Abstract
Electrical vestibular stimulation produces biphasic responses in muscles maintaining balance. The two components of these muscle responses (termed the short latency and medium latency components) are believed to be independent and elicited by vestibular stimuli of different frequencies. We tested these hypotheses by determining (a) if frequency-specific stimulation protocols could evoke independently the short and medium latency responses and (b) whether these two components are triggered by distinct brain regions with a fixed time delay, interacting around 10 Hz. First, subjects were provided 10–25 Hz, 0–10 Hz, and 0–25 Hz vestibular stimuli to selectively modulate the short latency, medium latency, or both components of the response; and second, they were provided twenty sinusoidal stimuli from 1 to 20 Hz with a 0–20 Hz control trial, designed to determine whether an interaction between the short and medium latency responses occurs at a specific stimulation frequency. Both the 0–10 Hz and 10–25 Hz vestibular stimuli elicited multiphasic waveforms, suggesting the short and medium latency components were not modulated independently by the frequency-specific stimuli. Sinusoidal vestibular stimuli evoked responses at the stimulated frequency but no evidence of a reflex component interaction was observed. Instead, summation of the responses evoked by each of the sinusoidal stimuli resembled the biphasic response to broad bandwidth stimuli. Due to the lack of interaction and linear contribution of all stimulus frequencies to both the short and medium latency responses, the present results support the use of broad bandwidth electrical vestibular signal for physiological or clinical testing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Galvanic and stochastic vestibular stimulation (GVS and SVS) entail the transmastoidal percutaneous application of a small electric current, which is used to probe vestibular function (GVS: Coats 1973; Nashner and Wolfson 1974; for review see Fitzpatrick and Day 2004; SVS: Fitzpatrick et al. 1996; Dakin et al. 2007). The applied electric current modulates the firing rate of the underlying vestibular afferents producing responses in muscles active in the maintenance of balance. In lower limb muscles, these responses exhibit a biphasic pattern with two opposing peaks, termed short (50–70 ms) and medium (100–120 ms) latency components (Britton et al. 1993; Dakin et al. 2007, 2010; Fitzpatrick and Day 2004; Fitzpatrick et al. 1994; Iles and Pisini 1992; Lee Son et al. 2008; Nashner and Wolfson 1974; Reynolds 2010). While the exact physiological mechanisms underlying these two peaks remain unclear, results from previous studies indicate they may derive from independent sources (Britton et al. 1993; Cathers et al. 2005; Dakin et al. 2007). Two main hypotheses have been formulated to account for the biphasic vestibulo-motor response: the short and medium latency components may originate from two distinct regions in the brain (the vestibulo and reticulospinal systems; see Britton et al. 1993) or from the stimulation of afferents from the otoliths and semicircular canals (Cathers et al. 2005). Recently, we proposed these two components may be preferentially elicited by specific vestibular stimulus frequency bandwidths (Dakin et al. 2007), which could provide a simple and effective way to assess the function and physiological relevance of the short and medium latency components in humans. In the present experiment, we developed specific electrical stimuli to determine whether altering the frequency content of an electrical vestibular stimulus could prove useful in dissociating the two vestibular response components.
The two components of the biphasic vestibulo-myogenic response may receive independent contributions from specific stimulus frequency bandwidths, as suggested by two regions (2–10 Hz and 11–20 Hz) of stronger coupling between SVS and muscle activity (Dakin et al. 2007). The time lags estimated by the slope of the SVS muscle phase function within these two bandwidths indicate that the 2–10 Hz bandwidth contributes primarily to the medium latency component and the 11–20 Hz bandwidth to the short latency component. These findings predict that an SVS stimulus within either the 0–10 Hz or 10–20 Hz bandwidth should modulate selectively either the short (10–20 Hz) or medium latency (0–10 Hz) peak of the vestibulo-myogenic response. Selective modulation of either response component would provide further support for the independence of the two components of the vestibular response. Therefore, the first aim of the current study was to determine whether distinct stimulus frequency bandwidths contribute to the individual peaks of the biphasic muscle response. We tested this hypothesis by designing stimuli within each of the two previously identified bandwidths in order to exclusively modulate the short (10–25 Hz stimulus) or medium latency (0–10 Hz stimulus) lower limb vestibulo-myogenic responses. We hypothesized that the short latency component of the muscle response would be triggered by the 10–25 Hz vestibular signals and the medium latency component by the 0–10 Hz signals.
The second aim of this study was to test the independence of the two components of the vestibulo-myogenic response by determining whether activation of distinct brain regions with a fixed central delay, as proposed by Britton et al. (1993), could account for the presence of a biphasic vestibulo-myogenic response. Assuming linear transmission of reflexes, a consequence of examining a system exhibiting two distinct reflexive responses triggered by brain regions with a fixed delay is that the two reflex components will exhibit a temporal overlap (described as reflex component interaction) at specific frequencies of stimulation. Specifically, the interaction of these reflex components will depend on the time delay that exists between these components. Matthews (1993) used this rationale to examine the components of the human stretch reflex, identifying perturbation frequencies (around 20 Hz) for the wrist muscles at which the short and long latency components of the stretch reflex exhibit a temporal overlap. This interaction between short and long latency stretch reflex components was demonstrated in two ways: (a) as abrupt changes in the phase frequency relationship between sinusoidal stimuli and associated electromyographic response and (b) as modulation of electromyographic activity at a frequency other than at the stimulation frequency. A similar interaction between the two vestibular response components may occur at specific frequencies of stimulation if the vestibulo-myogenic response originates from two independent central sources exhibiting a fixed delay between them (as proposed by Britton et al. 1993). For example, SVS–muscle coherence decreases at 10 Hz and exhibits a small inflection in phase at this frequency (Dakin et al. 2007), potentially reflecting an interaction between the short and medium latency vestibular response. The approximate 50 ms delay between the short and medium latency response components in the leg muscles (Britton et al. 1993) could be produced by two inputs to the motor neuron pool that are out of phase, resulting in destructive interference of the response to a 10 Hz vestibular stimulus. To test this second hypothesis, subjects were exposed to a series of sinusoidal stimuli (1–20 Hz) from which we examined the stimulus–EMG correlations and phase frequency function on a frequency by frequency basis. We hypothesized that, for vestibular stimuli around 10 Hz, an interaction between the short and medium latency response components would be observed, yielding an abrupt shift in phase of the stimulus—EMG phase frequency function as well as EMG modulation at frequencies other than the stimulus frequency.
Methods
Subjects
Twelve healthy subjects (7 men, 5 women) between the ages of 20 and 34 years, with no known history of neurological disease or injury participated in this study. The experimental protocol was explained to each subject and their written, informed consent was obtained. All procedures used in this study conformed to the standards of the Declaration of Helsinki and were approved by the University of British Columbia’s clinical research ethics board.
Vestibular stimuli
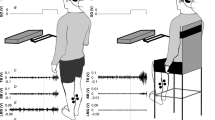
Vestibular stimulation was delivered using a binaural bipolar electrode configuration. Carbon rubber electrodes (9 cm2), coated with Spectra 360 electrode gel (Parker Laboratories, Fairfield, USA), were secured over participants’ mastoid processes with an elastic headband. Testing was performed in two separate experimental sessions (see below). Vestibular stimuli were generated on a PC computer using Labview software (National Instruments, Austin, USA) and were sent directly to a constant current isolation unit (Model 2200 Analog Stimulus Isolator: AM Systems, Carlsborg, WA) via a multifunction data acquisition board (PXI-6289, National Instruments, Austin, USA). The 0–10 Hz, 10–25 Hz, and 0–25 Hz stochastic signals were delivered as two 105 s trials that were normalized to provide similar amplitude at each frequency component, resulting in different RMS amplitudes for each bandwidth stimuli: 0.65 mA (0–10 Hz), 0.73 mA (10–25 Hz), and 0.98 mA (0–25 Hz). Frequency normalization of the stimuli ensured equal contribution for each frequency within the stimulus. Sinusoidal stimuli, on the other hand, had amplitudes of ±2 mA and were 90 s in length, while the comparison 0–20 Hz stochastic stimuli were also 90 s in length but ±4 mA in amplitude. The sinusoidal stimuli were provided with amplitudes of ±2 mA to ensure adequate responses at all frequencies but also small enough to limit the uncomfortable effects of the vestibular stimulus. The 0–20 Hz stochastic stimuli control trial was provided with an amplitude of ±4 mA to ensure an adequate response magnitude for comparison with the average of the sinusoidal stimuli (Dakin et al. 2007).
Testing protocol
During each testing session, participants were required to stand with their feet 2–3 cm apart (as measured at the medial malleoli). For each trial, participants were asked to keep their arms at their sides, stand relaxed, and keep their head turned to the right and eyes level, parallel to the floor. To maintain head position, participants were asked to focus on a target to the right of them. By maintaining this head position, the postural response to the vestibular stimulus was primarily aligned to the anterior–posterior directions, along the line of action of the left gastrocnemius (Cathers et al. 2005; Day and Fitzpatrick 2005; Lund and Broberg 1983). Electromyography (EMG) was collected for the left medial gastrocnemius as vestibular-evoked muscle responses are larger in the leg opposite to the direction the head is turned (Britton et al. 1993; Dakin et al. 2007).
To test our first hypothesis, ten subjects were exposed to three vestibular stimuli: a 0–10 Hz stimulus to modulate preferentially the medium latency response, a 10–25 Hz stimulus to modulate the short latency vestibular-evoked response, and a 0–25 Hz stimulus as a control. The bandwidths of the 0–10 Hz and 10–25 Hz stimuli were chosen based on the frequency range identified by the two slopes of the phase estimate described previously (Dakin et al. 2007). Participants were provided a total of six trials: two trials for each of the three stimulus bandwidths. Rest periods were provided at the request of the participant to avoid any sign of fatigue.
To test our second hypothesis, five participants (including two new participants) were provided twenty sinusoidal stimuli spanning 1–20 Hz and a 0–20 Hz SVS comparison control trial. Participants were tested for a total of twenty one trials which were collected on a separate day from the first experimental session. To reduce the number of vestibular stimuli for participants, we limited the sinusoidal stimuli to the 1–20 Hz bandwidth since SVS–muscle coherence is largest in this region (Dakin et al. 2007, 2010) and it encompasses the frequencies at which an interaction between reflex components may occur (~10 Hz).
Data collection and analysis
EMG was amplified (Gain ×5,000–20,000; Grass P511, Grass–Telefactor, West Warwick, USA) and band-pass filtered (30–1,000 Hz) prior to being digitized at 5,000 Hz for the stochastic stimuli and 8,192 Hz for the sinusoidal stimuli and associated 0–20-Hz control trial. EMG was sampled at 8,192 Hz for the sinusoids to allow Fast Fourier Transform (FFT) windows of a power of two (8,192 points). Using this sampling frequency and FFT window length, frequency values occur at each integer frequency, congruent with the sinusoidal stimuli that were delivered at integer frequencies. SVS trials within each testing session were time-locked to SVS onset and concatenated within each condition for each participant. The sinusoidal stimuli were delivered as a single trial and therefore within participant concatenation was not necessary. EMG data were full-wave rectified, concatenated within subjects, and concatenated across all subjects. Concatenated data were then used to estimate cross-covariance and phase functions between SVS (or sinusoidal stimuli) signal and EMG. For statistical analysis, dependent variables were extracted from the cross-covariance and phase functions estimated using concatenated data within participants, whereas the functions estimated using concatenated data across all participants were used for illustrative purposes only. Within subject cross-covariance and phase estimates provided timing and amplitude information regarding the time-domain characteristics of the identified coherent frequencies (Dakin et al. 2007; Rosenberg et al. 1989).
Cross-covariance functions and phase estimates were calculated using a Matlab script based on the method described by Rosenberg and colleagues (Rosenberg et al. 1989; Halliday et al. 1995). SVS–EMG cross-covariance functions were analyzed with resolutions of 0.076 Hz (13.1 s/segment) to identify the low frequency contributions. Sinusoidal data were analyzed with a resolution of 1 Hz (1 s/segment) to correspond to the frequency content of the sinusoidal stimuli which were at integer frequencies. By convention, anode right/cathode left currents are represented as a positive vestibular signal. Hence, a positive cross-covariance function indicates that anode right/cathode left currents induced muscle facilitation. The local maxima and minima in the muscle responses were estimated from the cross-covariance functions computed from the data concatenated for individual subjects.
To address our first hypothesis, the polarity and timing of the peaks (and troughs) obtained from the cross-covariance function for the 0–10 Hz and 10–25 Hz bandwidths were compared to those obtained following the broad bandwidth (0–25 Hz) vestibular signals. This comparison was made to determine whether a single component of the biphasic muscle response was triggered by the narrow bandwidth stimuli. Similarity between the 0–10 Hz and 0–25 Hz covariance functions were assessed using paired t-tests (statistical significance was set at P = 0.05). To address our second hypothesis, phase estimates were calculated for both the sinusoidal stimuli and the 0–20 Hz stochastic control stimuli. The phase estimates for the sinusoidal stimuli were extracted at each stimulus frequency, concatenated across frequencies, and realigned by values of 2π using the 1 Hz trial as a reference to obtain a continuous phase estimate. For illustrative purposes, the phase values obtained for the 0–20 Hz stochastic control trials are displayed as a continuous function of frequency (unwrapped) to provide an uninterrupted slope. As a secondary measure, we examined changes in EMG power to the sinusoidal stimuli. Similarly to Matthews (1993), if the two reflex components interact, it should be apparent as an increase in EMG power at frequencies other than the stimulus frequencies.
Results
Do 0–10 Hz and 10–25 Hz vestibular stimuli contribute independently to the short and medium latency components of the vestibulo-myogenic response?
The time-domain correlations between SVS and left medial gastrocnemius EMG revealed that the 0–10 Hz and 10–25 Hz bandwidth stimuli contribute to the shape and timing of both components of the vestibulo-myogenic response. The EMG–SVS cross-covariance obtained for the 0–10 Hz bandwidth exhibited spatial properties similar to those obtained for the 0–25 Hz vestibular stimuli, with both stimuli eliciting biphasic responses (Fig. 1). The timing of the two peaks, however, differed between stimuli. The short latency component of the 0–10 Hz cross-covariance occurred earlier than the corresponding peak for the 0–25 Hz control (50 ± 18 vs. 65 ± 6 ms), while the medium latency component of the 0–10 Hz response was later than the medium latency component of 0–25 Hz control (127 ± 18 vs. 115 ± 14 ms). This resulted in a longer period between the short latency peak and medium latency peak for the 0–10 Hz stimuli compared to the 0–25 Hz stimuli (77 ± 6 vs. 50 ± 13 ms; t (10) = 8.61, P < 0.05). On the other hand, the cross-covariance for the 10–25 Hz vestibular stimuli exhibited a triphasic response and was therefore not compared to the broad bandwidth responses (42 ± 7, 73 ± 5 and 106 ± 6 ms). The spatial and temporal characteristics of the second and third peaks of the triphasic response triggered by 10–25 Hz stimuli appear to contribute mainly to the short and medium latency components of the muscle response observed following 0–25 Hz stimuli (Fig. 1). Overall, neither the 0–10 Hz or 10–25 Hz stimuli independently contributed to short or medium latency component of the biphasic muscle response.
Stimuli, stimulus power spectra and 0–10 Hz, 10–25 Hz, and 0–25 Hz cross-covariance functions. a Five seconds of the 0–10 Hz, 10–25 Hz, and 0–25 Hz stimuli. b Power spectra for the 0–10 Hz, 10–25 Hz, and 0–25 Hz stimuli. c SVS–EMG cross-covariance functions for the 0–10 Hz, 10–25 Hz, and 0–25 Hz stimuli (n = 10). Neither the 0–10 Hz or 10–25 Hz stimuli evoked a single component of the biphasic response, although the 10–25 Hz stimulus appears to exhibit its greatest influence on the short component of the biphasic response
Are the two components of the vestibulo-myogenic response caused by a fixed central delay?
To test our second hypothesis, sinusoidal stimuli with frequencies ranging from 1 to 20 Hz were provided. We examined the phase between the muscle responses and vestibular stimuli as well as EMG power for indications of interactions in the vestibular-evoked muscle responses. All stimulus frequencies yielded increases in EMG power isolated to the stimulus frequency and not multiple frequencies as would be suggested by an interaction (Fig. 2b). However, stimulus frequencies between 4 and 12 Hz exhibited stronger entrainment of lower limb EMG than stimulus frequencies outside of this bandwidth (Figs. 2, 3). For stimulus frequencies exhibiting obvious entrainment, the association between the vestibular stimulus and the muscle response was detectable in the raw data (Fig. 3): an anode right/cathode left vestibular stimulus was associated with increased muscle activity about 90–100 ms later, while anode left/cathode right currents yielded a decrease (or absence) in gastrocnemius muscle activity with a similar delay. Similar entrainment was not evident in the 0–20 Hz stochastic trials (Fig. 3). All cross-covariance for the 1–20 Hz sinusoidal stimuli and associated EMG exhibited clear sinusoidal modulation at the stimulation frequency, correlating both prior to and following the zero time mark (Fig. 4a). Cross-covariance functions within the 4–15 Hz bandwidth displayed the largest modulation. None of the vestibular stimuli elicited muscle responses at frequencies other than the stimulation frequency, further indicating a lack of interaction between two central processes at a fixed delay (Fig. 2b).
Description of stimulus EMG entrainment. Rectified EMG for a stimulus frequency (3 Hz) that shows minimal EMG entrainment compared to a stimulus frequency (8 Hz) displaying stronger EMG entrainment. EMG from the 0–20 Hz control trial does not exhibit any obvious signs of entrainment to the stochastic stimulus
Cross-covariance functions for the sinusoidal stimuli. a Cross-covariance functions for the sinusoidal stimuli and the control broad bandwidth stimuli. Sinusoidal stimuli produce sinusoidal covariance functions that when averaged produce a biphasic response similarly to the 0–20 Hz. In gray are each subjects muscle response to the stimulus frequency listed on the left with the pooled response for all subjects superimposed in black (n = 5). Sinusoidal stimuli induce correlations in the cross-covariance function both following and preceding the zero time mark. Such pre-zero correlations result from a single period of the vestibular stimulus correlating not only with the response elicited by the stimulus but also with the response elicited by the stimulus period prior and the stimulus period post. b Superposition of the sinusoidal covariance functions displays the phase relationship between the different frequencies underlying the biphasic mean. Muscle responses to stimulus frequencies of 5 Hz and below (gray segmented lines) decrease the amplitude of the early latency response observed in the cross-frequency mean (black line). These plots represent the average response for all subjects (n = 5). Muscle responses to the 1–5 Hz stimuli are gray segmented lines, muscle responses to the 6–20 Hz stimuli are gray solid lines, and the mean muscle response across all stimulus frequencies are black solid lines
Examination of the vestibular stimuli–EMG phase relationship shows a comparable phase frequency function between the broad bandwidth stimulus and the individual sinusoidal stimuli over the 0–20 Hz frequency range (Fig. 5). For the 0–20 Hz stochastic trial, the slope of the phase frequency function over 0–10 Hz corresponds to a phase lag of 108 ms compared to 116 ms for the sinusoidal stimuli phase frequency function over the same frequency range (i.e., 0–10 Hz). For the 10–20 Hz interval, the slope of the 0–20 Hz broad bandwidth SVS indicated a phase lag of 71 ms compared to 77 ms for the sinusoidal stimuli (Fig. 5). Despite replicating the previously observed differences in the estimated time lags from the phase functions (Dakin et al. 2007), no prominent inflection in the phase functions was present for either stimulus protocols.
Are both peaks of the biphasic response rather a composite of all frequencies within the stimulus?
As the 0–10 Hz and 10–25 Hz stimuli did not independently modulate the short or medium latency responses, we examined whether the biphasic response is rather the net result of a broad bandwidth of stimulus frequencies. If this is true, the resulting averaged muscle response across all the individual sine waves should resemble the muscle response to broad bandwidth stimuli. To examine this, the cross-covariance functions for each of the sinusoidal stimuli were determined and averaged across frequencies within each subject. The timing and polarity of the mean of all frequencies were then compared to the cross-covariance from the 0–20 Hz control trial. Similarity between the mean sinusoidal response and the 0–20 Hz control trial would suggest that vestibular reflexes stimulated at all frequencies between 0 and 20 Hz summate linearly to contribute to both the short and medium latency response components.
Presumably, the relative phase for each frequency contributing to the average is important in determining the spatial and temporal characteristics of the biphasic profile of the average for the sinusoidal cross-covariance functions. Frequencies from 1 to 5 Hz, in this study, appeared to decrease the short latency component of the biphasic response as their cross-covariance function exhibits a single period for the duration of the biphasic response that is opposite in polarity to the short latency response (Fig. 4a, b). In contrast, frequencies from 6 to 13 Hz exhibit the correct period length, polarity, and phase to contribute to both the short and medium latency component of the biphasic response (Fig. 4a, b). Frequencies greater than 13 Hz exhibit multiple periods over the duration of the biphasic response, suggesting that they may contribute to shaping the contours of the net waveform, especially sharpening the peaks of the short and medium latency responses (Fig. 4a, b). When all the sinusoidal cross-covariance functions were superimposed their phase relationship becomes apparent highlighting how the biphasic waveform may result from the average of the sinusoidal cross-covariance functions (Fig. 4b).
When the cross-covariance functions to the sinusoidal stimuli were averaged across frequencies, the result was a biphasic response that approximated the spatial and temporal properties of the 0–20 Hz control cross-covariance function, exhibiting an early negative followed by later positive going peak (Fig. 4a). The timing for the short latency component, however, was significantly different from the control at 65 ± 6 ms (mean of sines) and 62 ± 6 ms (0–20 Hz control) (t (5) = 3.35, P < 0.05), while the timing of the medium latency component was not different from the control 104 ± 7 ms (mean) and 104 ± 10 ms (0–20 Hz control) (t (5) = 0.1, P > 0.05).
Discussion
The aim of the current study was to determine whether parameters of an electrical vestibular stimulus could be tuned to assess the function and physiology of the short and medium latency vestibulo-myogenic response. Specifically, we tested the hypotheses that (a) frequencies from the 0–10 Hz and 10–25 Hz bandwidths independently influence the short or medium latency components of the vestibular-evoked biphasic lower limb muscle response, and (b) the two components of the biphasic myogenic response, due to their fixed central delay, would interact at frequencies of stimulation near 10 Hz. The present results did not support either hypothesis. Instead, the results suggest that the biphasic lower limb muscle response is a linear composite of all frequencies within the stimulus bandwidth (0–20 Hz) and that the two components of the biphasic can not be explained by two independent sources generating muscle responses with a fixed delay between 25 and 500 ms.
Previously, we observed a dual sloped phase frequency relationship using a 0–50 Hz broad bandwidth stimuli (Dakin et al. 2007) which suggested, along with previous research (Britton et al. 1993; Cathers et al. 2005; Day and Fitzpatrick 2005), that the two peaks of the biphasic muscle response may be the product of separate physiological processes. The earlier of the two peaks appeared to be derived from frequencies in the 10–20 Hz bandwidth while the latter peak derived from frequencies in the 0–10 Hz bandwidth. These results led us to hypothesize that the frequency content of the stochastic stimuli could be adjusted to modulate independently the short or medium latency components of the biphasic response. This hypothesis, however, was proven incorrect as provision of low (0–10 Hz) or high (10–25 Hz) frequency stimuli did not independently modulate the short or medium latency components (Fig. 1). Instead, it appears that the spatial and temporal characteristics of the short and medium latency components of the lower limb vestibulo-myogenic response result from activation of vestibular afferents by a stimulus comprised of frequencies from a broader bandwidth (0–20 Hz) (Fig. 4) as indicated by linear summation of the individual sine waves. The lack of interaction between the two vestibular components to sinusoidal stimuli suggests that reducing the stimulus bandwidth to isolate components of the vestibular response does not provide any information that is not already provided by a broad bandwidth stimulus (0–20 Hz or 0–25 Hz). Instead, it appears more appropriate to use a large bandwidth vestibular signal (0–20 Hz or 0–25 Hz) for physiological or clinical testing due to increase subject comfort and reduced testing times. One exception, however, may include the possibility to reduce the magnitude of vestibular-evoked postural responses through reductions in the low frequency (<2 Hz) content of the stimuli (Dakin et al. 2010).
While no single peak of the biphasic response is contributed to exclusively by a specific frequency bandwidth, there does appear to be frequency bandwidths that contribute to specific attributes of the biphasic response. The biphasic shape of the myogenic response appears to be largely derived from frequencies between 6 and 13 Hz, while the higher frequencies sharpen the peaks of the response. This is evident in the 10–25 Hz trial (Fig. 1) where these frequencies appear to increase the amplitude and shorten the duration of the short latency component as well as shift the peak of the medium latency response earlier in time. On the other hand, frequencies below 6 Hz draw out the length of the medium latency response while attenuating the short latency response (Fig. 4b). Accordingly, we have previously observed that as lower frequencies are omitted from a vestibular stimulus (2–20 Hz), the amplitude of the short latency response increased (Dakin et al. 2010). The paradoxical contribution of low frequencies to the short and medium latency response components may explain the slope difference in the phase frequency relationship for frequencies below 8–10 Hz when compared to frequencies above 10 Hz between the stochastic vestibular stimuli and muscle response (c.f. Figs. 5, 6 in Dakin et al. 2007).
The two components of the vestibular-evoked muscle response also do not appear to interact with each other over stimulation frequencies of 1–20 Hz. Evidence of such an interaction would have manifested as an increase in EMG power at a frequency other than that of the stimulus frequency and a shift in the stimulus–EMG phase frequency function similar to those observed by Matthews (1993). In contrast, EMG activity was modulated only at the frequency of stimulation for each of the sinusoidal stimuli and the phase frequency function did not exhibit a marked shift in continuity. Therefore, our results do not support the premise that the two peaks of the biphasic myogenic response originate from two sources with a fixed time delay ranging between 25 and 500 ms. This interpretation would be appropriate for a linear system. Despite the non-linear input–output characteristics of individual motor neurons, the assumption of linearity is adequate for population of motor neurons (such as recorded with surface EMG here; Matthews 1993). This assumption is further supported by the observed similarity between the linear summation of the muscle responses evoked by sinusoidal stimuli between 0 and 20 Hz and those evoked by broadband stimuli over the same frequency range. Despite our failure to demonstrate an interaction between vestibular components, the two peaks could still originate from afferents of the semicircular canals and, although less likely, the otolith organs as proposed by Cathers et al. (2005). For example, it is possible that the delay between the two inputs may be less than 25 ms or that each of the individual components exhibit temporal features that are different than what can be inferred by their combined response in surface EMG. As well, the attenuated modulation of EMG to stimulus frequencies outside the 4–15 Hz bandwidth may mask signs of interaction in these frequencies. Strong evidence attributes the semicircular canals to the medium latency peak as this peak can be predictably modulated based on the orientation of the head and the assumption of non-specific activation of semicircular canal afferents (Cathers et al. 2005; Day and Fitzpatrick 2005; Mian and Day 2009). In contrast, the origin of the short latency component is not as clear. The directionality of the short latency vestibular response does not appear to correspond to the net activation of otolith afferents as predicted by the model proposed by Fitzpatrick and Day (2004) (Mian et al. 2010).
Limitations
Three methodological limitations must be considered when interpreting the results from this study. The first limitation pertains to the averaging of the muscle responses observed with 1–20 Hz sinusoids to replicate the results observed using the 0–20 Hz control stimulus. The 0–20 Hz stochastic stimulus contains power at non-integer frequencies. In order to most accurately replicate stochastic control condition, participants would have been required to be provided additional stimuli at intervals between each integer frequency making testing prohibitively long. The absence of non-integer stimulus frequencies likely led to the small difference (3 ms difference for the short latency component) in the average cross-covariance when compared to the 0–20 Hz stochastic control condition.
The second methodological limitation pertains to the use of continuous repetitive stimuli. While continuous vestibular stimuli provide an advantage over discrete stimuli, by reducing testing time and the amplitude of stimuli that can be used (Dakin et al. 2010), continuous repetitive stimuli likely induce correlated feedback into the muscle response. Sensory feedback correlated to the stimulus has been observed to influence vestibular responses with delays as late as 400 ms (Day and Guerraz 2007). Feedback occurring with shorter delays may be incorporated into the later aspects of the muscle response potentially interfering with interpretation of these later responses.
The third methodological limitation pertains to the use of surface EMG as a measure to identify the underlying synaptic contributions to the biphasic responses. The biphasic muscle response as observed through surface EMG is composed of two underlying motor phenomenon: individual motor unit firing behavior dictated by incoming synaptic currents and secondary ensemble phenomenon such as synchronization-related oscillations due to the high autocorrelation function of the individual motor units (Moore et al. 1970). Because surface EMG provides an estimate of the sum of the total muscle activity, it is difficult to isolate which component of it is caused by independent synaptic inputs versus synchronization-related behavior.
Conclusion
Our data demonstrate that selective bandwidth SVS cannot be used to selectively modulate the short or medium latency vestibular-evoked muscle response. Instead, the biphasic response as observed to a broad bandwidth stimulus (0–20 Hz) is the linear sum of all stimulus frequencies, with each frequency contributing to specific attributes of the short and medium latency vestibular-evoked muscle response. The lack of non-linear interaction in the vestibular responses evoked at frequencies between 0 and 20 Hz supports the use of large bandwidth vestibular stimuli for clinical and physiological testing.
References
Britton TC, Day BL, Brown P, Rothwell JC, Thompson PD, Marsden CD (1993) Postural electromyographic responses in the arm and leg following galvanic vestibular stimulation in man. Exp Brain Res 94:143–151
Cathers I, Day BL, Fitzpatrick RC (2005) Otolith and canal reflexes in human standing. J Physiol 563:229–234
Coats AC (1973) The variability of the galvanic body-sway test. Ann Otol Rhinol Laryngol 82:333–339
Dakin CJ, Lee Son GM, Inglis JT, Blouin JS (2007) Frequency response of human vestibular reflexes characterized by stochastic stimuli. J Physiol 583:1117–1127
Dakin CJ, Luu BL, van den Doel K, Inglis JT, Blouin JS (2010) Frequency-specific modulation of vestibular-evoked sway responses in humans. J Neurophysiol 103:1048–1056
Day BL, Fitzpatrick RC (2005) Virtual head rotation reveals a process of route reconstruction from human vestibular signals. J Physiol 567:591–597
Day BL, Guerraz M (2007) Feedforward versus feedback modulation of human vestibular-evoked balance responses by visual self-motion information. J Physiol 582:153–161
Fitzpatrick RC, Day BL (2004) Probing the human vestibular system with galvanic stimulation. J Appl Physiol 96:2301–2316
Fitzpatrick R, Burke D, Gandevia SC (1994) Task-dependent reflex responses and movement illusions evoked by galvanic vestibular stimulation in standing humans. J Physiol 478:363–372
Fitzpatrick R, Burke D, Gandevia SC (1996) Loop gain of reflexes controlling human standing measures with the use of postural and vestibular disturbances. J Neurophysiol 76:3994–4008
Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF (1995) A framework for the analysis of mixed time series/point process data- theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol 64:237–278
Iles JF, Pisini JV (1992) Vestibular-evoked postural reactions in man and modulation of transmission in spinal reflex pathways. J Physiol 455:407–424
Lee Son GM, Blouin JS, Inglis JT (2008) Short duration galvanic vestibular stimulation evokes prolonged balance responses. J Appl Physiol 105:1207–1210
Lund S, Broberg C (1983) Effects of different head positions on postural sway in man induced by a reproducible vestibular error signal. Acta Physiol Scand 117:307–309
Matthews PBC (1993) Interaction between short and long latency components of the human stretch reflex during sinusoidal stretching. J Physiol 462:503–527
Mian OS, Day BL (2009) Determining the direction of vestibular-evoked balance responses using stochastic vestibular stimulation. J Physiol 587:2869–2873
Mian OS, Dakin CJ, Blouin JS, Fitzpatrick RC, Day BL (2010) Lack of otolith involvement in balance responses evoked by mastoid electric stimulation. J Physiol 588:4441–4451
Moore GP, Segundo JP, Perkel DH, Levitan H (1970) Statistical signs of synaptic interaction in neurons. Biophys J 10:876–900
Nashner LM, Wolfson P (1974) Influence of head position and proprioceptive cues on short latency postural reflexes evoked by galvanic stimulation of the human labyrinth. Brain Res 67:255–268
Reynolds RC (2010) The effect of voluntary sway control on the early and late components of the vestibular-evoked postural response. Exp Brain Res 201:133–139
Rosenberg JR, Amjad AM, Breeze P, Brillinger DR, Halliday DM (1989) The fourier approach to the identification of functional coupling between neuronal spike trains. Prog Biophys Mol Biol 53:1–31
Acknowledgments
Funded by the Natural Sciences and Engineering Research Council of Canada (JSB). JSB received salary support from the Canadian Institutes of Health Research—Canadian Chiropractic Research Foundation and Michael Smith Foundation for Health Research; CJD received doctoral awards from the Natural Sciences and Engineering Research Council of Canada and the Michael Smith Foundation for Health Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dakin, C.J., Inglis, J.T. & Blouin, JS. Short and medium latency muscle responses evoked by electrical vestibular stimulation are a composite of all stimulus frequencies. Exp Brain Res 209, 345–354 (2011). https://doi.org/10.1007/s00221-011-2549-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-011-2549-7