Abstract
In this study, we explored whether gaze and posture would exhibit coordination with the motion of a presented visual stimulus, specifically with regard to the complexity of the motion structure. Fourteen healthy adults viewed a set of four visual stimulus motion conditions, in both self-selected and semi-tandem stance, during which the stimulus moved horizontally across a screen, with position updated to follow a sine, chaos, surrogate, or random noise trajectory. Posture was measured using a standard force platform in self-selected and semi-tandem stance conditions while gaze was recorded using image-based eye-tracking equipment. Cross-correlation confirmed the continuous coordination of gaze with each type of stimulus motion, with increasing lag as stimulus motion complexity increased. Correlation dimension and approximate entropy were used to assess the complexity of the measured gaze and posture behaviors, with these values compared against those of the actual stimulus via ANOVA and dependent t tests. We found that gaze behavior was particularly sensitive to the complexity of the stimulus motion, according to both metrics. Posture seemed to be unaffected by stimulus motion viewing; however, different stance conditions did exhibit differences in posture metrics. Our results support an evolving understanding of how vision is used for determining perception and action.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several researchers have provided groundwork to the investigation of gaze and postural coupling to motion of, and within, visual displays (Amblard and Carblanc 1980; Piponnier et al. 2009). Further, Wade and Jones (1997) discuss the value of Gibson’s presentation of a theory of ecological optics, wherein the interactions between the self and the environment were bi-directionally tuned to both produce and consume visual information about the movement behavior of the individual (Gibson 1966, 1979). Stoffregen et al. (2006) present compelling argument on the implicit entrainment of postural sway to the motion of a visual stimulus. In a moving room environment, participants were asked to stand with resistance to visual coupling of their posture to the motion of the room. Although entrainment was reduced, relative to an uninstructed condition, it was not eliminated completely. This suggests that both perceptual and nonperceptual factors influence coupling between postural sway and vision. Ravaioli et al. (2005) used a visual stimulus scaled across motion frequency to find concomitant modulation of postural sway. A similar effect is found from the eye-tracking literature, connecting stimulus motion qualities to properties of smooth pursuit eye movements (Born et al. 2002; Kowler 2011). Boccignone (2004) presents the notion that during free viewing, individuals appear variably motivated to gather information about the local environment. In a ‘low’ attention state, visual exploration tends to settle toward more localized fixations. More complicated or active visual environments exhibit greater mathematical complexity, which appears to compel increased gaze activity and evoke more complex scan paths. If the scan path becomes too erratic, model-able by an unconstrained walk, then little information can be stabilized. For these reasons, humans tend to demonstrate complex gaze behavior, aligning with the idea that a search state with maximum complexity provides the visual system access to an optimal amount and quality of information. Renninger et al. (2007) concur with this notion, but also present that individuals tend to employ a strategy during which local uncertainty is reduced during gaze task. Coordination of gaze and posture to stimulus motion is advantageous in that it helps to avoid excessive perturbing distraction, but also serves to maintain awareness of opportunities for purposeful behavior. Matching the motion of a presented stimulus, with both eye and body motion, may be most informative when particularly tuned to the complexity of the structure of motion of the presented stimulus. Such sensitivity may confer improved resolution to temporal accuracy of postural sway.
Dynamic systems theory (DST) provides a supplementary perspective, specifically a subset dealing with the description of coupled oscillators. Such a perspective allows us to explore the possibility that there is an absence of “information processing” proper. Instead, that a mode of behavior exists in which a direct linkage between what is seen and how we respond is available to us as the organizing factor in our motor output (Kay and Warren 2001). Such an explanation would help explain enumerable examples of coordination within and between interactive human systems of similar and dissimilar structure, organization, and even motivation (Richardson et al. 2008). DST provides a framework through which this online coupling of internal and external constraints (and their interactions) can be coordinated, serving as a means for the self-organization of human movement behavior (Kelso 1995; Thelen and Smith 1996). A critical argument made within the DST framework is that systems may tend to be organized around particular control parameters. These are factors defined by some system constraints which, when scaled up or down, provoke concomitant changes in the future state of the system. This has been exampled by Kay and Warren (2001), showing a direct linkage between oscillations of the visual environment and the observed oscillations of mediolateral sway during walking and showing dynamic coupling of posture and gait. However, their work was limited to changes only in the frequency domain and tested only the sensitivity to periodic oscillations, i.e., sinusoidal rhythms. However, the natural oscillatory behavior of human movement is best characterized by chaotic oscillations (Stergiou et al. 2004). Whether coupled system dynamics might be observable upon fluctuations of temporal complexity as a control parameter is still unknown.
We propose in the current investigation that the complexity of motion of a presented visual stimulus will elicit reciprocally complex gaze and posture motion. If complexity does indeed serve as a control parameter of these behaviors, we should see reciprocation of the qualities of the stimulus motion to be present in the viewer’s gaze and postural behaviors. Metrics have been developed to study behavioral dynamics by capturing the dynamic response of the system in response to an experimental dynamic stimulus. Such metrics include the calculation of correlation dimension (CorrDim), which allows the approximation of the system’s complexity (Newell et al. 2000; Grassberger and Procaccia 1983), and approximate entropy (ApEn), which summarizes the repetitive nature of the behavior of the system (Vaillancourt 2000; Pincus 1991). The current investigation used these metrics to assess the dynamics response of posture and gaze to the dynamics of a visual stimulus. We further inquire to whether the adoption of a semi-tandem stance modulates the sensitivity of the effect of stimulus motion on posture and gaze. Inherently less stable, semi-tandem stance may serve to amplify, or negate, entrainment of gaze and posture to stimulus motion.
Methods
Participants and procedures
Fourteen adults (4 male and 10 female, age 29.8 ± 10.5 years, height 1.638 ± 0.1 m, mass 67 ± 14.2 kg) attended a single data collection session during which synchronous measures of eye movement and standing posture were collected while viewing a moving point-light stimulus with various conditions of differing frequency structure. Informed consent was obtained prior to all experimental procedures, as approved by the University of Nebraska Medical Center Institutional Review Board. During the experiment, individuals stood atop an AMTI force platform (Advanced Mechanical Technology Inc., OR6-7, with MSA-6 amplifier) where center of pressure was collected at 50 Hz. This frequency was selected to allow real-time synchronization with the eye-tracking equipment, which is hard coded to deliver data at this rate. A red dot stimulus (25 pixel radius) was presented on a 55″ 1,920 × 1,200 pixel LCD monitor, with a black curtain surround to block sight of objects in the peripheral visual field. The faceLab 4.5 (Seeing Machines, Acton, MA, USA) eye-tracking equipment was used to track eye movements, also at 50 Hz. The faceLab eye-tracking system provides on-screen gaze coordinates, in both pixels and physical dimensions, in ‘real time.’ The system uses a head model to maintain dynamic regions of interest, which contains each of the eyes (binocular), coupled with IR retinal reflection and pupillometry to calculate gaze direction. In software, the ‘world model’ is constructed with the physical dimensions of the experimental setup. This affords the calculation of on-screen gaze coordinates from the head-to-screen distance and the gaze direction. Data collection and coordination were managed through custom software designed in Labview (National Instruments, Austin, TX, USA), including software synchronization of all data streams. Figure 1 provides a diagram of the setup, with Fig. 2 further detailing the dimensions of the stimulus display configuration.
Four stimulus conditions (detailed below) were viewed under two stance conditions (self-selected and semi-tandem), equaling eight total trials. Stimulus condition order was randomized within each stance condition block. It was randomly selected whether participants did all four self-selected stance trials first, or all four semi-tandem stance trials, counterbalanced such that half the participants did it each way. Figure 1 displays the general configuration of foot position for each stance type. For self-selected (A), participants were asked to stand comfortably. All participants freely chose an approximately shoulder width stance with feet even in the frontal plane. For semi-tandem (B), participants were asked to stand in the center of the plate with their dominant foot forward, with the heel of the dominant foot and the toe of the nondominant foot even in the frontal plane.
Trials lasted for 5 min each to ensure the capture of adequate lengths of data. Participants were given the instruction to stand quietly and attend to the motion of the stimulus until the end of the trial, as indicated by the investigator. Room lights were dimmed, and conversation was held to a minimum throughout each trial. However, participants were allowed to speak and move about freely in the time between conditions. Grid markings and tape placed on the surface of the force platform were used to realign the feet to ensure a similar stance for each condition.
Stimulus presentation
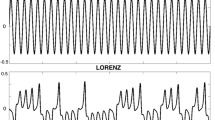
Participants were presented with four separate stimulus motion conditions, each defined by a different quality of motion of the stimulus. Each stimulus moved only horizontally and was positioned vertically central on the screen. The animations were constructed such that the position of the stimulus was updated at 50 Hz, above perceptive threshold of object motion, providing smooth oscillatory trajectories to which smooth pursuit eye movements could follow. The stimuli were designed to prevent saccadic motions, by keeping the velocity profile below 30°/s. Stimulus motion was defined to follow one of four main signal structures: sine, chaos, surrogate, and brown noise (Fig. 3). These particular signals were selected, as they span the spectrum of signal properties related to the temporal characteristics particularly interesting to the current investigation. As seen in Table 1, this set of stimuli provides incrementally increasing values of dimension and entropy, proceeding from the lowest values for the sine signal up to the largest for the brown noise signal. Each signal is comprised of 15,000 data points at 50 Hz, providing 5 mins of continuous point motion. Stimulus time series were constructed using both embedded and custom algorithms in Matlab (MathWorks, Natick, MA, USA). These data series were then accessed and displayed through the main Labview application onto the monitor.
Signals used to create the frequency structure of the motion of the point-light stimuli are shown in the first row. Data in rows two and three reflect the gaze and posture responses (respectively) from one representative participant. Of the 5 min trials, the middle 3 mins are shown. All data series are unit normalized, with ‘lower’ values representing leftward position and ‘higher’ values representing rightward position. Positions are all relative to the coordinated centers of the force plate and the LCD monitor
The sine signal was generated using the sin() function in Matlab, frequency 0.1 Hz. The chaos signal was produced from a model of the motion of a double pendulum. The x-axis position of the distal segment was extracted and used to produce the chaos signal, and was further processed to provide the surrogate signal, as well. The signal for the surrogate stimulus utilized a procedure, which applies a random number generated distributed noise to the frequency domain of the original time series (Theiler et al. 1992). In this case, it was the chaos signal from which a surrogate was produced. Ultimately, this was done as one means of producing a signal with random structure, which is yet restricted to a deterministic source. This is in contrast to the production of the brown noise signal, which was generated by the iterative addition of a random perturbation to the original point position. It is this spectrum of particular signal generation schemes that provides us the scale of signal properties seen in Table 1. The median frequency (computed using fft() function in Matlab) of each stimulus was between 0.1 and 0.2 Hz, with frequency dispersion <1 Hz, except for the brown noise signal. Brown noise is characterized by the display of 1/f 2 power spectral density, causing a bias toward a lower median frequency with broader frequency dispersion. Spectral and temporal metrics varied according to the nature of each signal structure. Velocity profiles were bound ± 30°/s.
Data processing
Gaze and postural data were recorded at 50 Hz, throughout the entire 5 min duration of stimulus condition presentation. Gaze data were recorded as the on-screen pixel coordinate toward which the participant was looking at each time point throughout the trial. Mediolateral center of pressure was recorded as the measure of posture. For both signals, only the horizontal component of motion was considered, as the stimulus signal was set to move only in the horizontal direction.
To avoid the influence of novelty, the first minute of data was eliminated, as was the fifth minute, due to possible influence of fatigue. The middle 3 mins (9,000 data points) of each segment were submitted for further processing. Characteristics of gaze and posture were calculated using custom Matlab software (MathWorks, Natick, MA, USA). Coupling of each measure, gaze and posture, to the stimulus signal was tested via cross-correlation metrics of maximum correlation (Max) and the time lag (Lag) at which Max occurred (Fig. 4). CorrDim and ApEn properties were calculated to determine the responsive changes, due to viewing the various structures of stimulus motion.
Cross-correlation group means and standard deviations, showing coupling ‘strength’ of each behavior with the stimulus motion, gaze (left) and posture (right). Maximum correlation values are shown across the top. Lag values (bottom) are reported in number of data points. Sampled at 50 Hz, 50 data points are equal to 1 s of experiment time. *Indicates significant difference, p < 0.001
Correlation dimension (CorrDim)
Calculating the CorrDim of a data series provides a representative of the number of active degrees of freedom of the system being evaluated (Newell et al. 2000). This is related to the general complexity of the given behavior of the system. Generally, it is taken that greater complexity is associated with more difficult to solve system organizations. This is often the case in less optimized performance of a novice to a given task. However, according to Bernstein (1967), experts tend to return to a higher complexity state as a means of expanding their repertoire of possible movement strategies. CorrDim was calculated in Matlab, using the algorithm presented by Grassberger and Procaccia (1983). Each measure was submitted to the CorrDim calculation with embedding dimension parameter previously determined via false nearest-neighbor algorithm (Abarbanel 1996). This is done to ensure that each time series is embedded in its own most appropriate state space, prior to further analysis. Values consistently ranged between 6 and 9 for the measured time series.
The use of CorrDim is considered valid mathematically with 10^D data points (Grassberger and Procaccia 1983). For the gaze signals expressing CorrDim ≈3, this would require 1,000 data points. In this experiment, 9,000 data points were considered for analysis. CorrDim for posture was 4–6, thus leading to the need from 10,000 to as many as 1,000,000 data points. At 50 Hz, the consideration of 9,000 data points is very close to the lower limit. Furthermore, at 50 Hz, to fulfill the upper limit of this requirement would require more than 333 h of continuous data collection, which is unreasonable for posture. It should also be mentioned that the 10^D estimation is based on known mathematical data and may not be appropriate for biological data. The requirements of this algorithm with respect to biological data are presently under investigation in our laboratory.
Approximate entropy (ApEn)
ApEn characterizes the temporal structure of variability within the signal, the degree to which a signal remains self-similar or dissimilar through time (Vaillancourt 2000). Higher values indicate that the behavior is irregular and lower values indicate maintained consistency. ApEn was computed using algorithms by Pincus (1991), implemented in Matlab, using parameter values m = 2 and r = 0.2 for all analyses. Effectively, the algorithm compares the logarithmic probability of the occurrence of an m + 1 length vector, relative to the probability of occurrence of an m length vector in the data.
Statistical analysis
Separate statistical analyses were conducted for Max, Lag, ApEn, and CorrDim for each data type (Gaze and Posture), each utilizing a 2 × 4 repeated-measures (stance × stimulus) ANOVA. Follow-up dependent t tests were used to elucidate identified differences. Statistical tests were conducted with an alpha set at 0.05.
Results
Cross correlations
Similar results were found in self-selected and semi-tandem stance conditions, with no interaction effects. Repeated-measures ANOVA revealed that Max was similar across all stimulus conditions for gaze (F 3,39 = 0.611, p = 0.612, with 95 % CI from 0.958 to 0.987 across all conditions) and posture (F 3,39 = 0.390, p = 0.761, with 95 % CI from −0.091 to 0.331 across all conditions). Figure 4 shows group data, in the self-selected stance condition. High correlation values for gaze response indicate tight coupling to the stimulus motion. Low values for postural response indicate weak coupling of postural sway to the motion of the stimulus.
Values of Lag differed for gaze in response to each stimulus (F 3,39 = 139.6, p < 0.000), with post hoc indicating progressively longer delays across the stimulus set (group average values; sine = 123 ± 1.9, chaos = 127 ± 1.2, surrogate = 129 ± 1.5, brown noise = 131 ± 1.9). With a sampling frequency of 50 Hz, each increment in Lag represents 20 ms, suggesting that the brown noise stimulus required an additional 160 ms in order to produce reciprocal gaze following patterns. With such low values of Max for posture, the follow-up consideration of Lag is generally less meaningful; however, a significant difference was found (F 3,39 = 4.86, p = 0.006). Post hoc indicates that this is due to a dramatically lower Lag in response to the brown noise stimulus (near 112, compared to values near 300 for each of the other stimulus conditions).
Gaze
A main effect was found for stimulus condition. Gaze behavior was found to be different across stimulus conditions for both CorrDim (F 3,39 = 4.64, p = 0.007) and ApEn (F 3,39 = 21.1, p < 0.001). However, neither a main effect for stance nor any interaction (stimulus and stance) was found (p > 0.05; Fig. 5). A breakdown of pair-wise comparisons in Table 2 indicates that the ApEn of gaze was greater when viewing the chaos or surrogate stimulus, but did not differ between the sine and brown noise conditions. CorrDim showed to only differ significantly between the chaos and surrogate conditions.
Posture
Participants’ posture responses show a significant main effect for stance condition (CorrDim, F 1,13 = 67, p < 0.001; ApEn, F 1,13 = 23.7, p < 0.001), but with no main effect for stimulus condition. All values of CorrDim and ApEn were lower for semi-tandem stance compared to self-selected stance. No interactions (stimulus by stance) were identified for ApEn or CorrDim.
Discussion
The results of this study do not fully support the stated hypothesis that the complexity of motion structure of the visual stimulus would elicit reciprocally complex gaze and posture behaviors. We do provide evidence to the extent that the viewers did coordinate gaze with the motion of each stimulus via cross-correlation. Further, complexity of the visual stimulus was identified to elicit reciprocal complexity in the response of the smooth pursuit gaze behavior, via ApEn. However, even with provision of modified stance, intended to amplify the possibility of entrainment, we did not find evidence that stimulus motion was a direct informant on the organization of posture. Moreover, these results suggest that these two systems (gaze and posture) are potentially independently operational in response to the visual stimulus motion. These results are surprising given the amount of evidence, which has been advanced that vision plays a regulative role on the organization of posture, both in standing (Stoffregen et al. 2006; Jeka et al. 2006; Giveans et al. 2011) and during walking (O’Connor and Kuo 2009; Kay and Warren 2001). It is possible that the task demands of our experiment were not sufficiently challenging to elicit changes in postural behavior (Stoffregen et al. 2000, 2007). Similarly, it is possible that the use of stimulus motion information was neither obligatory nor afforded any direct benefit to the organization of posture.
Potentially, nothing about our task prompted an obligation for the individual to modulate posture according to visual motion information in such a way as previous experiments, e.g., moving room scenarios. Our inclusion of the semi-tandem stance condition was intended to supply enhanced postural challenge to participants in order to prime the individuals into increased reliance on the visual stimulus to provide motion information (Ravaioli et al. 2005). With regard to visual information dependence, this change in posture did not confer sufficient deficit or direct negative effect on the individual. It did, however, seem that semi-tandem stance successfully challenged participants, with many self-reports of being “more difficult to stand tandem.” All values of CorrDim and ApEn were lower for semi-tandem stance compared to self-selected stance. It is uncertain from this study whether this is due to an impact on the strategies employed for postural control, as measured by CorrDim or ApEn, or whether these results suggest some fundamental difference in the way these postures must be organized for success within the particular constraints. The semi-tandem stance provided a smaller area of the base of support. This spatial constraint may have had at least some effect on the opportunity for posture to evolve as it would in self-selected stance, thus displaying different dynamics. Kirby et al. (1987) found a slew of evidence that sway amount is modified by foot position and corroborated more recently by Nejc et al. (2010). Further, Wang and Newell (2012) have identified altered time evolutionary characteristics of varied foot position stances.
Alternative to the ‘obligation’ argument for postural response to visual motion information, it might be that reciprocity scales with attention regulation, as has been shown with attempted suppression of attention (Stoffregen et al. 2006). It is possible in our experiment that participants were suppressing attention to the stimuli based on some inferred expectation of the task. No exact instructions were given to participants on how to coordinate their activities during each trial. However, it could be that persons developed their own expectations of what was anticipated from their postural behavior and constructed a set of self-constraints which, when applied across all trials, produced unnaturally uniform postural behaviors. If this were the case, it may have been beneficial to have provided more explicit directions to participants to pay close attention to the structure of variability of motion of the stimulus motion. Doing such may have drawn increased attention to the task and increased the likelihood of reciprocity of the stimulus motion complexity upon posture.
As discussed by Dokka et al. (2010), a standing viewer will incorporate information about self-motion along with information about environmental motion and consider each of these experiences in the process to determine appropriate postural response. It seems as such that the visual stimulus used in our experiment did better to provoke gaze following (for the intent of ‘vision for perception’), but was not sufficiently motivating to breach the stability afforded by vestibular and somatosensation, thereby not reaching the threshold for consideration as ‘vision for action,’ as described by Goodale (1992; Milner 2008). Perhaps, in our case, the motion of the visual stimulus was not sufficient to convince the viewer that they were experiencing self-motion. This result could be understood in that the information from vision was not sufficiently overwhelming of the information gained via the other sensory modalities (Jeka et al. 1998). Ultimately, we are inclined to conclude that our stimulus signal did not obviously impact the viewer’s egocentric perspective. In previous work, equal salience and strength of entrainment have not been found for stimulus motion of all frequencies (Kay and Warren 2001; Stoffregen et al. 2007). Possibly, the same effect is true here, and reciprocity does not occur for all values of complexity. Future work might show that a particular type or bandwidth of complexity may do better to elicit reciprocally complex posture.
In contrast, though, results indicate that gaze does exhibit reciprocally complex dynamics in response to a wide array of stimulus motion types. We presented four separate stimulus conditions, spanning the spectrum of complexity from absolutely periodic (sine) to as random as possible (given the nature of the task, brown noise). The ApEn measure reveals that gaze behavior emulated the temporal structure of variability present in the stimulus motion. Gaze ApEn was increased in accord with moderate stimulus ApEn (chaos and surrogate), yet exhibited less temporal variability in response to both very low (sine) and very high (brown noise) stimulus ApEn. Low temporal variability is expected in response to the sine stimulus and highlights the flexibility of the visual system to attend to simple and complex motions. This flexibility is important and confers high ecological value, providing that a single sensory system is modifiable for use in information acquisition under a variety of natural conditions (Boccignone 2004). This system can be endogenously constrained by modifying the attention state and exogenously by the ‘outcome value’ or affordance of utilizing the available visual information to guide movements (Thaler and Goodale 2010 and Thaler and Todd 2010; Dokka et al. 2010).
In response to the brown noise stimulus, which has the highest temporal variability, the gaze ApEn resembles that which is seen in response to the sine stimulus. Why would the gaze dynamics revert to a simpler motion strategy in response to the brown noise stimulus? It may be that the gaze behavior cannot present such high temporal variability as is exhibited by the brown noise stimulus. Alternately, there may be a limit on the sensitivity of the system or a critical threshold of stimulus motion complexity that can be emulated. We argue that it is a factor of the ‘value’ of the information presented by the different stimulus motions. The chaos and surrogate stimuli express deterministic variability, appearing random but deriving from an inherently meaningful source (which has been shown to approximate biological motion, Stergiou et al. 2004). However, the brown noise stimulus exhibits nondeterministic variability, which is essentially truly random, and has practically zero information content. Thus, it may be more appropriate to ignore the temporal structure of the brown noise stimulus during gaze following.
These results collectively suggest that an optimal range of complexity of stimulus motion exists that compels entrainment of gaze behavior. This finding coincides with previous assertions that persons are particularly successful when behaviors exhibit an optimal complexity (Stergiou et al. 2006; Haworth et al. 2013). Moreover, these results fall directly in line with the suggestions of Boccignone (2004) that a complex scan path might deliver the highest level of information. From this current work, we clearly observed changes in gaze behavior in response to stimulus motion, scaled across various complexity structures.
Potential applications of such a finding could include training/retraining of vision as an informer of the state of the body within its local environment. Glasauer et al. (2005) presented the notion that visual motion could be used to improve the postural sway of patients with vestibulopathy, an approach that was later extended by Laurens et al. (2010). Summary results of these experiments suggest that a stationary, large-field pattern provides information toward the stabilization of posture. This was true whether or not the eyes were pursuing a moving target or fixated on a stationary target. Guerraza and Bronsteinb (2008) discuss the current debate on whether it is ocular or extra-ocular factors which dominate in the regulation of standing posture. They conclude with the argument that the concurrent function of these two systems is what allows for such dynamic functional behavior of individuals. It seems certain that further developments in our understanding of this system will come from studies based on the inclusion of complexity and its role in optimization of system behavior (Renaud et al. 2003, 2007). Continued investigation along this line will provide additional insight into the intrinsic and environmental properties, which serve to modulate behavior in real time. Furthermore, by identifying these properties, continued growth of the empirical information base becomes available. It is this outcome which will continue to drive further understanding of the internal organization of the brain (and the human being as a whole organism) and the coordination of complex behavior.
Conclusion
It is hoped that the results of this study will be additive to the efforts of others in providing a firm base of research from which the use of visual information on subsequent movement behaviors may be fully understood. Zentgraf et al. (2011) put it well to acknowledge that the use of vision and the enactment of movement behavior are necessarily embedded within complex environments. Specifically, these environments are characterized by complex dynamic interactions amongst internal and external factors to the individual. Furthermore, by housing knowledge of these intricate interactions and the possession of higher level, meta-cognitive regulation of perception–action behavior, individuals are primed to have a “clear” awareness of their self-regulation of outcome behaviors. It is precisely for this reason that we should continue to be diligent in our approach to understanding the full organization of the visuomotor system.
References
Abarbanel HDI (1996) Analysis of observed chaotic data. Springer, New York
Amblard B, Carblanc A (1980) Role of foveal and peripheral visual information in maintenance of postural equilibrium in man. Percept Motor Skills 51:903–912
Bernstein N (1967) The co-ordination and regulation of movement. Pergamon Press, New York
Boccignone F (2004) Modelling gaze shift as a constrained random walk. Phys A 331:207
Born RT, Pack CC, Zhao R (2002) Integration of motion cues for the initiation of smooth pursuit eye movements. In: Hyönä J, Munoz DP, Heide W, Radach R (eds) Prog Brain Res, vol. 140. Elsevier Science
Dokka K, Kenyon RV, Keshner EA, Kording KP (2010) Self versus environment motion in postural control. PLoS Comput Biol 6:e1000680
Gibson J (1966) The senses considered as perceptual systems. Houghton Mifflin Co, Boston
Gibson J (1979) The ecological approach to visual perception. Houghton Mifflin Co, Boston
Giveans MR, Yoshida K, Bardy B, Riley M, Stoffregen TA (2011) Postural sway and the amplitude of horizontal eye movements. Ecol psychol 23:247–266
Glasauer S, Schneider E, Jahn K, Strupp M, Brandt T (2005) How the eyes move the body. Neurology 65:1291–1293
Goodale MA, Milner AD (1992) Separate visual pathways for perception and action. Trends Neurosci 15:20–25
Grassberger P, Procaccia I (1983) Characterization of strange attractors. Phys Rev Lett 50:346–349
Guerraza M, Bronsteinb AM (2008) Ocular versus extraocular control of posture and equilibrium. Clin Neurophysiol 38:391–398
Haworth J, Vallabhajosula S, Tzetzis G, Stergiou N (2013) Optimal variability and complexity: a novel approach for management principles. In: Banerjee S (ed) Chaos and complexity theory for management: nonlinear dynamics. IGI Global, USA
Jeka JJ, Oie K, Schöner G, Dijkstra T, Henson E (1998) Position and velocity coupling of postural sway to somatosensory drive. J Neurophysiol 79:1661–1674
Jeka J, Allison L, Saffer M, Zhang Y, Carver S, Kiemel T (2006) Sensory reweighting with translational visual stimuli in young and elderly adults: the role of state-dependent noise. Exp Brain Res 174:517–527
Kay BA, Warren WH (2001) Coupling of posture and gait: mode locking and parametric excitation. Biol Cybern 85:89–106
Kelso JAS (1995) Dynamic patterns: the self-organization of brain and behavior. MIT Press, Cambridge
Kirby RL, Price NA, MacLeod DA (1987) The influence of foot position on standing balance. J Biomech 20(4):423–427
Kowler E (2011) Eye movements: the past 25 years. Vision Res 51:1457–1483
Laurens J, Awai L, Bockisch CJ, Hegemann S, van Hedel HJA, Dietz V, Straumann D (2010) Visual contribution to postural stability: interaction between target fixation or tracking and static or dynamic large-field stimulus. Gait Posture 31:37–41
Milner AD, Goodale MA (2008) Two visual systems re-viewed. Neuropsychologia 46:774–785
Nejc S, Jernej R, Loefler S, Kern H (2010) Sensitivity of body sway parameters during quiet standing to manipulation of support surface size. J Sports Sci Med 9:431–438
Newell KM, Challis S, Morrison S (2000) Dimensional constraints on limb movements. Hum Mov Sci 19:175–201
O’Connor SM, Kuo AD (2009) Direction-dependent control of balance during walking and standing. J Neurophysiol 102:1411–1419
Pincus (1991) Approximate entropy as a measure of system complexity. P Natl Acad Sci USA 88:2297–2301
Piponnier JC, Hanssens JM, Faubert J (2009) Effect of visual field locus and oscillation frequencies on posture control in an ecological environment. J Vision 9:13
Ravaioli E, Oie KS, Kiemel T, Chiari L, Jeka JJ (2005) Nonlinear postural control in response to visual translation. Exp Brain Res 160:450–459
Renaud P, Décarie J, Gourd SP, Paquin LC, Bouchard S (2003) Eye-Tracking in immersive environments: a general methodology to analyze affordance-based interactions from oculomotor dynamics. Cyberpsychol Behav 6:519
Renaud P, Chartier S, Albert G, Décarie J, Cournoyer LG, Bouchard S (2007) Presence as determined by fractal perceptual-motor dynamics. Cyberpsychol Behav 10:122
Renninger LW, Verghese P, Coughlan J (2007) Where to look next? Eye movements reduce local uncertainty. J Vision 7:6
Richardson D, Dale R, Shockley K (2008) Synchrony and swing in conversation: coordination, temporal dynamics and communication. In: Wachsmuth I, Lenzen M, Knoblich G (eds) Embodied communication. Oxford University Press, Oxford, pp 75–93
Stergiou N, Buzzi UH, Kurz MJ, Heidel J (2004) Nonlinear tools in human movement. In: Stergiou N (ed) Innovative analyses of human movement. Human Kinetics, Champaign, pp 63–90
Stergiou N, Harbourne RT, Cavanaugh JT (2006) Optimal movement variability: a new theoretical perspective for neurologic physical therapy. J Neurol Phys Ther 30:120–129
Stoffregen TA, Pagulayan RJ, Bardy BG, Hettinger LJ (2000) Modulating postural control to facilitate visual performance. Hum Mov Sci 19:203–220
Stoffregen TA, Hove P, Schmit J, Bardy BG (2006) Voluntary and involuntary postural responses to imposed optic flow. Mot Control 10:24–33
Stoffregen TA, Bardy BG, Bonnet CT, Hove P, Oullier O (2007) Postural sway and the frequency of horizontal eye movements. Mot Control 11:86–102
Thaler L, Goodale MA (2010) Beyond distance and direction: the brain represents target locations non-metrically. J Vision 10(3):1–27
Thaler L, Todd JT (2010) Evidence from visuomotor adaptation for two partially independent visuomotor systems. J Exp Psychol Hum Percept Perform 36(4):924–935
Theiler J, Eubank S, Longtin A, Galdrikian B, Farmer JD (1992) Testing for nonlinearity in time series: the method of surrogate data. Physica D 58:77–94
Thelen E, Smith LB (1996) A dynamic systems approach to the development of cognition and action. MIT Press/Bradford Books Series in Cognitive Psychology, Cambridge
Vaillancourt N (2000) The dynamics of resting and postural tremor in Parkinson’s disease. Clin Neurophysiol 111:2045–2056
Wade MG, Jones G (1997) The role of vision and spatial orientation in the maintenance of posture. Phys Ther 77:619–628
Wang Z, Newell KM (2012) Phase synchronization of foot dynamics in quiet standing. Neurosci Lett 507:47–51
Zentgraf K, Munzert J, Bischoff M, Newman-Norlund RD (2011) Simulation during observation of human actions: theories, empirical studies, applications. Vision Res 51:827–835
Acknowledgments
This work was supported by National Institutes of Health (Grant Number K25HD047194), National Institute of Disability and Rehabilitation Research (Grant Numbers H133G040118, H133G080023), the Nebraska Research Initiative, NASA Nebraska Space Grant Fellowship, and a Dennis Weatherstone Pre-doctoral Fellowship from Autism Speaks (Grant Number 7070).
Conflict of interest
There is no conflict of interest associated with this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haworth, J.L., Vallabhajosula, S. & Stergiou, N. Gaze and posture coordinate differently with the complexity of visual stimulus motion. Exp Brain Res 232, 2797–2806 (2014). https://doi.org/10.1007/s00221-014-3962-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-014-3962-5