Abstract
Transspinal (or transcutaneous spinal cord) stimulation is a promising noninvasive method that may strengthen the intrinsic spinal neural connectivity in neurological disorders. In this study we assessed the effects of cervical transspinal stimulation on the amplitude of leg transspinal evoked potentials (TEPs), and the effects of lumbosacral transspinal stimulation on the amplitude of arm TEPs. Control TEPs were recorded following transspinal stimulation with one cathode electrode placed either on Cervical 3 (21.3 ± 1.7 mA) or Thoracic 10 (23.6 ± 16.5 mA) vertebrae levels. Associated anodes were placed bilaterally on clavicles or iliac crests. Cervical transspinal conditioning stimulation produced short latency inhibition of TEPs recorded from left soleus (ranging from − 6.11 to -3.87% of control TEP at C-T intervals of -50, -25, -20, -15, -10, 15 ms), right semitendinosus (ranging from − 11.1 to -4.55% of control TEP at C-T intervals of -20, -15, 15 ms), and right vastus lateralis (ranging from − 13.3 to -8.44% of control TEP at C-T intervals of -20 and − 15 ms) (p < 0.05). Lumbosacral transspinal conditioning stimulation produced no significant effects on arm TEPs. We conclude that in the resting state, cervical transspinal stimulation affects the net motor output of leg motoneurons under the experimental conditions used in this study. Further investigations are warranted to determine whether this protocol may reactivate local spinal circuitry after stroke or spinal cord injury and may have a significant effect in synchronization of upper and lower limb muscle synergies during rhythmic activities like locomotion or cycling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transspinal (or transcutaneous spinal cord) stimulation is a promising non-invasive method that may strengthen the intrinsic spinal neural connectivity in upper motor neuron lesions and promote physiological synergistic interactions between arm and leg muscles during locomotion, cycling or other rhythmic motor activities. Paired transspinal stimulation of cervical and lumbosacral spinal cord segments may constitute an approach to strengthen the intrinsic spinal neuronal connectivity between arms and legs. This thesis is supported by the segmental effects of transspinal stimulation and the neurophysiological evidence on arm and leg neural coupling and interlimb coordination discussed herewith. Cervical transspinal stimulation (1 ms pulse, supra motor threshold; intensity = 94.21 ± 26.14 mA; mean ± SD) produces a short (0, 5 ms) and medium (45, 50, 55 ms) latency soleus H-reflex facilitation (Islam et al. 2020) that parallels the facilitation of leg transspinal evoked potentials (TEPs) by ulnar nerve conditioning stimulation, with and without volitional leg motor activity, in people with spinal cord injury (Atkinson et al. 2020). However, this is opposite to the reported depression of epidural stimulation-produced TEPs (Angeli and Gerasimenko 2023). In a similar manner, arm TEP amplitudes increase following fibular nerve stimulation or lumbosacral transspinal stimulation (Atkinson et al. 2022). The ascending and descending neuromodulation effects of lumbosacral transspinal stimulation are further supported by the significant changes on cortical and corticospinal excitability, and temporal summation of leg motor evoked potentials (MEPs) and leg TEPs on surface electromyogram (Knikou 2014; Knikou et al. 2015; Dixon et al. 2016; Steele et al. 2021).
Neuromodulation of networks subserving arm and leg coupling is well established. Both ipsilateral and contralateral rhythmic arm movements decrease soleus H-reflex excitability in seated and standing humans (Knikou 2007), while static contralateral arm flexion and extension produces inhibition and facilitation in the ipsilateral soleus tendon reflex, respectively (Delwaide et al. 1977). Coupling of cervical and lumbosacral enlargements is apparent during movement, with interlimb cutaneous reflexes of arms and legs to be modulated in a walking speed-dependent manner (Klarner et al. 2020) and cutaneous afferents to modulate muscle activity throughout the body with forelimb-to-hindlimb being stronger compared to hindlimb-to-forelimb neuronal pathways (Pearcey and Zehr 2019). Short, medium, and long latency reflexes produced by cutaneous stimulation of the wrist or ankle strongly support for movement coordination via interlimb reflexes at rest (Zehr et al. 2001) and during rhythmic activity (Zehr and Haridas 2003). Furthermore, the lumbosacral cord potentials with an onset latency of 12 ms produced by median nerve stimulation at or above motor threshold (Sarica and Ertekin 1985) support directly for neuronal pathways connecting cervical and lumbosacral spinal cord segments.
Cervical and lumbosacral transspinal stimulation may activate α motor neurons innervating arm and leg muscles via similar pathways. Cervical transspinal stimulation produces concurrently TEPs in arm and forearm muscles, with the flexor carpi radialis (FCR) TEP displaying a latency half that of the FCR H-reflex (Einhorn et al. 2013; Oh et al. 2022). This finding mirrors the soleus TEP latency that is close to half of the soleus H-reflex latency (Knikou 2013). Both arm and leg TEPs are prone to post-activation depression (Milosevic et al. 2019; Fleming et al. 2023) and follow a sigmoid recruitment curve (Skiadopoulos et al. 2022). Moreover, both cervical and lumbosacral transspinal stimulation produce an early and prolonged FCR and soleus H-reflex depression, respectively (Einhorn et al. 2013; Knikou and Murray 2018), in agreement with the medium latency inhibitory postsynaptic potentials exerted on soleus motor neurons following lumbosacral transspinal stimulation (Yildiz et al. 2024).
Collectively, the main research question in this study was to examine to what extent transspinal stimulation over the cervical and lumbosacral spinal cord segments can affect simultaneously the net output of different groups of motor neurons innervating arm and leg muscles bilaterally. The specific objectives of this study were to establish the effects of suprathreshold cervical transspinal stimulation on TEPs recorded bilateral from knee/ankle muscles, and the effects of suprathreshold lumbosacral transspinal stimulation on TEPs recorded bilateral from forearm/arm muscles.
Materials and methods
Subjects
Fourteen (6 women) (27.9 ± 9.8; mean ± SD) healthy and physically active subjects participated in the study. Each participant signed an informed consent form before enrollment to the study. Individuals with history of neurological, muscular, or psychiatric disorders, and wearing pacemakers were excluded from the study.
Study Design
A single-group repeated-measures study design was used to evaluate the effects of suprathreshold cervical and lumbosacral transspinal stimulation on leg and arm TEPs, respectively. Two experimental protocols were completed. The protocols were approved by the Institutional Review Board (IRB) of the City University of New York (CUNY). All experimental procedures were conducted in compliance with the Declaration of Helsinki and CUNY IRB-wide regulations and guidelines. In the first protocol, the effects of cervical transspinal stimulation on leg TEPs were investigated. The second protocol aimed to investigate the effects of lumbosacral transspinal stimulation on arm TEPs. The order of the protocols was randomized across subjects and were completed in two recording sessions on the same day, with a minimum of 30 min of rest in between.
For both protocols, two control sequences were recorded. Subjects reported no pain but some discomfort during stimulation, with short-lived effects. Blood pressure and heart rate were measured three times (initial, intermediate, final) at each experimental session, and both remained unaltered. Given the distance between the cervical and lumbosacral spinal cord levels, we did not expect to encounter occlusion effects as reported with multi-site transspinal stimulation (Tran et al. 2024).
Surface electromyography (EMG)
Following standard preparation (skin was dry shaved, abraded, and cleaned with alcohol), single differential bipolar surface electrodes (common mode rejection ratio > 100dB at 40 Hz, input impedance > 100,000 MΩ) with fixed inter-electrode distance of 2 cm (MA-300, Motion Lab Systems Inc., Baton Rouge, LA, USA) were used to record myoelectric signals from both arms and/or legs while at rest from biceps brachii (BIC), triceps brachii (TRIC), FCR, extensor carpi radialis (ECR), vastus lateralis (VL), semitendinosus (ST), soleus (SOL), and tibialis anterior (TA) muscles. Surface electrodes were secured with Tegaderm transparent film (3 M Healthcare, St. Paul, MN, USA). All EMG signals were low-pass filtered with a cut-off frequency of 1,000 Hz (MA-300, Motion Lab Systems Inc., Baton Rouge, LA, USA), recorded at a sample rate of 2,000 Hz using 1401 plus analog-to-digital interface running Spike 2 (Cambridge Electronics Design Ltd., England, UK), and saved in a personal computer as Spike 2 data files for off-line analysis.
Transspinal stimulation
We delivered cervical and lumbosacral transspinal stimulation according to methods we have previously used in our laboratory (Einhorn et al. 2013; Knikou 2013, 2014; Knikou et al. 2015; Skiadopoulos et al. 2022). With subjects seated at the edge of a treatment table, the Cervical 3 spinous process was identified via palpation. A single reusable self-adhered cathode electrode (10.2 × 5.1 cm2, Uni-Patch, Massachusetts, USA) was placed at midline along the vertebrae equally between the left and right paravertebral sides covering from Cervical 3 to Cervical 7 / Thoracic 1 vertebrae. A pair of interconnected anode electrodes (same type as the cathode) was placed on either side of the clavicles. For lumbosacral transspinal stimulation, the Thoracic 10 spinous process was identified via palpation and anatomical landmarks. A single reusable self-adhered cathode electrode (10.2 × 5.1 cm2, Uni-Patch, Massachusetts, USA) was placed at midline along the vertebrae equally between the left and right paravertebral sides covering from Thoracic 10 to Lumbar 1–2 vertebrae levels. A pair of interconnected anode electrodes (same type as the cathode) was placed on either side of the iliac crests. Transspinal stimulation was delivered via a constant current stimulator (DS7A or DS7AH, Digitimer Ltd., Welwyn Garden City, UK) with single monophasic 1 ms pulses at 0.2 Hz triggered by Spike 2 scripts (CED Ltd., Cambridge, UK).
Experimental protocols
Effects of cervical transspinal stimulation on leg TEPs
In this protocol the leg TEPs evoked by lumbosacral transspinal stimulation were conditioned by cervical transspinal stimulation. Subjects were supine with knee joints flexed at 30 degrees, while lumbosacral transspinal stimulation intensity was increased progressively, and the right and left SOL TEPs were observed on a digital oscilloscope (Tektronix Inc., USA). Optimal cathode position referred to the position when the TEPs in the right and left SOL muscles occurred at similar intensities and had similar shapes. For each subject while supine with knees and hips flexed at 30°, the stimulation intensity that the right soleus TEP was equal to 100 mV peak-to-peak amplitude on the oscilloscope was termed as TEP threshold and corresponded to 20.5 ± 1.8 mA across subjects. Ten TEPs at 0.2 Hz from leg muscles were recorded at an average of 1.6 ± 0.34 SOL TEP (23.6 ± 16.5 mA) threshold under control conditions and following cervical transspinal conditioning stimulation delivered at twice the right FCR TEP threshold. Conditioned leg TEPs were recorded randomly at C-T intervals ranging from negative 50 to positive 50 ms with 5 ms increment steps (21 total C-T intervals).
Effects of lumbosacral transspinal stimulation on arm TEPs
In this protocol the arm TEPs evoked by cervical transspinal stimulation were conditioned by lumbosacral transspinal stimulation. The experimental procedures were exactly as outlined before, with the main difference being that the test and conditioning stimuli were the cervical and lumbosacral transspinal stimulation, respectively. Optimal cathode position referred to when the TEPs in the right and left FCR muscles occurred at similar thresholds, and at increasing intensities the shapes of the action potentials were similar. For each subject while supine and arms placed parallel to the body, the stimulation intensity that the right FCR TEP was equal to 100 mV peak-to-peak amplitude on the oscilloscope was termed as FCR TEP threshold and corresponded to 11.5 ± 0.9 mA, across subjects. Ten TEPs at 0.2 Hz from arm muscles were recorded at 1.85 ± 0.46 FCR TEP threshold under control conditions and following lumbosacral transspinal conditioning stimulation delivered at twice the right SOL TEP threshold (28 ± 1.94 mA). Conditioned arm TEPs were recorded randomly at C-T intervals ranging from negative 50 to positive 50 ms with 5 ms increment steps (21 total C-T intervals).
Data analysis
The onset latency was measured from the waveform average of control arm and leg TEPs for each muscle and subject, at a point that the first deflection from baseline was present. All control and conditioned responses (arm TEPs, leg TEPs) were measured as the area under the rectified curve because there are no differences when measured as peak-to-peak amplitude or as area (Knikou and Taglianetti 2006). Markers were visually placed at each full-wave rectified sweep from the onset latency until the response returned to baseline (Spike 2, CED Ltd., UK). The conditioned responses at each C-T interval were expressed as a percentage of the mean value of the homonymous control response. The mean value of each normalized conditioned TEP from each subject was grouped based on the C-T interval and muscle.
Shapiro-Wilk’s test for normal distribution was established. According to the normality results, non-parametric tests were used for inference. A Sign test was conducted to determine whether there was a significant difference in the latency values of each TEP between the left and right muscles. For each muscle separately, a non-parametric Friedman test (Conover and Iman 1981) was performed to test the effect of transspinal stimulation on TEPs. When necessary, this was followed by an exact non-parametric many-to-one post-hoc test (Eisinga et al. 2017) with false discovery rate correction, to establish statistically significant differences between control and conditioned TEPs. The non-parametric Skillings-Mack test was used for missing data (Skillings and Mack 1981). Kendall’s W coefficient of concordance was reported as the effect size (W < 0.10 negligible, W < 0.25 small, W < 0.40 moderate, otherwise large effect). Rank biserial correlation (rrb) was used as effect size for significant post-hoc test (0 being the lowest and 1 being the highest effect size) (Cureton 1956). All statistical tests were performed at α = 0.05. Results are reported as median and interquartile range (Mdn [Q1, Q3]). All tests were performed using the PMCMRplus and effect size packages for the R statistical language (Ben-Shachar et al. 2020; Pohlert 2023; R Core Team 2023).
Results
The median latency for each TEP was not statistically significant different between the left and right BIC (6.75 [6.6, 7.5] vs. 6.9 [6.6, 7,1] ms; S = 3, p = 0.09), TRIC (7 [6.4, 8.3] vs. 7.7 [6.7, 8.8] ms; S = 4, p = 0.54), ECR (11.0 [10.2, 12.2] vs. 10.6 [10.0, 12.4] ms; S = 5, p = 1), FCR (10.4 [9.5, 11.3] vs. 9.85 [9.02, 10.6] ms; S = 8, p = 0.38), VL (9.2 [9.0, 9.8] vs. 9.0 [8.1, 9.0] ms; S = 2, p = 1), ST (13.6 [13.6, 14.2] vs. 14 [14, 14.3] ms; S = 2, p = 1), TA (19.0 [19.0, 20] vs. 19.0 [16.7, 20.1] ms; S = 2, p = 1), and SOL (19.9 [19.5, 20.2] vs. 20.0 [19.5, 20.6] ms; S = 4, p = 1). This indicates that the cathodal stimulating electrode was placed on the spinal process with an equal distance from the left and right paravertebral muscles and that transsynaptic activation of motoneurons occurred via similar pathways in the left and right sides. The latencies are consistent to our previous reports for arm (Einhorn et al. 2013) and leg (Knikou et al. 2015) TEPs.
Effects of cervical transspinal conditioning stimulation on leg TEPs
Raw waveform averages of TEPs recorded from leg muscles under control conditions (black lines) and following cervical transspinal conditioning stimulation (red lines) are shown for each C-T interval tested from a representative subject (C09) in Fig. 1.
Raw waveform averages of TEPs recorded bilaterally from the right and left SOL, TA, ST, and VL muscles under control conditions (black lines) and following cervical transspinal conditioning stimulation (red lines) at each conditioning-test (C-T) interval tested for one subject (C09). The waveform average of TEPs is shown for each C-T interval tested ranging from negative 50 to positive 50 ms. SOL: soleus, TA: tibialis anterior, ST: semitendinosus, VL: vastus lateralis, TEPs: transspinal evoked potentials
The overall effects of cervical transspinal conditioning stimulation on leg TEPs for all subjects are depicted in Fig. 2. The conditioned TEPs are normalized to the mean amplitude of the homonymous control TEP and subtracted from 100 for ease recognition of changes from zero. The conditioned right VL (F(21, 210) = 1.96, p = 0.009; Kendall’s W = 0.16) was significantly different from control values at the C-T intervals of -20 ms (right VL TEP = -13.3 [-32.4, -4.66]; rrb = 0.79; p = 0.04) and − 15 ms (right VL TEP = -8.34 [-13.3, 7.35]; rrb = 0.91; p = 0.04) (Fig. 2). Statistically significant differences of the conditioned TEPs among C-T intervals for the left VL were found (F(21, 210) = 1.63, p = 0.045; Kendall’s W = 0.14), but the conditioned left VL TEP was not different from control values (p > 0.05). The conditioned right ST TEP was significantly different from control values (F(21, 252) = 2.15, p = 0.003; Kendall’s W = 0.15) at the C-T intervals of -20 ms (-11.1 [-23, 3.5]; rrb = 0.08; p = 0.04), -15 ms ( -4.55 [-19.1, -0.68]; rrb = 0.91; p = 0.02), and 15 ms (-5.76 [-18.6, 4.6]; rrb = 0.21; p = 0.01). For the left ST TEP despite the significant overall effect among conditioned TEPs (F(21, 252) = 1.86, p = 0.01; Kendall’s W = 0.13), no significant differences from control TEP values were found (p > 0.77; Fig. 2).
Effects of cervical transspinal stimulation on leg TEPs. TEPs recorded from the right and left SOL, TA, ST, and VL muscles following single pulse cervical transspinal conditioning stimulation from all subjects as a percentage of median amplitude of the homonymous control TEP and subtracted from 100 for ease recognition of changes from zero. For all cases, conditioned TEPs are shown for conditioning-test intervals ranging from negative to positive 50 ms. Red denotes significant differences between control and conditioned TEPs based on the Friedman test followed by many-to-one post-hoc test with false discovery rate correction. Error bars denote the IQR. SOL: soleus, TA: tibialis anterior, ST: semitendinosus, VL: vastus lateralis, TEPs: transspinal evoked potentials
The conditioned TEPs recorded from the left SOL muscle were significantly different from control TEP values (F(21, 273) = 2.53, p < 0.001; Kendall’s W = 0.16), and were decreased at the C-T intervals of -50 ms (-4.59 [-7.4, 0.77]; rrb = 0.85; p = 0.04), -25 ms ( -4.95 [-5.78, 0.59]; rrb = 0.58; p = 0.04), -20 ms ( -6.11 [-7.43, -1.50]; rrb = 0.70; p = 0.002), -15 ms ( -4.54 [-6.80, -0.07]; rrb = 0.66; p = 0.04), -10 ms ( -3.87 [-11.2, 0.17]; rrb = 0.60; p = 0.04), and at 15 ms (-4.97 [-7.71, 1.18]; rrb = 0.77; p = 0.02) (Fig. 2). No significant differences were observed between control and conditioned TEPs (p ≥ 0.05 for each; Fig. 2) for the right SOL and left TA despite the significant overall effect among conditioned TEPs for the right SOL (F(21, 273) = 1.80, p = 0.01; Kendall’s W = 0.12), and left TA (F(21, 273) = 2.23, p = 0.002; Kendall’s W = 0.15). The right SOL and left TA conditioned TEPs at 50 ms were significantly different from those recorded at negative C-T intervals supporting for a time-dependent modulation. Last, no significant effects were observed for the right TA (F(21, 273) = 1.41, p = 0.11; Kendall’s W = 0.10) (Fig. 2). These results suggest a short latency depression of the net motor neuron output for legs following cervical transspinal stimulation.
Effects of lumbosacral transspinal stimulation on arm TEPs
Raw waveform averages of TEPs recorded from arm muscles under control conditions (black lines) and following lumbosacral transspinal conditioning stimulation (red lines) are shown for each C-T interval tested from a representative subject (C09) in Fig. 3.
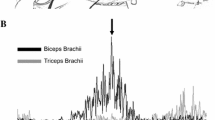
Non-rectified raw waveform averages of TEPs recorded bilaterally from the left and right TRIC, BIC, FCR, and ECR muscles under control conditions (black lines) and following single pulse lumbosacral transspinal conditioning stimulation (red lines) for one subject (C09). The waveform average of TEPs is shown for each conditioning-test interval tested ranging from negative 50 to positive 50 ms. TRIC: triceps bracchii, BIC: biceps bracchii, FCR: flexor carpi radialis, ECR: extensor carpi radialis, TEPs: transspinal evoked potentials
The mean amplitude of the arm TEPs in response to lumbosacral transspinal conditioning stimulation as a percentage of the homonymous control TEP are shown in Fig. 4. The conditioned TEPs are normalized to the mean amplitude of the homonymous control TEP and subtracted from 100 for ease recognition of changes from zero. Friedman test showed that the conditioned right TRIC (F(21, 252) = 0.60, p = 0.92; Kendall’s W = 0.05), left TRIC (F(21, 252) = 1.09, p = 0.39; Kendall’s W = 0.08), right BIC (F(21, 252) = 1.19, p = 0.26; Kendall’s W = 0.09), left BIC (F(21, 252) = 0.93, p = 0.55; Kendall’s W = 0.07), right ECR (F(21, 252) = 1.15, p = 0.29; Kendall’s W = 0.08), left ECR (F(21, 252) = 0.59, p = 0.93; Kendall’s W = 0.05), right FCR (F(21, 252) = 0.64, p = 0.88; Kendall’s W = 0.05), and left FCR (F(21, 252) = 0.64, p = 0.88; Kendall’s W = 0.05) TEPs were not statistically significant different from the homonymous control TEP at any C-T interval tested. These results suggest that lumbosacral transspinal stimulation did not affect the net motor output of the cervical spinal cord in resting healthy subjects.
Effects of lumbosacral transspinal stimulation on arm TEPs. Conditioned TEPs recorded from the right and left ECR, FCR, TRIC, and BIC muscles following single pulse lumbosacral transspinal conditioning stimulation from all subjects as a percentage of the homonymous median value of the control TEP subtracted from 100 for easy recognition of differences from zero. No statistically significant differences between control and conditioned TEPs were found. Error bars denote the IQR. ECR: extensor carpi radialis, FCR: flexor carpi radialis, TRIC: triceps brachii, BIC: biceps brachii, TEPs: transspinal evoked potentials
Discussion
In this study, we found that cervical transspinal stimulation resulted in short latency inhibition of TEPs recorded from the left SOL (-50, -25, -20, -15, -10, 15 ms), right ST (-20, -15, 15 ms), and right VL (-20, -15 ms) muscles out of the eight knee and ankle muscles recorded bilaterally. In contrast, lumbosacral transspinal stimulation did not produce significant modulation on the amplitude of TEPs recorded from arm and forearm muscles.
Important parameters in a paired stimulation protocol are the interval between the two stimuli and their intensity. The conduction time between Cervical 7 and Thoracic 12 for central cutaneous pathways is 7 ms (Jenner and Stephens 1982), while supramaximal median and posterior tibial nerve stimulation produces dorsum cord action potentials at lower cervical and thoracic levels with a latency of 6.5 ± 0.1 ms (Ertekin 1976). The first slow component of the action potential lasts up to 29.2 ms, while when the first slow component occurs at a latency of 11.1 ± 0.2 ms it lasts up to 36 ± 0.5 ms (Ertekin 1976). These results suggest that excitation of afferents from mixed peripheral nerves produce short and medium latency action potentials in the dorsum spinal cord. Thus, transspinal stimulation which also activates simultaneously afferents of different diameter produces neuronal activity in the dorsum spinal cord that in turn can potentially activate one of the longest loops, the transcortical continuation of ascending pathways reaching the somatosensory and motor cortices (Yamawaki et al. 2021). These pathways support for engagement of transcortical reflexes following transspinal stimulation.
Cervical transspinal stimulation produced depression of leg TEPs from − 20 to -15 ms C-T interval, while a short latency inhibition at C-T interval of 15 ms was observed in right ST and left SOL TEPs (Fig. 2), which agrees to the short (10–20 ms) latency soleus H-reflex inhibition following median nerve stimulation (Kagamihara et al. 2003). It needs though to be mentioned that when analysis was done based on peristimulus times histograms, inhibition or inhibition-facilitation was found (Kagamihara et al. 2003). The short and medium latency depression and facilitation of TEPs may involve different neuronal pathways with the former be attributed to activity of long descending propriospinal neuronal activity, and the latter to activation of transcortical loops as seen in monkeys with cerebellectomy or spinal hemisection when direct motor and reflex responses are present (Ruegg and Chofflon 1983). Based on the long-latency (80 to 400 ms) facilitation of the soleus H-reflex by brachial plexus or forelimb nerve stimulation (Meinck 1976), it remains to establish the full time-dependent modulation profile of leg TEPs following cervical transspinal conditioning stimulation.
The non-significant effects on left VL and ST TEPs (Fig. 2) may partly be related to the stimulation intensity strength. TEPs follow a sigmoid recruitment curve similar to the SOL H-reflex (Knikou 2008; Skiadopoulos et al. 2022), but the stimulation intensity was based on the right SOL TEP threshold and not to that of the knee flexor and extensors TEPs, which appear first at lower stimulation intensities compared to the SOL and TA TEPs that appear at higher stimulation intensities. The sensitivity of TEPs to inhibition and/or facilitation has not been established when evoked at different intensities along the recruitment curve as is known for the SOL H-reflex (Crone et al. 1985, 1990). We evoked leg TEPs at 1.6 ± 0.34 SOL TEP threshold intensities, which might have been higher or lower within the recruitment curve for TEPs recorded from other muscles, or within the SOL across subjects. While TEPs represent the net excitatory and inhibitory postsynaptic potentials of motor neurons (Yildiz et al. 2024), and are not monosynaptic H-reflexes, a systematic investigation of their susceptibility to inhibition and facilitation as a function of stimulation intensity relative to the maximal M-wave is warranted.
There were no significant effects on arm/forearm TEPs following lumbosacral transspinal conditioning stimulation (Fig. 4). It is worth testing the same effects during walking on a motorized treadmill given that neurons connecting spinal cord enlargements have diverse synaptic inputs and roles in locomotion (Brown et al. 2024).
Conclusion
In this study we evaluated the net motor output of cervical and lumbosacral spinal segments following lumbosacral and cervical transspinal conditioning stimulation, respectively. A short latency depression of leg TEPs following cervical and lumbosacral transspinal conditioning stimulation were evident. Delineation of the type and duration of postsynaptic potentials exerted on α motor neurons via single motor unit recordings during paired cervical and lumbosacral transspinal stimulation, as well as recordings of TEPs during rhythmic activity such as walking or cycling will enable us to understand better the neuronal interactions and optimally use this protocol in upper motor neuron lesions.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors upon request.
References
Angeli CA, Gerasimenko Y (2023) Combined cervical transcutaneous with lumbosacral epidural stimulation improves voluntary control of stepping movements in spinal cord injured individuals. Front Bioeng Biotechnol 11:1073716. https://doi.org/10.3389/fbioe.2023.1073716
Atkinson DA, Sayenko DG, D’Amico JM et al (2020) Interlimb conditioning of lumbosacral spinally evoked motor responses after spinal cord injury. Clin Neurophysiol 131:1519–1532. https://doi.org/10.1016/j.clinph.2020.03.021
Atkinson DA, Steele AG, Manson GA et al (2022) Characterization of interlimb interaction via transcutaneous spinal stimulation of cervical and lumbar spinal enlargements. J Neurophysiol 127:1075–1085. https://doi.org/10.1152/jn.00456.2021
Ben-Shachar M, Lüdecke D, Makowski D (2020) Effectsize: estimation of effect size indices and standardized parameters. J Open Source Softw 5(56):2815. https://doi.org/10.21105/joss.0281
Brown BL, Anil N, States G et al (2024) Long ascending propriospinal neurons are heterogenous and subject to spinal cord injury induced anatomic plasticity. Exp Neurol 373:114631. https://doi.org/10.1016/j.expneurol.2023.114631
Conover WJ, Iman RL (1981) Rank transformations as a bridge between parametric and nonparametric statistics. Am Stat 35:124–129. https://doi.org/10.2307/2683975
Crone C, Hultborn H, Jespersen B (1985) Reciprocal Ia inhibition from the peroneal nerve to soleus motoneurones with special reference to the size of the test reflex. Exp Brain Res 59. https://doi.org/10.1007/BF00230924
Crone C, Hultborn H, Mazieres L et al (1990) Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Exp Brain Res 81. https://doi.org/10.1007/BF00230098
Cureton EE (1956) Rank-biserial correlation. Psychometrika 21(3):287–290. https://doi.org/10.1007/BF02289138
Delwaide PJ, Figiel C, Richelle C (1977) Effects of postural changes of the upper limb on reflex transmission in the lower limb. Cervicolumbar reflex interactions in man. J Neurol Neurosurg Psychiatry 40:616–621. https://doi.org/10.1136/jnnp.40.6.616
Dixon L, Ibrahim MM, Santora D, Knikou M (2016) Paired associative transspinal and transcortical stimulation produces plasticity in human cortical and spinal neuronal circuits. J Neurophysiol 116:904–916. https://doi.org/10.1152/jn.00259.2016
Einhorn J, Li A, Hazan R, Knikou M (2013) Cervicothoracic multisegmental transpinal evoked potentials in humans. PLoS ONE 8:e76940. https://doi.org/10.1371/journal.pone.0076940
Eisinga R, Heskes T, Pelzer B et al (2017) Exact p-values for pairwise comparison of Friedman rank sums, with application to comparing classifiers. BMC Bioinformatics 18:1–18. https://doi.org/10.1186/s12859-017-1486-2
Ertekin C (1976) Studies on the human evoked electrospinogram. Acta Neurol Scand 53:3–20. https://doi.org/10.1111/j.1600-0404.1976.tb04321.x
Fleming N, Taylor C, Etzelmueller M et al (2023) Contralateral selectivity of upper-limb motor pools via targeted stimulation of the cervical spinal cord. Biomedicines 11:332. https://doi.org/10.3390/biomedicines11020332
Islam MA, Zaaya M, Comiskey E et al (2020) Modulation of soleus H-reflex excitability following cervical transspinal conditioning stimulation in humans. Neurosci Lett 732:135052. https://doi.org/10.1016/j.neulet.2020.135052
Jenner JR, Stephens JA (1982) Cutaneous reflex responses and their central nervous pathways studied in man. J Physiol 333:405–419. https://doi.org/10.1113/jphysiol.1982.sp014461
Kagamihara Y, Hayashi A, Masakado Y, Kouno Y (2003) Long-loop reflex from arm afferents to remote muscles in normal man. Exp Brain Res 151:136–144. https://doi.org/10.1007/s00221-003-1436-2
Klarner T, Pearcey GEP, Sun Y et al (2020) Changing coupling between the arms and legs with slow walking speeds alters regulation of somatosensory feedback. Exp Brain Res 238:1335–1349. https://doi.org/10.1007/s00221-020-05813-y
Knikou M (2007) Neural coupling between the upper and lower limbs in humans. Neurosci Lett 416:138–143. https://doi.org/10.1016/j.neulet.2007.01.072
Knikou M (2008) The H-reflex as a probe: pathways and pitfalls. J Neurosci Methods 171:1–12. https://doi.org/10.1016/j.jneumeth.2008.02.012
Knikou M (2013) Neurophysiological characterization of transpinal evoked potentials in human leg muscles. Bioelectromagnetics 34:630–640. https://doi.org/10.1002/bem.21808
Knikou M (2014) Transpinal and transcortical stimulation alter corticospinal excitability and increase spinal output. PLoS ONE 9:e102313. https://doi.org/10.1371/journal.pone.0102313
Knikou M, Murray LM (2018) Neural interactions between transspinal evoked potentials and muscle spindle afferents in humans. J Electromyogr Kinesiol 43:174–183. https://doi.org/10.1016/j.jelekin.2018.10.005
Knikou M, Taglianetti C (2006) On the methods employed to record and measure the human soleus H-reflex. Somatosens Mot Res 23:55–62. https://doi.org/10.1080/08990220600702715
Knikou M, Dixon L, Santora D, Ibrahim MM (2015) Transspinal constant-current long-lasting stimulation: a new method to induce cortical and corticospinal plasticity. J Neurophysiol 114:1486–1499. https://doi.org/10.1152/jn.00449.2015
Meinck HM (1976) Descending long-loop reflexes in the human spinal cord I. Facilitation of the triceps surae H reflex following stimulation of forelimb afferences (author’s transl). EEG EMG Z Elektroenzephalogr Elektromyogr Verwandte Geb 7:146–150
Milosevic M, Masugi Y, Sasaki A et al (2019) On the reflex mechanisms of cervical transcutaneous spinal cord stimulation in human subjects. J Neurophysiol 121:1672–1679. https://doi.org/10.1152/jn.00802.2018
Oh J, Steele AG, Varghese B et al (2022) Cervical transcutaneous spinal stimulation for spinal motor mapping. iScience 25:105037. https://doi.org/10.1016/j.isci.2022.105037
Pearcey GEP, Zehr EP (2019) We are upright-walking cats: human limbs as sensory antennae during locomotion. Physiology 34:354–364. https://doi.org/10.1152/physiol.00008.2019
Pohlert T (2023) PMCMRplus: Calculate pairwise multiple comparisons of mean rank sums extended. R package version 1.9.10
R Core Team (2023) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Ruegg DG, Chofflon M (1983) Peripheral and transcortical loops activated by electrical stimulation of the tibial nerve in the monkey. Exp Brain Res 50–50. https://doi.org/10.1007/BF00239193
Sarica Y, Ertekin C (1985) Descending lumbosacral cord potentials (DLCP) evoked by stimulation of the Mdn nerve. Brain Res 325:299–301. https://doi.org/10.1016/0006-8993(85)90327-0
Skiadopoulos A, Pulverenti TS, Knikou M (2022) Physiological effects of cathodal electrode configuration for transspinal stimulation in humans. J Neurophysiol 128:1663–1682. https://doi.org/10.1152/jn.00342.2022
Skillings JH, Mack GA (1981) On the use of a Friedman-type statistic in balanced and unbalanced block designs. Technometrics 23:171–177. https://doi.org/10.1080/00401706.1981.10486261
Steele AG, Atkinson DA, Varghese B et al (2021) Characterization of spinal sensorimotor network using transcutaneous spinal stimulation during voluntary movement preparation and performance. J Clin Med 10:5958. https://doi.org/10.3390/jcm10245958
Tran K, Steele A, Crossnoe R et al (2024) Multi-site lumbar transcutaneous spinal cord stimulation: when less is more. Neurosci Lett 820:137579. https://doi.org/10.1016/j.neulet.2023.137579
Yamawaki N, Raineri Tapies MG, Stults A et al (2021) Circuit organization of the excitatory sensorimotor loop through hand/forelimb S1 and M1. eLife 10:e66836. https://doi.org/10.7554/eLife.66836
Yildiz N, Cecen S, Sancar N et al (2024) Postsynaptic potentials of soleus motor neurons produced by transspinal stimulation: a human single motor unit study. J Neurophysiol. https://doi.org/10.1152/jn.00077.2024
Zehr EP, Haridas C (2003) Modulation of cutaneous reflexes in arm muscles during walking: further evidence of similar control mechanisms for rhythmic human arm and leg movements. Exp Brain Res 149:260–266. https://doi.org/10.1007/s00221-003-1377-9
Zehr PE, Collins DF, Chua R (2001) Human interlimb reflexes evoked by electrical stimulation of cutaneous nerves innervating the hand and foot. Exp Brain Res 140:495–504. https://doi.org/10.1007/s002210100857
Acknowledgements
We thank Dr. Marie-Pascale Côté for helpful comments on an earlier version of this manuscript.
Funding
Research reported in this publication was supported by the New York State Department of Health (NYSDOH) under Contract Numbers C35594GG and C38333GG awarded to Maria Knikou. The content is solely the responsibility of the author(s) and does not necessarily represent the official views of the New York State Department of Health.
Author information
Authors and Affiliations
Contributions
AMSA: performed experiments, analyzed data, prepared figures, and approved the final version of the manuscript. AS: performed experiments, analyzed data, prepared figures, and approved the final version of the manuscript. MK: conception and design of research, performed experiments, analyzed data, prepared figures, interpreted results of experiments, wrote the first draft of the manuscript, approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The studies involving human participants were reviewed and approved by the Institutional Review Board of the City University of New York. All participants provided their written informed consent to participate in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Communicated by Winston D Byblow.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sayed Ahmad, A.M., Skiadopoulos, A. & Knikou, M. Interactions between arm and leg neuronal circuits following paired cervical and lumbosacral transspinal stimulation in healthy humans. Exp Brain Res 242, 2229–2239 (2024). https://doi.org/10.1007/s00221-024-06891-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-024-06891-y