determined as the sum of the vectors generated by all vestibular afferents) is aligned primarily in the sagittal plane with rotations about the ankles. Sample raw EVS, medial gastrocnemius (MG) electromyography (EMG), and anterior-posterior forces (APF) data segments are provided. Sample cumulant density (time domain) and coherence (frequency domain) functions for MG EMG with (black) and without (gray) elevated cognitive load are also provided. Values exceeding 95% confidence limits (black dashed lines) are considered statistically significant
For each trial, surface EMG (Blue Sensor M, M-00-S, Ambu A/S Ballerup, Denmark) was sampled from the right medial gastrocnemius (MG) (Dakin et al. 2007; Dalton et al. 2014; Dakin et al. 2016) and tibialis anterior (TA) (Dalton et al. 2014). Anterior–posterior ground-body forces (APF) (Pastor et al. 1993; Mian and Day 2009; Dalton et al. 2014) and total anterior–posterior COP displacement were also measured. All data were sampled at 2048 Hz.
Vestibular stimulation
A continuous current that varied randomly in both amplitude (±2.50 mA; root mean square (RMS) = 1.13 mA) and frequency (0–20 Hz) was generated using LabVIEW software (National Instruments, Austin, TX, USA) and delivered via an isolated bipolar constant current stimulator (±10 V input, ±10 mA output, DS5, Digitimer Ltd, Welwyn Garden City, UK) through carbon rubber electrodes (contact area: 9 cm2) coated in Spectra 360 electrode gel (Parker Laboratories, Fairfield, NJ, USA) over the mastoid processes. The electrodes were secured using Durapore tape (3 M, St. Paul, MN, USA) and an elastic bandage. EVS signals containing amplitudes and frequencies similar to those used here have been shown to evoke a significant postural reflex (Dakin et al. 2007; Luu et al. 2012; Forbes et al. 2013; Dalton et al. 2014). The randomization of these EVS waveform properties generates a continually dynamic balance response and thus allows for characterization of the vestibular-evoked balance response across a range of frequencies and amplitudes (Dakin et al. 2007; Mian and Day 2009; Reynolds 2010; Dalton et al. 2014).
Surface electromyography
Surface EMG was sampled from the right leg because EVS-evoked muscle responses are larger in the leg opposite the direction of head rotation (Britton et al. 1993; Dakin et al. 2007). Prior to electrode placement, the skin surface was cleaned with an alcohol swab. Electrodes were placed in line with the muscle fiber orientation in a bipolar arrangement (~2-cm center-to-center inter-electrode distance). A ground electrode was placed over the right medial malleolus. The MG EMG electrodes were placed centrally over the muscle belly, and the TA EMG electrodes were placed over the proximal portion of the muscle ~2 cm lateral to the anterior tibial border and ~7 cm distal to the tibial tuberosity. The EMG signals were pre-amplified (×1000; NL844, Digitimer Ltd, Welwyn Garden City, UK), amplified (×2 NL820A, Digitimer Ltd, Welwyn Garden City, UK) and band-pass-filtered (30–1000 Hz; NL136 and NL144, Digitimer Ltd, Welwyn Garden City, UK).
Anterior–posterior ground-body forces
Anterior–posterior ground-body forces were measured using a triaxial force plate (OR6-5-2000, Advanced Mechanical Technology Inc., Watertown, MA, USA). Force data were mean-removed and low-pass-filtered digitally (Butterworth filter, 30 Hz; MATLAB, MathWorks, Natick, MA, USA).
Cognitive task
Two random two-digit integers (10–99) were generated and randomly assigned to be added or subtracted (LabVIEW, National Instruments, Austin, TX, USA). Beginning with the onset of EVS, one equation was delivered verbally by an investigator every 10 s for a total of 18 questions per trial. Participants responded verbally and were not given any indication as to the accuracy of their responses until they completed all trials. Failure to respond within the allotted 10 s was recorded as an incorrect response. This cognitive task is a novel variation of math-based tasks used previously to alter quiet standing balance control (Stelmach et al. 1990; Vuillerme and Vincent 2006).
Data analysis
The APF and EMG signals were time-locked to the onset of EVS. The final 2 s for the APF and 1 s for EMG were removed for each trial to ensure that the EVS signal spanned all segments used for analysis. These data records (APF, 178 s and EMG, 179 s) were used to estimate the relationships between the EVS input and the electrophysiological [full-wave-rectified EMG (Dakin et al. 2014)] and kinetic (APF) output. Relationships were characterized in the time (cumulant density) and frequency (coherence and gain) domains using MATLAB (MathWorks, Natick, MA, USA) functions derived from a multivariate Fourier analysis (EMG: segment length = 1.0 s, resolution = 1.0 Hz; APF: segment length: 2.0 s, resolution: 0.5 Hz) (NeuroSpec 2.0: http://www.neurospec.org). Concatenated data from all participants were used for visualization of the cumulant density function and statistical analysis of coherence. All participants demonstrated significant medium-latency responses for EVS–APF relationships. Owing to technical issues, EVS–MG EMG was not reported for 1 participant. For EVS–TA EMG, medium-latency peak amplitudes did not exceed the 95% confidence intervals in five participants. These data were omitted from their respective statistical analyses (EVS–APF: n = 14; EVS–MG: n = 13; EVS–TA EMG: n = 9).
The cumulant density function reveals an association pattern between the EVS and outcome variables in the time domain. Amplitudes of each function were normalized by the product of the vector norms of the EVS signal and the outcome variable. This normalization likens the cumulant density function to a cross-correlation (−1 < r < 1) and allows for comparison of data regardless of output amplitude (Dakin et al. 2010; Luu et al. 2012).
Coherence estimates were derived for each participant to explore the frequency bandwidth of the EVS-evoked motor responses between dual and single-task groups. Coherence represents a measure of the linear relationship between the EVS and an outcome variable across a range of frequencies. For every frequency data point, coherence varies from 0 (no linear relationship) to 1 (perfect linear relationship) (Rosenberg et al. 1989; Halliday et al. 1995). To help describe significant coherence frequencies of the vestibular-evoked response, gain-frequency functions were calculated. For each muscle and APF, gain estimates were normalized to the mean gain at the lowest frequency (EMG: 1 Hz; APF: 0.5 Hz) and as such is a unit-less metric. The gain function represents the magnitude of the motor output as it relates to the input signal (EVS). It is also used to identify muscle-dependent filtering behavior; as the frequency increases, the gain function tends to decrease (Forbes et al. 2013). To compare elevated cognitive load with minimal cognitive load, point-wise 95% confidence limits were constructed for each condition (Fig. 3). When the gain 95% confidence limits did not overlap, the corresponding frequencies were considered statistically different.
Total distance traveled for anterior–posterior COP was calculated to assess balance variability between trials. Data were digitally down-sampled to 100 Hz and total distance travelled was determined as \(\sum {\left| {{\text{COP}}_{n} - {\text{COP}}_{n - 1} } \right|}\), where n is any given anterior–posterior COP data point.
Statistical analysis
To determine values that varied significantly from zero for coherence and cumulant density functions, 95% confidence limits were calculated from the total number of data segments per participant (Rosenberg et al. 1989; Halliday et al. 1995). To assess the main effect of cognitive load on EVS–EMG and EVS–APF cumulant density functions, participant’s peak short- and medium-latency cumulant density values were compared across the increased cognitive load and minimal cognitive load trials using paired t tests (SPSS 22.0, IBM, North Castle, NY, USA). A post hoc power calculation was performed on the differences between elevated and minimal cognitive load conditions for EVS–APF medium-latency peak amplitudes to ensure power was satisfactory to detect differences (1 − β = 0.90). The EVS–APF medium-latency peak amplitude response was chosen for the power analysis because it represents the net summation of all muscles pertaining to the vestibular-evoked balance response (Pastor et al. 1993; Mian and Day 2009). Additionally, differences between task condition for total anterior–posterior COP displacement as well as cognitive task performance with and without EVS were compared using paired t tests. Significance for all analyses was set at p < 0.05. Data are reported as means ± standard deviations.
Differences in coherence between minimal and elevated cognitive load conditions were assessed with the “Difference of Coherence” subroutine in NeuroSpec 2.0 (Rosenberg et al. 1989; Amjad et al. 1997). This analysis tests the assumption that the coherence estimates are equal within a normally distributed variance by comparing the standardized differences between coherence of the two trials and 95% confidence limits. Any frequencies where the standardized difference of coherence exceeded the 95% confidence limits were considered statistically different (Rosenberg et al. 1989; Amjad et al. 1997).
Results
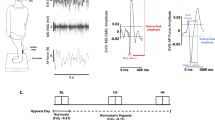
Medium-latency peak amplitudes were 13, 13, and 54% greater with elevated cognitive load compared to without for EVS–APF, EVS–MG, and EVS–TA, respectively (p < 0.05) (Fig. 2). There were no detectable differences between the conditions for short-latency peak amplitudes for any outcome measure (p > 0.05; Fig. 2).
Peak amplitude for short- (left) and medium-latency (right) responses for all outcome measures. Mean responses are depicted in black and individual responses are in gray. Medium-latency peak amplitudes were greater during the dual-task (DT; elevated cognitive load) compared with the single-task (ST; minimal cognitive load) for anterior–posterior forces (APF), medial gastrocnemius (MG), and tibialis anterior (TA) (* p < 0.05)
Significant EVS–APF coherence values spanned similar operational frequencies for both conditions (Fig. 3). With respect to EVS–EMG coherence estimates, significant frequencies occurred up to ~20 Hz (Fig. 3). While the operational bandwidth frequencies were not different between cognitive load conditions, the difference of coherence function depicted larger coherence values with elevated cognitive load compared to minimal cognitive load. These differences reached significance for EVS–APF at 3, 4, 6, and 8 Hz and EVS–MG EMG at 9 and 15 Hz (Fig. 3). The difference of coherence between conditions was not significant for EVS–TA EMG. Initially, the gain increased for both cognitive load conditions and peaked at frequencies ≤2 Hz for APF and MG EMG and declined towards lower values thereafter. For the elevated cognitive load condition, the gain function displayed values that were greater for EVS–APF (2 Hz) and EVS–MG EMG (5–7, 8–10, 13 and 18–21 Hz) than with minimal cognitive load. For EVS–TA EMG, gain was greater for frequencies ≤12 Hz, between 13 and 15 Hz, and between 16 and 19 Hz.
Vestibular-evoked frequency responses calculated from concatenated data from all participants for the elevated cognitive load condition (black) and minimal cognitive load condition (gray). Coherence functions (left column) reached significance for both cognitive load conditions such that values exceeded the 95% confidence limits (dashed black line) for anterior-posterior forces (APF), medial gastrocnemius (MG), and tibialis anterior (TA). The middle column depicts the difference of coherence estimates between the elevated cognitive load and minimal cognitive load conditions. Negative difference of coherence values indicate greater coherence during the elevated cognitive load condition compared to the minimal cognitive load condition; whereas positive values denote the opposite. Values exceeding 95% confidence limits (gray dashed lines) are considered statistically significant. Gain estimates (right column) were enhanced for multiple frequencies for MG and TA. The dark and light shaded areas represent point-wise 95% confidence limits for the elevated cognitive load and minimal cognitive load conditions, respectively. Cognitive load conditions were considered statistically different from each other when the confidence limits did not overlap
Mean total anterior–posterior COP displacement was 263.4 ± 141.7 cm (1.5 ± 0.8 cm/s) for the no cognitive task condition compared to 260.9 ± 141.6 cm (1.4 ± 0.8 cm/s) when performing the cognitive task (p = 0.42). Cognitive task accuracy was unchanged with and without EVS (p = 0.15). On average, participants responded to the cognitive task math questions with 63.5 ± 20.1% accuracy when standing while exposed to EVS and 68.8 ± 15.3% accuracy when standing without EVS.
Discussion
The aim of the present study was to assess the effects of increased cognitive load on the vestibular control of quiet standing. This overarching aim was accomplished by assessing the relationship between EVS and motor responses in the time (cumulant density) and frequency (coherence and gain) domains with and without a secondary cognitive task (mental arithmetic). While cognitive task accuracy was maintained regardless of the presence of EVS, the elevated and minimal cognitive task EVS–motor output relationships support our hypotheses, such that peak medium-latency amplitudes, EVS–APF and EVS–MG EMG coherence, and EVS–EMG gain functions were greater with increased cognitive load compared with minimal cognitive load. Further, short-latency peak amplitude was unchanged between conditions. Our results support the model that vestibular control of quiet standing balance may be subject to modification by higher-order brain structures involved in cognitive processing. Further, this augmented sensorimotor balance strategy may help to compensate for acute neuromuscular alterations associated with increased cognitive demand or divided attention, thus supplementing postural control under such conditions.
The cumulant density function represents an associative relationship between the EVS input and motor output. As such, a greater value for cumulant density represents an enhanced motor response to the same vestibular error signal. This time-dependent function for an EVS-evoked motor response is biphasic, and exhibits short- and medium-latency peaks (Nashner and Wolfson 1974; Iles and Pisini 1992; Britton et al. 1993; Fitzpatrick et al. 1994; Dakin et al. 2007). The timing and amplitude of both responses in the present study are consistent with those demonstrated previously (Dakin et al. 2010; Dalton et al. 2014; Horslen et al. 2014).
Compared to quiet standing alone, the medium-latency peak amplitude was significantly elevated with the addition of a cognitive task for both EVS–EMG relationships. The medium-latency peak amplitude for the net whole-body postural response (as measured by APF) was also greater for the cognitive task condition. These results demonstrate that the vestibular-evoked postural response is indeed altered with increased cognitive demand and thus provide evidence for a connection between brain structures involved in cognitive processing and the vestibular control of balance. Furthermore, the elevated medium-latency peak amplitude in combination with the limited change in the short-latency peak amplitude demonstrated by all outcome measures while standing with an elevated cognitive load supports the concept of independent origins or propagation pathways associated with each peak of the biphasic response (Britton et al. 1993; Dakin et al. 2010; Mian et al. 2010). These data also provide specificity regarding the location of the neural alterations during upright balance control with an elevated cognitive load. It has been proposed that the short- and medium-latency responses are the result of unique afferent signal propagation pathways (i.e., direct vestibulospinal pathway for the short-latency and indirect reticulospinal for the medium-latency) (Britton et al. 1993; Welgampola and Colebatch 2001). In this context, it is likely that increased cognitive demand results in enhanced sensitivity to vestibular afferent signaling at the brainstem or cortices (Britton et al. 1993). Previous studies have proposed that the vestibular nuclei are a likely site for increased sensitivity or excitation of the vestibular control of balance (Horslen et al. 2014; Naranjo et al. 2016). These neural alterations may explain augmented VOR responses seen with increased postural threat, as vestibular signals passing through the vestibular nuclei also drive the VOR response (Naranjo et al. 2016).
Even though five participants did not demonstrate significant EVS–TA EMG responses in the time domain, those participants who did exhibit a significant response showed lower peak amplitudes and greater variability compared to the EVS–MG EMG and EVS–APF relationships. These responses may be explained by the fact that a muscle must be involved in maintaining upright balance in order for EVS to evoke a significant response (Britton et al. 1993; Fitzpatrick et al. 1994; Fitzpatrick and Day 2004). For example, EVS-evoked responses diminish in plantar flexor muscles when participants are moved from a standing to a seated position (Britton et al. 1993). Similarly, EVS will evoke a response in upper limb muscles if they are used for balance control (Britton et al. 1993; Luu et al. 2012). Due to the relative ease of the balance task used in this study (quiet standing on a firm surface) it is possible that the TA was not actively involved in maintaining balance, thus diminishing its EVS-evoked motor responses. It is also possible that the TA was only involved in balance control intermittently such as during larger center of mass excursions. Despite the variability seen in these data, the EVS–TA response demonstrated here is consistent with that seen in the past (Dalton et al. 2014).
The coherence function assesses the correlation between EVS and outcome measures across a range of frequencies and the gain function describes the magnitude of the relationship. Accordingly, these functions reveal the operational frequency bandwidth of the vestibular error for evoking a significant motor response and the strength of the association between the vestibular error signal and the subsequent motor response. Our results indicate no difference in the operational bandwidth frequencies between the cognitive load conditions. However, there was increased EVS–APF and EVS–MG EMG coherence, and increased gain at multiple frequencies for the EMG variables with increased cognitive load, which signifies greater reliance on vestibular control of quiet standing balance under this condition. Time and frequency domain data regarding the vestibular control of balance are likely interconnected. It is suggested that the medium-latency response is composed primarily of low frequencies and the short-latency response is composed of higher frequencies (Dakin et al. 2007); however, these muscle responses may be a composite of all stimulus frequencies (Dakin et al. 2011). As with the cumulant density function, elevated coherence and gain may result from increased excitability of the vestibular nuclei through the corticospinal/reticulospinal pathway. However, it is unknown whether other sensory pathways also exhibit increased excitability during a dual-task paradigm.
The increased cumulant density and coherence function amplitudes reflect greater relationships between EVS and the motor output in both the time and frequency domains while standing with elevated cognitive load compared to standing alone. It is also important to consider that total distance traveled for anterior–posterior COP remained similar between trials, thus offering support for the likelihood that increased vestibular control of balance acts in opposition to neuromuscular deficits associated with increased cognitive load (e.g., increased intra-cortical inhibition and delayed muscle onset time for reactive balance control) (Teasdale et al. 1993; McIlroy et al. 1999; Holste et al. 2015). Such a relationship may explain previous studies, which have reported no deficits in quiet standing when comparing conditions of minimal cognitive load to those with elevated cognitive load (Kerr et al. 1985; Brown et al. 1999).
The aforementioned physiology provides a viable explanation by which vestibular control of balance is enhanced with the addition of a cognitive task. However, alternative neural pathways must be acknowledged. Foremost, there could be an increase in excitability at the vestibular cortex. This cortex is capable of evoking a postural response via projections through sensory cortices to the motor cortex (Staab et al. 2013). Therefore, it is possible that elevated EVS-evoked motor responses seen with increased cognitive load are the result of increased cortical excitability rather than the vestibular nuclei. Increased EVS-evoked motor responses may also be the result of more excitable lower motor neuron pools, though this is contradicted by the fact that medium-latency peak amplitudes were larger with a cognitive task than without, while short-latency peak amplitudes were unchanged.
A dual-task experimental paradigm may be affected by several factors including difficulty of the motor task, difficulty of the cognitive task, and each participant’s aggregate comfort with performing both tasks in unison. The primary limitation of this protocol was individual comfort with a math-based cognitive task. Participants who are more comfortable performing mental arithmetic may have devoted fewer cognitive resources to the cognitive task, and thus reallocated fewer resources away from motor task performance. Additionally, these participants may not have utilized the allotted 10-s response window and thus spent less time under true conditions of elevated cognitive load. Future studies would benefit from using a continuous math-based cognitive task or measuring individual response times. Lastly, even though our EVS signal contained frequencies between 0 and 20 Hz, the power spectrum of the EVS was not evenly distributed across the frequency bandwidth. Thus, future studies may want to ensure normalization of the power spectrum across all frequencies to ensure equal distribution of the stimulus bandwidth. Nevertheless, these limitations did not hinder our ability to demonstrate differences in the vestibular control of balance with increased cognitive load.
Conclusion
The current findings illustrate augmented vestibular control of standing balance in both the time and frequency domains when cognitive load is increased. These modifications may stem from increased sensitivity of the vestibular nuclei or other physiological mechanisms including excitation of the vestibular cortex. However, it remains equivocal if this elevated sensitivity is specific towards vestibular signals or rather towards other sensory signals involved in standing balance control. Irrespective of the mechanism, these data not only provide further evidence for previously theorized connections between the vestibular control of balance and higher-order brain structures involved in cognitive processing, but also help to explain a disconnect in the dual-task literature, which depicts neuromuscular deficits with minimal manifestation of those deficits in standing balance control.
References
Amjad AM, Halliday DM, Rosenberg JR, Conway BA (1997) An extended difference of coherence test for comparing and combining several independent coherence estimates: theory and application to the study of motor units and physiological tremor. J Neurosci Methods 73:69–79
Bourke PA, Duncan J, Nimmo-Smith I (1996) A general factor involved in dual-task performance decrement. Q J Exp Psychol Sect A Hum Exp Psychol 49:525–545
Britton TC, Day BL, Brown P, Rothwell JC, Thompson PD, Marsden CD (1993) Postural electromyographic responses in the arm and leg following galvanic vestibular stimulation in man. Exp Brain Res 94:143–151
Brown LA, Shumway-Cook A, Woollacott MH (1999) Attentional demands and postural recovery: the effects of aging. J Gerontol A Biol Sci Med Sci 54:M165–M171
Cathers I, Day BL, Fitzpatrick RC (2005) Otolith and canal reflexes in human standing. J Physiol 563:229–234
Cullen KE (2012) The vestibular system: multimodal integration and encoding of self-motion for motor control. Trends Neurosci 35:185–196
Dakin CJ, Lee Son G, Inglis JT, Blouin J-S (2007) Frequency response of human vestibular reflexes characterized by stochastic stimuli. J Physiol 583:1117–1127
Dakin CJ, Luu BL, van den Doel K, Inglis JT, Blouin J-S (2010) Frequency-specific modulation of vestibular-evoked sway responses in humans. J Neurophysiol 103:1048–1056
Dakin CJ, Inglis JT, Blouin J-S (2011) Short and medium latency muscle responses evoked by electrical vestibular stimulation are a composite of all stimulus frequencies. Exp Brain Res 209:345–354
Dakin CJ, Dalton BH, Luu BL, Blouin J-S (2014) Rectification is required to extract oscillatory envelope modulation from the surface electromyographic signals. J Neurophysiol 112:1685–1691
Dakin CJ, Héroux ME, Luu BL, Inglis JT, Blouin J-S (2016) Vestibular contribution to balance control in the medial gastrocnemius and soleus. J Neurophysiol 115:1289–1297
Dalton BH, Blouin J-S, Allen MD, Rice CL, Inglis JT (2014) The altered vestibular-evoked myogenic and whole-body postural responses in old men during standing. Exp Gerontol 60:120–128
Dalton BH, Rasman BG, Inglis JT, Blouin J-T (2017) The internal representation of head orientation differs for conscious perception and balance control. J Physiol (in Press)
Day BL, Cole J (2002) Vestibular-evoked postural responses in the absence of somatosensory information. Brain 125:2081–2088
Day BL, Fitzpatrick RC (2005) Virtual head rotation reveals a process of route reconstruction from human vestibular signals. J Physiol 567:591–597
Fitzpatrick RC, Day BL (2004) Probing the human vestibular system with galvanic stimulation. J Appl Physiol 96:2301–2316
Fitzpatrick RC, Burke D, Gandevia SC (1994) Task-dependent reflex responses and movement illusions evoked by galvanic vestibular stimulation in standing humans. J Physiol 478:363–372
Forbes PA, Dakin CJ, Vardy AN, Happee R, Siegmund GP, Schouten AC, Blouin J-S (2013) Frequency response of vestibular reflexes in neck, back, and lower limb muscles. J Neurophysiol 110:1869–1881
Goldberg JM, Smith CE, Fernández C (1984) Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. J Neurophysiol 51:1236–1256
Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF (1995) A framework for the analysis of mixed time series/point process data—theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol 64:237–278
Holste KG, Yasen AL, Hill MJ, Christie AD (2015) Motor cortex inhibition is increased during a secondary cognitive task. Mot Control 20:380–394
Horslen BC, Dakin CJ, Inglis JT, Blouin J-S, Carpenter MG (2014) Modulation of human vestibular reflexes with increased postural threat. J Physiol 592:3671–3685
Iles JF, Pisini JV (1992) Vestibular-evoked postural reactions in man and modulation of transmission in spinal reflex pathways. J Physiol 455:407–424
Jahn K, Naessl A, Schneider E, Strupp M, Brandt T, Dieterich M (2003) Inverse U-shaped curve for age dependency of torsional eye movement responses to galvanic vestibular stimulation. Brain 126(7):1579–1589
Kerr B, Condon SM, McDonald LA (1985) Cognitive spatial processing and the regulation of posture. J Exp Psychol Hum Percept Perform 11:617–622
Kim J, Curthoys IS (2004) Responses of primary vestibular neurons to galvanic vestibular stimulation in the anaesthetized guinea pig. Brain Res Bull 64:265–271
Lund S, Broberg C (1983) Effects of different head positions on postural sway in man induced by a reproducible vestibular error signal. Acta Physiol Scand 117:307–309
Luu BL, Inglis JT, Huryn TP, Van der Loos HFM, Croft EA, Blouin J-S (2012) Human standing is modified by an unconscious integration of congruent sensory and motor signals. J Physiol 590:5783–5794
Maki BE, McIlroy WE (1996) Influence of arousal and attention on the control of postural sway. J Vestib Res 6:53–59
Massion J (1998) Postural control systems in developmental perspective. Neurosci Biobehav Rev 22:465–472
McIlroy WE, Norrie RG, Brooke JD, Bishop DC, Nelson AJ, Maki BE (1999) Temporal properties of attention sharing consequent to disturbed balance. Neuroreport 10:2895–2899
Mian OS, Day BL (2009) Determining the direction of vestibular-evoked balance responses using stochastic vestibular stimulation. J Physiol 587:2869–2873
Mian OS, Dakin CJ, Blouin J-S, Fitzpatrick RC, Day BL (2010) Lack of otolith involve- ment in balance responses evoked by mastoid electrical stimulation. J Physiol 588:4441–4451
Naranjo EN, Allum JHJ, Inglis JT, Carpenter MG (2015) Increased gain of vestibulospinal potentials evoked in neck and leg muscles when standing under height-induced postural threat. Neuroscience 293:45–54
Naranjo EN, Cleworth TW, Allum JHJ, Inglis JT, Lea J, Westerberg BD, Carpenter MG (2016) Vestibulo-spinal and vestibulo-ocular reflexes are modulated when standing with increased postural threat. J Neurophysiol 115:833–842
Nashner LM, Wolfson P (1974) Influence of head position and proprioceptive cues on short latency postural reflexes evoked by galvanic stimulation of the human labyrinth. Brain Res 67:255–268
Pashler H (1994) Dual-task interference in simple tasks: data and theory. Psychol Bull 116:220–244
Pastor MA, Day BL, Marsden CD (1993) Vestibular induced postural responses in Parkinson’s disease. Brain 116:1177–1190
Reynolds RF (2010) The effect of voluntary sway control on the early and late components of the vestibular-evoked postural response. Exp Brain Res 201:133–139
Rosenberg JR, Amjad AM, Breeze P, Brillinger DR, Halliday DM (1989) The fourier functional approach to the identification of coupling between neuronal spike trains. Prog Biophys Mol Biol 53:1–31
Sigman M, Dehaene S (2008) Brain mechanisms of serial and parallel processing during dual-task performance. J Neurosci 28:7585–7598
Staab JP, Balaban CD, Furman JM (2013) Threat assessment and locomotion: clinical applications of an integrated model of anxiety and postural control. Semin Neurol 33:297–306
Stelmach GE, Zelaznik HN, Lowe D (1990) The influence of aging and attentional demands on recovery from postural instability. Aging Clin Exp Res 2:155–161
Teasdale N, Bard C, LaRue J, Fleury M (1993) On the cognitive penetrability of postural control. Exp Aging Res 19:1–13
van der Kooij H, Jacobs R, Koopman B, Grootenboer H (1999) A multisensory integration model of human stance control. Biol Cybern 80:299–308
Vuillerme N, Vincent H (2006) How performing a mental arithmetic task modify the regulation of centre of foot pressure displacements during bipedal quiet standing. Exp Brain Res 169:130–134
Wardman DL, Taylor JL, Fitzpatrick RC (2003) Effects of galvanic vestibular stimulation on human posture and perception while standing. J Physiol 551:1033–1042
Welgampola M, Colebatch J (2001) Vestibulospinal reflexes: quantitative effects of sensory feedback and postural task. Exp Brain Res 139:345–353
Wilson VJ, Boyle R, Fukushima K, Rose PK, Shinoda Y, Suqiuchi Y, Uchino Y (1995) The vestibulocollic reflex. J Vestib Res 5:147–170
Yardley L, Gardner M, Bronstein A, Davies R, Buckwell D, Luxon L (2001) Interference between postural control and mental task performance in patients with vestibular disorder and healthy controls. J Neurol Neurosurg Psychiatry 71:48–52
Acknowledgements
The authors would like to thank all volunteers for their participation and Wendy Peters and Jonathan Wallace for help with data analysis. B.H. Dalton was supported by a New Investigator Grant from the Medical Research Foundation as part of the Oregon Health and Science University Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McGeehan, M.A., Woollacott, M.H. & Dalton, B.H. Vestibular control of standing balance is enhanced with increased cognitive load. Exp Brain Res 235, 1031–1040 (2017). https://doi.org/10.1007/s00221-016-4858-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-016-4858-3