Abstract
The afferent volley generated during neuromuscular electrical stimulation (NMES) can increase the excitability of human corticospinal (CS) pathways to muscles of the leg and hand. Over time, such increases can strengthen CS pathways damaged by injury or disease and result in enduring improvements in function. There is some evidence that NMES affects CS excitability differently for muscles of the leg and hand, although a direct comparison has not been conducted. Thus, the present experiments were designed to compare the strength and specificity of NMES-induced changes in CS excitability for muscles of the leg and hand. Two hypotheses were tested: (1) For muscles innervated by the stimulated nerve (target muscles), CS excitability will increase more for the hand than for the leg. (2) For muscles not innervated by the stimulated nerve (non-target muscles), CS excitability will increase for muscles of the leg but not muscles of the hand. NMES was delivered over the common peroneal (CP) nerve in the leg or the median nerve at the wrist using a 1-ms pulse width in a 20 s on, 20 s off cycle for 40 min. The intensity was set to evoke an M-wave that was ~15% of the maximal M-wave in the target muscle: tibialis anterior (TA) in the leg and abductor pollicis brevis (APB) in the hand. Ten motor-evoked potentials (MEPs) were recorded from the target muscles and from 2 non-target muscles of each limb using transcranial magnetic stimulation delivered over the “hotspot” for each muscle before and after the NMES. MEP amplitude increased significantly for TA (by 45 ± 6%) and for APB (56 ± 8%), but the amplitude of these increases was not different. In non-target muscles, MEPs increased significantly for muscles of the leg (42 ± 4%), but not the hand. Although NMES increased CS excitability for target muscles to the same extent in the leg and hand, the differences in the effect on non-target muscles suggest that NMES has a “global” effect on CS excitability for the leg and a “focused” effect for the hand. These differences may reflect differences in the specificity of afferent projections to the cortex. Global increases in CS excitability for the leg could be advantageous for rehabilitation as NMES applied to one muscle could strengthen CS pathways and enhance function for multiple muscles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuromuscular electrical stimulation (NMES) is a common rehabilitation tool used to help restore movement following damage to central motor pathways (Sheffler and Chae 2007). Over time, NMES can strengthen corticospinal (CS) pathways to affected muscles leading to improvements in movement that outlast NMES training sessions (Liberson et al. 1961; Knash et al. 2003; Conforto et al. 2002; Everaert et al. 2010). These enduring improvements in function occur concomitantly with increased CS excitability that is driven, at least in part, by the electrically evoked afferent volley from muscles in both the leg (Knash et al. 2003; Kido and Stein 2004) and hand (Conforto et al. 2002; Hoffman and Field-Fote 2007). However, there is some evidence that NMES may affect CS excitability differently for muscles of the leg versus the hand. When applied to the leg, NMES may have a relatively weak but non-specific or “global” effect that increases CS excitability for multiple muscles (Kido and Stein 2004; Mang et al. 2010). In contrast, for the hand, NMES may have a stronger but more specific or “focused” effect that increases CS excitability for only muscles innervated by the stimulated nerve (target muscles) (Ridding et al. 2000, 2001). Unfortunately, differences in stimulation parameters between studies of the leg and hand make it difficult to compare results between the studies. Thus, the present experiments were designed to directly compare the strength and specificity of changes in CS excitability induced by NMES for muscles of the leg and hand when using the same stimulation parameters.
When NMES is applied to increase CS excitability for leg muscles, it is typically delivered at relatively high intensities that generate M-waves between 15 and 50% of the maximal M-wave (M max), frequencies between 25 and 200 Hz, a range of duty cycles, and durations of ~30–45 min. This type of NMES generates contractions in the target muscles that can be used for rehabilitation and to assist with functional movements. Delivered in this way, all studies showed that NMES increased CS excitability for target muscles (Khaslavskaia et al. 2002; Knash et al. 2003; Kido and Stein 2004; Khaslavskaia and Sinkjaer 2005; Mang et al. 2010), but the effect on CS excitability for leg muscles not innervated by the stimulated nerve (non-target muscles) was less consistent. Two studies reported no change in CS excitability for non-target leg muscles (Khaslavskaia et al. 2002; Knash et al. 2003), while 2 other studies reported significant increases (Kido and Stein 2004; Mang et al. 2010). In contrast, when NMES is applied to increase CS excitability for hand muscles, it is delivered at low intensities (near motor threshold), low frequencies (10–30 Hz), duty cycles involving short bursts of stimulation (i.e. 500 ms on–500 ms off), and for up to 2 h. This type of stimulation is designed to “prime” CNS circuits and increase CS excitability for subsequent rehabilitation sessions without producing large or functional contractions (Hoffman and Field-Fote 2007). Using this type of stimulation, CS excitability increased for the target muscles, but did not change for non-target hand muscles in close proximity (Ridding et al. 2000, 2001; McKay et al. 2002; Pitcher et al. 2003).
Generally, increases in stimulation intensity enhance the afferent drive generated by NMES (Smith et al. 2003) and increase CS excitability more than lower intensity NMES (Khaslavskaia et al. 2002). However, despite applying NMES at higher intensities and frequencies when activating the leg muscles compared with the hand muscles, CS excitability for target muscles increased similarly (range: ~40–100%). Therefore, we predict that when stimulation parameters are the same, NMES will increase CS excitability more for a target hand muscle than for a target leg muscle. Furthermore, the evidence that NMES can increase CS excitability in non-target leg muscles, but not in non-target hand muscles, suggests that NMES may have a global effect on CS excitability for leg muscles and a focused effect for hand muscles. Presently, it is unclear whether the differences in the strength and specificity of NMES on CS excitability for leg and hand muscles are due to physiological differences in the neural circuits that control the leg and hand or are simply due to methodological differences in the stimulation parameters used between studies.
In this study, we compared the effect of NMES of the common peroneal (CP) nerve in the leg and the median nerve at the wrist on CS excitability for target and non-target muscles when using the same stimulation parameters in the same participants. The CP and median nerves were chosen because they have been studied previously and are commonly stimulated for rehabilitation of dorsiflexion and grasping, respectively. We hypothesized that for target muscles, motor-evoked potentials (MEPs) evoked by transcranial magnetic stimulation (TMS) over the motor cortex would increase more after NMES of the hand than NMES of the leg. We also hypothesized that NMES applied to the leg would increase MEPs for non-target muscles, consistent with a global effect of NMES on CS excitability for leg muscles, while NMES applied to the hand would increase MEPs only for the target muscle, consistent with a focused effect of NMES on CS excitability for hand muscles. The results of this study contribute to a growing body of knowledge about how the afferent volley generated during NMES alters CS excitability in humans.
Methods
Eleven men and three women ranging in age from 19 to 47 years old and with no known neurological disorders participated in this study. All participants gave written informed consent prior to testing. The experimental procedures were approved by the Human Research Ethics Board at the University of Alberta. Subjects were seated with their backs and necks supported. All subjects participated in 2 separate ~3-h testing sessions at least 48 h apart in which NMES was applied to the CP nerve to activate tibialis anterior (TA) in the leg on one occasion and the median nerve to activate abductor pollicis brevis (APB) in the hand on the other occasion. The order of testing sessions was randomized for each participant. The time of day of each session was the same for each subject to reduce the potential confounding effect of diurnal changes in CNS excitability (Lagerquist et al. 2006; Tamm et al. 2009). When NMES was applied over the CP nerve, the hip, knee, and ankle angles were maintained at ~110°, 100°, and 90°, respectively. Padded restraints were secured around the right foot to minimize movement, and the left foot was placed on a foot rest. When NMES was applied over the median nerve, the shoulder, elbow, and wrist angles were maintained at ~15°, 120°, and 180°, respectively. The elbow was supported by an arm support, and the hand was placed on a hand rest in a relaxed position. Subjects were instructed to avoid consumption of caffeine 12 h prior to testing sessions and during a session to eliminate the influence of caffeine on CNS excitability (Walton et al. 2003) and to refrain from intense physical activity 12 h prior to the testing sessions.
Electromyography (EMG)
When NMES was applied to the CP nerve, EMG was recorded from TA, soleus, and vastus medialis (VM) of the right leg. When NMES was applied to the median nerve, EMG was recorded from APB, FDI, and extensor carpi ulnaris (ECU) of the right hand (see Fig. 1a). All EMG was recorded using bipolar (2.25 cm2) surface recording electrodes (Vermed Medical, Bellow Falls, Vermont) with the exception of EMG from FDI, which was recorded with electrodes trimmed down to ~1 cm2. EMG signals were pre-amplified (1,000×) and band-pass filtered at 10–1,000 Hz (NeuroLog System; Digitimer, Welwyn Garden City, Hertfordshire, England). Data were sampled at 2,000 Hz with a 12-bit A/D converter (National Instruments, Austin, Texas). During the collection of MEPs, data were recorded in 450-ms sweeps from 100 ms before to 350 ms after stimulus delivery.
Schematics of electrode placements and stimulation patterns used for experimental sessions. a shows that when CP nerve stimulation was applied, TA was considered to be the target muscle, soleus the adjacent muscle, and VM the remote muscle. When median nerve stimulation was applied, APB was considered to be the target muscle, FDI the adjacent muscle, and ECU the remote muscle. b shows the stimulation pattern delivered for NMES. Each gray box represents a period of NMES followed by a black line representing a rest period. NMES was delivered at an intensity of ~15% M max, a frequency of 100 Hz, and a pulse width of 1 ms for a total duration of 40 min
Neuromuscular electrical stimulation (NMES)
NMES was applied over the right CP nerve near the fibular head or the right median nerve at the wrist using bipolar round (3.2 cm) neurostimulation electrodes (Axelgaard Manufacturing Co., Ltd.) placed at the site that evoked a response (M-wave or H-reflex) at the lowest stimulation intensity in TA and APB, respectively. With these stimulation sites, TA and APB were considered to be the target muscles because they are innervated by the stimulated nerves. Muscles not innervated by the stimulated nerves were considered to be the non-target muscles and were categorized as either adjacent or remote muscles relative to the target muscle. Soleus and FDI were considered to be the adjacent muscles, and VM and ECU, the remote muscles, when NMES was applied to the CP and median nerves, respectively (see Fig. 1a).
Rectangular pulses of 1 ms duration were delivered from a Digitimer (DS7A, Hertfordshire, England) constant current stimulator for all experimental sessions. The stimulation was adjusted to an intensity at which a single stimulus evoked an M-wave that was ~15% M max in the target muscle (TA or APB) during a single trial in which 5 stimuli were delivered 3–5 s apart. NMES was then delivered at this intensity with a stimulation frequency of 100 Hz for 40 min in a 20 s on, 20 s off cycle (see Fig. 1b; Mang et al. 2010). A 1-ms pulse width was utilized because wide pulse NMES increases the afferent volley to the CNS and can enhance the central or “reflexive” contribution to electrically evoked contractions (Lagerquist and Collins 2008, 2010). Likewise, a frequency of 100 Hz was utilized because we have previously shown that 100-Hz NMES increases CS excitability more than 10, 50, or 200-Hz NMES (Mang et al. 2010). To eliminate any effect of voluntary contraction on CS excitability, participants were instructed to remain relaxed while NMES was applied.
Transcranial magnetic stimulation (TMS)
To test the excitability of the CS pathway, MEPs were evoked using TMS (Magpro R30; Medtronic Inc., Minneapolis, Minnesota) applied with a parabolic (Medtronic MMC-140, Minneapolis, Minnesota) or figure-of-eight coil (Medtronic MC-B70, Minneapolis, Minnesota). The parabolic coil was used in 6 subjects because it was difficult to evoke MEPs in leg muscles with the figure-of-eight coil. For a given subject, the same coil was used in both sessions. All MEPs were evoked, while subjects were relaxed. In the experimental session involving CP nerve stimulation, separate trials were conducted in which MEPs were evoked from the optimal stimulation sites (“hotspots”) for right TA, soleus, and VM. In the experimental session involving median nerve stimulation, MEPs were evoked in separate trials from the hotspots for right APB, FDI, and ECU. The hotspots were found before the application of NMES by moving the coil over the left motor cortex to find the site that elicited the largest amplitude MEP in the muscle of interest at the lowest intensity of stimulation. Using a Brainsight image-guided stimulation system (Rogue Research, Montreal, Quebec), the sites were recorded and the coil was manually held in place to maintain position and orientation (precision: ±3 mm) during measurement trials. Resting MEP thresholds were then determined by finding the lowest intensity that produced MEPs of at least 50 μV in 4 out of 8 stimuli. The intensity of TMS was then set at 120% of the threshold for each muscle for the measurements. Ten MEPs were evoked from each hotspot and recorded from each muscle immediately before (Pre) and after (Post) the prolonged period of NMES. The MEPs were evoked at an inter-stimulus interval that varied randomly between 4 and 6 s. The order in which muscles were tested was randomized before and after the NMES for each session.
Maximal M-waves (M max)
To determine M max for the target muscles, stimulation intensity was increased over 5 stimuli from below motor threshold to 1.5–2 times the minimum current required to evoke M max. M max was calculated as the largest M-wave evoked in the target muscle. The amplitude of M max from the target muscle was tested on 3 occasions before and after the delivery of NMES. Occasionally, the amplitude of M max was reduced in APB following the NMES. In these instances, the recording electrodes were replaced and M max was re-collected. In some instances, there were still small variations in M max, despite replacement of the electrodes. Thus, all MEPs in target muscles were normalized to the M max recorded nearest in time to the collection of the MEPs.
Data analyses
Changes in CS excitability induced by NMES were determined by quantifying and comparing the group averages of the 10 MEPs evoked before and after NMES. MEPs recorded from TA and APB were measured peak-to-peak and normalized to M max. As we did not measure M max in the non-target muscles (soleus, VM, FDI, and ECU), these MEPs were left in mV. To ensure all MEPs were obtained at rest, MEP data were inspected post hoc and discarded if the EMG during the 1 s before the TMS exceeded 2 standard deviations of the average baseline signal recorded at rest before the stimulation. Of the 1,680 MEPs evoked from 14 subjects, 17 MEPs (~1% of the total responses) were removed from the analyses based on this criterion. Data from the trials in which 5 stimuli at the selected NMES intensity were delivered were also inspected post hoc to ensure that NMES intensity was set correctly (to ~15% M max) for the target muscle and that there was minimal to no activation of the non-target muscles by the electrical stimulus.
A two-way repeated measures analysis of variance (RM-ANOVA) test was used to compare NMES-evoked changes in MEP amplitude between the leg and hand for the target muscles. The factors were Limb (2 levels: leg and hand) and Time (2 levels: Pre and Post). To test whether NMES had a global or focused effect on CS excitability in the leg and hand, separate two-way RM-ANOVAs were used to examine NMES-evoked changes in MEP amplitude for non-target muscles of each limb. For these ANOVAs, the factors were Muscle (2 levels: adjacent and remote) and Time (2 levels: Pre and Post). For all tests, the significance level was set at P < 0.05. As no significant interactions were found and each main effect had only 2 levels, post hoc analyses were not necessary. All descriptive statistics are reported as mean ± standard error.
Results
MEP amplitude for target muscles increased following the NMES over both the CP nerve and median nerve, but the magnitude of these increases was not different. NMES over the CP nerve also increased MEP amplitude for non-target muscles, while median nerve NMES did not alter MEP amplitude for non-target muscles.
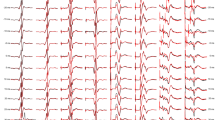
Figure 2 shows mean MEP waveforms (n = 10) recorded from one subject before and after NMES applied to the CP (Panel a) and median nerves (Panel b). MEPs are shown for the target muscles (TA and APB), the adjacent muscles (soleus and FDI), and the remote muscles (VM and ECU). After NMES of the CP nerve in the leg, MEPs increased by 69% for the target muscle, 98% for the adjacent muscle, and 2% for the remote muscle in the leg. After NMES of the median nerve at the wrist, MEPs increased by 81% for the target muscle, 15% for the adjacent muscle, and decreased by 9% in the remote muscle. Although changes in MEP amplitude were not tested for statistical significance in individual subjects, in this subject MEPs appear to increase markedly for both the target muscle and the adjacent muscle in the leg, but only for the target muscle in the hand.
Changes in MEP amplitude in the target, adjacent, and remote muscles following NMES of the leg and hand in a single subject. a shows the mean MEP waveforms (n = 10) before (Pre; gray line) and after (Post; black line) CP nerve stimulation. b shows the mean MEP waveforms (n = 10) before (Pre; gray line) and after (Post; black line) median nerve stimulation
Target muscles
MEP amplitude for the target muscles averaged across the group before (Pre) and after (Post) NMES was applied to the CP and median nerves are shown in Fig. 3a. Mean MEP amplitude before (Pre) and after (Post) NMES for the target muscle of the leg and hand for each subject is shown in Panels b and c, respectively. The RM-ANOVA revealed a main effect of Time [F (1, 13) = 11.1, P < 0.01], no main effect of Limb [F (1, 13) = 1.0, P = 0.33], and no interaction [F (1, 13) = 0.85, P = 0.37]. Thus, MEPs in the target muscle were significantly increased following NMES of both the leg (45 ± 6% increase) and hand (56 ± 8% increase), but the magnitude of these increases was not different.
MEP amplitude Pre-(gray) and Post-NMES (black) averaged across the group for the target muscles following NMES of the leg and hand. Asterisks in a represent significant increases from control (Pre-NMES). Error bars represent one standard error. b and c show mean MEP amplitude of the target muscle Pre-and Post-NMES for the leg and hand, respectively, for each subject
Non-target muscles
MEP amplitude for non-target muscles averaged across the group before (Pre) and after (Post) NMES was applied to the CP and median nerves are shown in Fig. 4a. For non-target muscles of the leg, the RM-ANOVA revealed a main effect of Time [F (1, 13) = 12.6, P < 0.01], no main effect of Muscle [F (1,13) = 2.4, P = 0.15], and no interaction [F (1, 13) = 0.84, P = 0.38]. These results show that NMES of the leg significantly increased MEPs in the non-target muscles (by 42 ± 4%), but the magnitude of these increases was not different between the adjacent (47 ± 5%) and remote (36 ± 3%) muscles. For non-target muscles of the hand, there was no main effect of Time [F (1, 13) = 0.05, P = 0.83], a main effect of Muscle [F (1, 13) = 9.1, P = 0.01 (not shown)], and no interaction [F (1, 13) = 0.11, P = 0.75]. Thus, although MEPs for both non-target muscles were elicited at a TMS intensity of 120% MEP threshold, the amplitude of MEPs in mV was different between the adjacent and remote muscles at all time points. Nonetheless, MEP amplitude did not change following NMES for either of the non-target muscles of the hand (30 ± 8 and 14 ± 5% for adjacent and remote muscles, respectively). MEP amplitude Pre- and Post-NMES for the non-target muscles of the leg and hand for each subject are shown in Panels b and c of Fig. 4, respectively. As there were no interactions between Time and Muscle, the data for adjacent and remote muscles have been collapsed in the figures to show the average changes in MEPs for non-target muscles from Pre- to Post-NMES.
MEP amplitude Pre-(gray) and Post-NMES (black) averaged across the group for the non-target muscles following NMES of the leg and hand. Asterisks in a represent significant increases from control (Pre-NMES). Error bars represent one standard error. b and c show mean MEP amplitude of the non-target muscle Pre-and Post-NMES for the leg and hand, respectively, for each subject
M-wave amplitudes of target and non-target muscles during NMES
Data from trials in which the NMES intensity was adjusted to the appropriate intensity were inspected post hoc to ensure that intensity was set correctly and that there was minimal to no M-wave elicited in the non-target muscles by the electrical stimulus. The average M max across the group for TA when the CP nerve was stimulated was 4.98 mV. Averaged across the group, the intensity of stimulation utilized for NMES of the CP nerve elicited an M-wave of 750 uV in TA (~15% M max). The average M-wave elicited in soleus at this intensity was 110 uV, and there was no M-wave elicited in VM. The average M max across the group for APB when the median nerve was stimulated was 8.0 mV. Averaged across the group, the intensity of stimulation utilized for NMES of the median nerve elicited an M-wave of 1.2 mV in APB (~15% M max). The average M-wave elicited in FDI at this intensity was 180 uV, and there was no M-wave elicited in ECU.
Discussion
The present experiments were designed to compare the strength and specificity of changes in CS excitability when NMES was applied to the leg and the hand. The main findings were that NMES of the leg and hand increased CS excitability for target muscles to the same extent, but only NMES applied to the leg increased CS excitability for non-target muscles. These results support the idea that NMES has a global effect on CS excitability for leg muscles and a focused effect for hand muscles.
CS excitability changes for target muscles
It is well documented that NMES increases CS excitability for the target muscle in both the leg and hand. CP nerve stimulation in the leg increased CS excitability for the target muscle (TA) by ~40–50% in some studies (Knash et al. 2003; Kido and Stein 2004; Khaslavskaia and Sinkjaer 2005) and by ~100% in others (Khaslavskaia et al. 2002; Mang et al. 2010). Similarly, ulnar nerve stimulation in the hand increased CS excitability for the target muscle (FDI) by ~50% in two studies (Ridding et al. 2000; McKay et al. 2002) and by ~100% in another study (Ridding et al. 2001). Although the increases in CS excitability induced by NMES for muscles of the leg and hand were similar in the aforementioned studies, the stimulation parameters used in these studies were vastly different. NMES of the leg was applied with high intensities (up to 50% M max) and frequencies (up to 200 Hz) for ~30–45 min. In contrast, NMES of the hand was applied with low intensities (near motor threshold) and frequencies (10 Hz) for 2 h. Presently, our hypothesis that when stimulation parameters were the same, CS excitability of the target muscle would increase more following NMES of the hand than NMES of the leg was not supported.
The similarity in the effect of NMES on the target muscle in the leg and hand could reflect a “ceiling effect” in the extent to which CS excitability can be increased by NMES. Previously, when stimulating the CP nerve with the same stimulation parameters utilized in this study, we found that CS excitability for TA was elevated by the twenty-fourth minute of stimulation and did not increase further over the remainder of the 40 min of stimulation (Mang et al. 2010). When stimulating the ulnar nerve in the hand for 2 h with lower intensity and lower frequency parameters than are used for the leg, McKay et al. (2002) found that increases in CS excitability plateaued after ~45–60 min. These studies suggest that a ceiling effect exists for both leg and hand muscles for the effect of NMES on CS excitability. If the ceiling was reached in the present study, our data suggest that it is the same for the leg and hand. However, further research is required to identify the NMES parameters that maximize increases in CS excitability and do so in the shortest amount of time.
CS excitability changes for non-target muscles
The present study is the first designed to investigate NMES-induced increases in CS excitability in non-target muscles of the leg and hand in the same participants using the same stimulation parameters. Our hypothesis that NMES would have a global effect on CS excitability for leg muscles and a focused effect for hand muscles was supported by our results. NMES of the CP nerve in the leg increased CS excitability significantly for the target muscle (TA) and non-target muscles (soleus and VM), while NMES of the median nerve at the wrist increased CS excitability significantly for only the target hand muscle (APB) and not for non-target muscles (FDI, ECU). It is also important to note that some individuals had particularly robust increases in CS excitability for non-target leg muscles following NMES, while others did not (see panels b and c of Figs. 3 and 4). Similar inter-subject variability for the effect of NMES on CS excitability has been described previously (Kaelin-Lang et al. 2002), suggesting that NMES has a greater effect on CS excitability for some individuals than for others. The lack of an effect of NMES on CS excitability for non-target muscles of the leg found in previous studies (Khaslavskaia et al. 2002; Knash et al. 2003) may have been due to insufficient statistical power to identify such changes, as the effects on non-target muscles were tested in relatively few subjects in those studies. Alternatively, by stimulating over the hotspot on the motor cortex for non-target muscles in the present study, which has not been done previously, we may have increased our ability to detect changes in CS excitability for non-target muscles. Regardless, the presently observed increases in CS excitability for non-target leg muscles could have been due to the spread of the electrical current from the stimulation site to activate afferents and efferents of non-target muscles; however, this is unlikely because CS excitability increased for non-target muscles in the leg but not in the hand, despite the further distance between the stimulation site and the non-target muscles for the leg compared with the hand. The small M-waves recorded in soleus and FDI may have been due to cross talk between EMG channels or activation of axons in the antagonist nerve. Thus, we cannot discount the possibility that increased CS excitability in soleus was due to activation of afferents in the tibial nerve, although CS excitability did not change for FDI despite recording of small M-waves similar to those observed in soleus. Also, this explanation cannot account for changes in CS excitability in VM following CP nerve stimulation as no M-waves were elicited in VM, and it is unlikely that current could spread from the CP nerve at the fibular head to activate afferents in VM above the knee. Thus, instead of an issue of current spread during NMES, we suggest that CS excitability increases in non-target leg muscles but not in non-target hand muscles, reflect differences in the neural circuits that control the leg and hand.
Studies investigating the afferent conditioning of MEPs by a preceding peripheral nerve stimulus suggest that CNS excitability is affected differently by afferent input received from the leg and hand (Deletis et al. 1992; Roy and Gorassini 2008). These studies show that when a peripheral nerve stimulus precedes a TMS stimulus, the resulting MEP is either inhibited or facilitated depending on the conditioning (peripheral nerve stimulus)-test (TMS) interval. The facilitation of MEPs was due to increases in both cortical and spinal excitabilities (Poon et al. 2008) and occurred for both target and non-target muscles (Deletis et al. 1992; Roy and Gorassini 2008). However, the facilitation appears to spread to non-target muscles to a greater degree for muscles of the leg (Deletis et al. 1992; Roy and Gorassini 2008) than the hand (Classen et al. 2000). This global effect of afferent input on excitatory circuits in leg motor cortex, when compared with a more focused effect for hand muscles, is consistent with the findings in the present study and could reflect more diffuse excitatory afferent projections to motor cortex from leg muscles than from hand muscles. Alternatively, the differences we observed in the leg and hand could be explained by differences in the mechanisms underlying cortical plasticity for hand and leg muscles; however, such mechanistic differences have not been addressed experimentally.
Location of excitability changes
Changes in MEP amplitude measured in the present study could be due to changes in the excitability of cortical circuits, spinal circuits, or both, but the experiments were not designed to distinguish between them. Previously, all studies have shown that cortical excitability of target muscles changes following NMES (Ridding et al. 2000; Stefan et al. 2000; Mang et al. 2010), with some evidence for spinal excitability changes as well (Khaslavskaia et al. 2002).
Implications
Afferent feedback and its effect on CS excitability is thought to play an important role in improvements in motor performance (Ziemann et al. 2001) and the acquisition of new motor skills (Pascual-Leone et al. 1995) in individuals with and without neurological injury. Our results show that afferent feedback from the leg has global effects on CS excitability compared with focused effects for hand muscles. If these results reflect more diffuse excitatory sensory projections from the leg compared with more specific projections from the hand, it may be that there are also differences in the mechanisms that underlie how afferent feedback from the leg and hand is utilized when humans learn new motor tasks. For example, more specific sensory projections from the hand to motor cortex than from the leg could contribute to the greater skillfulness of the hand for developing fine motor skills.
NMES is commonly used for treatment of impaired dorsiflexion and grasping following CNS injury (Sheffler and Chae 2007). Improvements in dorsiflexion (Knash et al. 2003; Everaert et al. 2010) and grasping (Conforto et al. 2002) that outlast the stimulation are associated with increases in CS excitability for the involved muscles. If enhancing CS excitability leads to functional improvements, then a better understanding of how NMES affects CS excitability for different muscles will be important for maximizing the efficacy of NMES therapies. Our data suggest that NMES can increase CS excitability to the same extent for the target muscles of both the leg and hand. Thus, rehabilitative benefits associated with increased CS excitability could be realized to a similar extent for the target muscle when applied to both the leg and hand. The global increases in CS excitability following NMES of the leg could be advantageous for rehabilitation as NMES applied to activate just one muscle could strengthen CS pathways and produce enduring functional benefits for multiple muscles.
References
Classen J, Steinfelder B, Liepert J, Stefan K, Celnik P, Cohen LG, Hess A, Kunesch E, Chen R, Benecke R, Hallett M (2000) Cutaneomotor integration in humans is somatotopically organized at various levels of the nervous system and is task dependent. Exp Brain Res 130:48–59
Conforto AB, Kaelin-Lang A, Cohen LG (2002) Increase in hand muscle strength of stroke patients after somatosensory stimulation. Ann Neurol 51:122–125
Deletis V, Schild JH, Beric A, Dimitrijevic MR (1992) Facilitation of motor evoked potentials by somatosensory afferent stimulation. Electroencephalogr Clin Neurophysiol 85:302–310
Everaert DG, Thompson AK, Chong SL, Stein RB (2010) Does functional electrical stimulation for foot drop strengthen corticospinal connections? Neurorehabil Neural Repair 24(2):168–177
Hoffman LR, Field-Fote EC (2007) Cortical reorganization following bimanual training and somatosensory stimulation in cervical spinal cord injury: a case report. Phys Ther 87:208–223
Kaelin-Lang A, Luft AR, Sawaki L, Burstein AH, Sohn YH, Cohen LG (2002) Modulation of human corticomotor excitability by somatosensory input. J Physiol 540:623–633
Khaslavskaia S, Sinkjaer T (2005) Motor cortex excitability following repetitive electrical stimulation of the common peroneal nerve depends on the voluntary drive. Exp Brain Res 162:497–502
Khaslavskaia S, Ladouceur M, Sinkjaer T (2002) Increase in tibialis anterior motor cortex excitability following repetitive electrical stimulation of the common peroneal nerve. Exp Brain Res 145:309–315
Kido TA, Stein RB (2004) Short-term effects of functional electrical stimulation on motor-evoked potentials in ankle flexor and extensor muscles. Exp Brain Res 159:491–500
Knash ME, Kido A, Gorassini M, Chan KM, Stein RB (2003) Electrical stimulation of the human common peroneal nerve elicits lasting facilitation of cortical motor-evoked potentials. Exp Brain Res 153:366–377
Lagerquist O, Collins DF (2008) Stimulus pulse width influences H-reflex recruitment but not H(max)/M(max) ratio. Muscle Nerve 37(4):483–489
Lagerquist O, Collins DF (2010) Influence of stimulus pulse width on M-waves, H-reflexes, and torque during tetanic low-intensity neuromuscular stimulation. Muscle Nerve 42(6):886–893
Lagerquist O, Zehr EP, Baldwin ER, Klakowicz PM, Collins DF (2006) Diurnal changes in the amplitude of the Hoffmann reflex in the human soleus but not in the flexor carpi radialis muscle. Exp Brain Res 170:1–6
Liberson WT, Holmquest HJ, Scot D, Dow M (1961) Functional electrotherapy: stimulation of the peroneal nerve synchronized with the swing phase of the gait of hemiplegic patients. Arch Phys Med Rehabil 42:101–105
Mang CS, Lagerquist O, Collins DF (2010) Changes in corticospinal excitability evoked by common peroneal nerve stimulation depend on stimulation frequency. Exp Brain Res 203:11–20
McKay D, Brooker R, Giacomin P, Ridding M, Miles T (2002) Time course of induction of increased human motor cortex excitability by nerve stimulation. NeuroReport 13:1271–1273
Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M (1995) Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol 74:1037–1045
Pitcher JB, Ridding MC, Miles TS (2003) Frequency-dependent, bi-directional plasticity in motor cortex of human adults. Clin Neurophysiol 114:1265–1271
Poon DE, Roy FD, Gorassini MA, Stein RB (2008) Interaction of paired cortical and peripheral nerve stimulation on human motor neurons. Exp Brain Res 188:13–21
Ridding MC, Brouwer B, Miles TS, Pitcher JB, Thompson PD (2000) Changes in muscle responses to stimulation of the motor cortex induced by peripheral nerve stimulation in human subjects. Exp Brain Res 131:135–143
Ridding MC, McKay DR, Thompson PD, Miles TS (2001) Changes in corticomotor representations induced by prolonged peripheral nerve stimulation in humans. Clin Neurophysiol 112:1461–1469
Roy FD, Gorassini MA (2008) Peripheral sensory activation of cortical circuits in the leg motor cortex of man. J Physiol 586(Pt 17):4091–4105
Sheffler LR, Chae J (2007) Neuromuscular electrical stimulation in neurorehabilitation. Muscle Nerve 35:562–590
Smith GV, Alon G, Roys SR, Gullapalli RP (2003) Functional MRI determination of a dose-response relationship to lower extremity neuromuscular electrical stimulation in healthy subjects. Exp Brain Res 150:33–39
Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J (2000) Induction of plasticity in the human motor cortex by paired associative stimulation. Brain 123(Pt 3):572–584
Tamm AS, Lagerquist O, Ley AL, Collins DF (2009) Chronotype influences diurnal variations in the excitability of the human motor cortex and the ability to generate torque during a maximum voluntary contraction. J Biol Rhythms 24:211–224
Walton C, Kalmar J, Cafarelli E (2003) Caffeine increases spinal excitability in humans. Muscle Nerve 28:359–364
Ziemann U, Muellbacher W, Hallett M, Cohen LG (2001) Modulation of practice-dependent plasticity in human motor cortex. Brain 124:1171–1181
Acknowledgments
This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada. The authors thank Dr. Ying Cui for statistical consultation and Mr. Alejandro Ley for his technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mang, C.S., Clair, J.M. & Collins, D.F. Neuromuscular electrical stimulation has a global effect on corticospinal excitability for leg muscles and a focused effect for hand muscles. Exp Brain Res 209, 355–363 (2011). https://doi.org/10.1007/s00221-011-2556-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-011-2556-8