Abstract
The aim of this study was to examine corticomotor excitability and plasticity following repetitive thumb abduction training in left and right hands of young and old adults. Electromyographic recordings were obtained from the abductor pollicis brevis (APB) muscle of 12 young (aged 18–27 years) and 14 old (aged 63–75 years) adults. Motor training consisted of 300 ballistic abductions of the thumb to maximize peak abduction acceleration, with each hand tested in a separate session. Transcranial magnetic stimulation (TMS) over the primary motor cortex (M1) was used to assess changes in contralateral APB motor-evoked potentials (MEPs) and short-interval intracortical inhibition (SICI) before and after training. For young and old adults, APB MEP amplitude increased for both hands after training, which is indicative of use-dependent plasticity. However, the increase in MEP amplitude was 21% (P = 0.04) greater in the left (non-dominant) hand compared with the right (dominant) hand. This occurred despite a 40% greater improvement in peak thumb abduction acceleration (motor learning) for the right hand in young subjects compared with the left hand in young subjects (P < 0.04) and the right hand in old subjects (P < 0.01). Furthermore, no difference in use-dependent plasticity was observed between young and old adults, and SICI remained unchanged following ballistic training for both hands in all subjects. These findings suggest that there is greater strengthening of corticomotor circuits for control of the left compared with the right hand during simple ballistic thumb training and that an age-related decline in motor learning was observed only in the dominant hand. In contrast to previous studies, these data also indicate that young and old adults can demonstrate similar use-dependent corticomotor plasticity during this simple thumb-training task.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The human brain, in particular the primary motor cortex (M1), has the potential to functionally and structurally reorganize following physiological (learning) and pathological (injury or disease) events (see Sanes and Donoghue 2000 for review). As a result, in addition to having the essential function of controlling voluntary movement, M1 is a crucial site for use-dependent plasticity, such as learning new motor skills and recovery of motor function after injury (Pascual-Leone et al. 1995; Nudo et al. 1996). In the context of motor learning, use-dependent plasticity can be assessed in humans by examining the change in the muscle-evoked potential (MEP) from electromyography (EMG) recordings following transcranial magnetic stimulation (TMS) of M1. An increase in the MEP after motor training reflects the increased excitability of corticospinal and spinal motor neurons (Muellbacher et al. 2001; Ziemann et al. 2001), which is thought to occur through long-term potentiation (LTP)-like mechanisms (Bütefisch et al. 2000). Although several factors are known to influence motor system plasticity (see Ridding and Ziemann 2010 for review), limited information exists on differences in use-dependent plasticity with hand preference and advancing age. These factors could contribute to differences in use-dependent plasticity in a diverse subject population and have important implications for therapeutic interventions in neurological patients.
The motor system between left and right brain hemispheres is not symmetrical, with anatomical and physiological differences between sides potentially contributing to asymmetries in hand performance (Amunts et al. 1996; Guye et al. 2003; Ilic et al. 2004; Triggs et al. 1997; Volkmann et al. 1998). However, few studies have examined the extent of corticomotor plasticity in left and right hemispheres and whether this has implications for improved motor performance. Studies involving artificially induced (Ridding and Flavel 2006) and use-dependent plasticity (Garry et al. 2004; Gallasch et al. 2009) have generally shown no hemispheric differences in MEP facilitation in young subjects, although repeat performance of a goal-directed movement task results in more sustained facilitation in the left hand (Gallasch et al. 2009). Recent evidence suggests that artificially induced and use-dependent plasticity involve overlapping and functionally relevant cortical circuits (Ziemann et al. 2004; Stefan et al. 2006), but the functional implications of any hemispheric differences in use-dependent plasticity for motor skill learning are unknown.
Both structural and functional plasticity in response to motor training are altered with advancing age. For example, there is reduced structural plasticity of cortical gray matter in older adults when learning a novel motor skill (Boyke et al. 2008), and there is an age-related decrease in the ability of M1 to reorganize in response to motor training (Sawaki et al. 2003; Rogasch et al. 2009). More recently, we have shown reduced use-dependent plasticity and motor learning following 30 min of ballistic thumb abduction training in older adults, with these differences unrelated to the extent of improvement in motor performance in young or old subjects (Rogasch et al. 2009). However, these previous studies have focused on the right (preferred) hand to assess use-dependent plasticity with advancing age, and there are no studies that have compared use-dependent plasticity in left and right hands of older adults.
The aim of this study was to examine the extent of use-dependent plasticity following repetitive thumb abduction training for M1 control of the right (dominant) and left (non-dominant) hands of young and old adults. The repetitive ballistic thumb abduction task was selected as it is commonly used to induce use-dependent plasticity in young subjects (e.g., Muellbacher et al. 2001; Ziemann et al. 2001), and there is recent evidence from TMS studies indicating improvements in ballistic motor performance are dependent on adaptations in M1 (Muellbacher et al. 2001, 2002; Carroll et al. 2008). Using the ballistic thumb-training task, we hypothesize that use-dependent corticomotor plasticity and motor learning will be reduced with advancing age, and there will be greater use-dependent plasticity in the right (non-dominant hand) hemisphere compared with the left (dominant hand) hemisphere in all subjects.
Materials and methods
Experiments were performed on the right and left hand of 27 subjects with no known history of peripheral or neurological impairment. Data were excluded from one young male subject as he displayed excessive thumb flexion throughout the training task (standard deviation 3× greater than the mean of all subjects), suggesting that it was not performed correctly. Therefore, data were analyzed from twelve young (7 women, 5 men; 22 ± 2 years; range 18–27 years) and fourteen older subjects (7 women, 7 men; 67 ± 4 years; range 63–75 years). All subjects were strongly right handed (Laterality Quotient (LQ); Young: median LQ = 0.82, range 0.6–1.0; Old: median LQ = 0.92, range 0.6–1.0) as assessed by the Edinburgh Handedness Questionnaire (Oldfield 1971), and no subjects reported long-term skilled use of the hands, such as playing a musical instrument (Rosenkranz et al. 2007b). Subjects also completed the long version of the International Physical Activity Questionnaire (IPAQ), consisting of 31 items describing the extent of leisure time physical activity involving aerobic exercises such as running, cycling, and walking (Craig et al. 2003; Fogelholm et al. 2006). All subjects gave written informed consent prior to participation in the study, which was approved by the University of Adelaide Human Research Ethics Committee.
Experimental arrangement
The experimental setup used for these studies has been described previously (Rogasch et al. 2009). Subjects were seated comfortably with either their right or left shoulder abducted approximately 45° to allow the hand and arm to rest on a manipulandum. Experiments were conducted in the afternoon, and each hand was tested on a different day separated by at least 2 weeks, with the hand examined first selected randomly. For all measures other than training, the forearm was pronated and the palm was facing down on the manipulandum. Surface EMG was recorded from the abductor pollicis brevis (APB) and abductor digiti minimi (ADM) muscles throughout the experiment using bipolar surface electrodes (Ag–AgCl, 4 mm diameter) placed ~2 cm apart using a muscle belly-tendon configuration. A grounding strap placed around the elbow was used as a common reference for all electrodes. The EMG signals were amplified (×100–1000), bandpass filtered (high pass at 13 Hz, low pass at 1000 Hz), digitized online at 2 kHz with a CED interface system (Cambridge Electronic Design Co. LTD, UK) and recorded onto computer for offline analysis. The EMG signals of both muscles were displayed on an oscilloscope to assist the subject in maintaining EMG silence when required.
Experimental procedures
At the beginning of each experiment, maximal thumb abduction force (maximum voluntary contractions; MVCs) was measured, and resting and active motor thresholds were determined using TMS (see below). To examine the extent of corticomotor plasticity in left and right hands of young and old adults, corticomotor excitability (input–output curves; IO curves) and short-interval intracortical inhibition (SICI curves) were assessed using TMS before and after a motor training task. To examine training-induced changes in peripheral neuromuscular processes, maximum compound muscle action potentials (M-waves) were also recorded before and after training in the majority of these subjects. The order of these measurements for all subjects was M-waves, IO curves and SICI before training, whereas it was M-waves, SICI (5 min after training) and IO curve (10 min) after training. This order was selected to obtain SICI measurements as close as possible to the training, as SICI can return to baseline levels within 15 min (see Garry et al. 2004), whereas MEP amplitude can remain elevated for up to an hour after the intervention (Muellbacher et al. 2001; Ziemann et al. 2001).
MVC
The hand was positioned with the palm facing down, and the proximal phalanx of the thumb was placed in a metal ring attached to a load cell (LC1205-K020, A&D Mercury Pty Ltd, Australia) to facilitate measurement of thumb abduction force. Maximum thumb abduction force was exerted by the subject for 3 s against the force transducer with verbal encouragement provided by the experimenters. Several MVC trials were performed, with a minimum of 30-s rest between trials, until the peak force from two trials were within 10% of each other, and the MVC with the largest thumb abduction force was used for the assessment of muscle strength. Visual feedback of thumb abduction force was displayed on an oscilloscope, and the subject was monitored in each trial to ensure that proximal limb muscles were not contributing to the thumb abduction force. Force signals were amplified (×1000), digitized online (2 kHz) via a CED 1401 interface (Cambridge Electronic Design, UK) and stored on computer for offline analysis.
TMS
TMS was applied using a figure-of-eight coil (external wing diameter 90 mm) with two Magstim 200 magnetic stimulators connected with a Magstim Bistim unit (Magstim, Whitland, Dyfed, UK). The coil was held tangentially to the skull with the handle pointing backward and laterally at an angle of 45° to the sagittal plane. With this coil orientation, current flow within the cortex was induced in a posterior–anterior direction. The coil was positioned at the optimal scalp position over the appropriate hemisphere for eliciting a MEP in the relaxed APB muscle. The optimal scalp position was then marked for reference. TMS was delivered at 0.2 Hz for all conditions, and optimal coil position was continually monitored throughout the experiment.
RMT and AMT
Resting motor threshold was determined as the minimum stimulus intensity required to elicit a MEP in the relaxed APB of at least 50 μV in 3 out of 5 consecutive trials. Active motor threshold was defined as the minimum stimulus intensity required to elicit a MEP in the APB muscle of at least 200 μV in 3 out of 5 consecutive trials during low-level voluntary thumb abduction (10% MVC). Both RMT and AMT are expressed relative to 100% maximum stimulator output (MSO), and the stimulus intensity was altered in 1% increments of MSO throughout this process until the appropriate threshold level was achieved.
IO curve
The intensities used to construct the TMS IO curves were determined for each individual according to their RMT before ballistic thumb training. Seven TMS intensities of 100, 110, 120, 130, 140, 160, and 180% of RMT were recorded for each subject at rest. TMS pulses that included obvious EMG during the pre-stimulus period (100 ms before TMS) were discarded, and the TMS pulse repeated at the end of the IO curve block. A single IO curve block consisted of 56 stimuli (8 stimuli at each intensity) with the order of presentation of the seven conditions pseudo-randomized throughout the trial. MEP amplitudes were measured for each TMS pulse to calculate the mean MEP amplitude for each TMS intensity.
SICI
Short-interval intracortical inhibition (SICI) was assessed using a paired-pulse TMS paradigm consisting of a subthreshold conditioning stimulus that preceded a suprathreshold test stimulus by 3 ms (Kujirai et al. 1993). The test stimulus intensity was set to produce a MEP amplitude of ~1 mV in resting APB before training, whereas the conditioning stimulus was randomized as 70, 80, or 90% of AMT. The test stimulus intensity remained constant before and after training. Each data block consisted of ten trials for each of four conditions; test stimulus alone and SICI at 70, 80, and 90% AMT, with the order of presentation randomized throughout the trials (40 trials total). The conditioned MEP amplitude was expressed as a percentage of the unconditioned test MEP amplitude to calculate the influence of the conditioning stimulus on SICI circuits.
M-waves
Maximal M-waves from the APB muscle were recorded from both left and right hands in 10 young and 10 old subjects. Supramaximal electrical stimulation was administered to the median nerve at the wrist using a constant current stimulator (DS7A, Digitimer, UK) via bipolar surface electrodes, separated by 20 mm, with the cathode proximal. Stimuli were square wave pulses with 100 μs pulse duration. Stimulator intensity was set at 120% of that required to elicit a maximal M-wave response from APB. The M-wave responses from five stimuli were recorded before and immediately after training in these subjects.
Motor training task
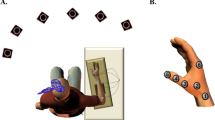
The motor training task was similar to that described previously (Rogasch et al. 2009), which required the subject to maximize peak thumb abduction acceleration (TAAcc) during ballistic movement of the thumb. Subjects sat with their forearm placed in a custom designed splint, and their arm abducted at the shoulder and bent at approximately 90° at the elbow. The splint was designed to have the forearm placed in a neutral position (between pronation and supination) with the thumb free to move, while the other digits were immobilized. Two blocks of 150 ballistic thumb abduction movements (total of 300 trials) paced at 0.5 Hz by an audible tone from a metronome were performed in each session. Subjects rested their thumb for 30 s after ten trials and for 5 min between the first and second blocks to avoid fatigue. A biaxial accelerometer (sensitivity ± 6 g, LIS3L06AL, STMicroelectronics, Switzerland) placed over the interphalangeal joint of the thumb was used to assess thumb acceleration in the abduction–adduction plane. Thumb acceleration > +3 m s−2 in the abduction–adduction plane triggered a recording sweep of ± 500 ms, and each movement recorded acceleration data in the abduction–adduction plane, along with EMG from the APB and ADM muscles. Continual verbal encouragement and visual feedback of thumb abduction acceleration displayed on a computer screen was provided to the subject throughout training to improve and maximize thumb acceleration. Acceleration signals were digitized online at 2 kHz, using a CED interface system and recorded on computer for offline analysis.
Data analysis
All MEP and M-wave trials that contained any pre-stimulus EMG (100 ms before stimulation) were discarded from analysis. MEP and M-wave amplitudes were measured peak-to-peak in each individual trial and averaged for each condition. For paired-pulse TMS, conditioned MEPs were expressed as a percentage of the mean test-alone MEP in each block to quantify the effectiveness of SICI. Maximum force was calculated during the MVC and maximum APB EMG was assessed as the mean rectified EMG 500 ms before and after peak force.
For each movement trial, a baseline period from 400 to 200 ms before abduction acceleration was used to calculate the mean baseline acceleration in the abduction–adduction plane. The mean acceleration over this baseline period was subtracted so that baseline acceleration equaled 0 m s−2, and the peak TAAcc was then assessed. Abduction acceleration for each block of 150 ballistic thumb abduction movements was subdivided into blocks of 50 trials representing a start, middle and end (six segments of 50 trials in total) for detailed analysis. To assess improvement in peak TAAcc (motor learning), each block of 50 trials was normalized to the first 10 contractions. APB EMG was quantified for one in ten movement trials by obtaining the average rectified EMG from movement onset to peak TAAcc. Movement onset was defined as the time when EMG increased by more than 3 standard deviations above baseline (−400 to 200 ms before movement).
Statistical analysis
A paired t-test was performed to compare baseline TAAcc between hands, and an unpaired t-test was performed to compare baseline TAAcc and IPAQ scores between age groups. A Mann–Whitney U test was used to compare non-parametric handedness scores between young and old adults. Two-way ANOVA (Hand, Age) was used to analyze subject characteristics of maximum thumb abduction force, maximum M-wave amplitude, RMT, AMT, and MEP test-alone TMS intensity before training. A three-way ANOVA (Hand, Age, Time) was used to examine M-wave amplitude and test MEP amplitude from SICI data. A three-way ANOVA (Hand, Age, Time) was also used to examine TAAcc improvement and mean rectified EMG during acceleration. A four-way ANOVA was used to analyze IO curve (Hand, Age, Time, TMS intensity) and SICI (Hand, Age, Time, Conditioning intensity). A Fisher’s LSD post hoc test that performed all possible comparisons was used to analyze significant main effects and interactions where necessary. The significance level was set at P < 0.05 for all comparisons, and all group data are provided as mean ± SEM.
Results
All subjects were comfortable with the TMS and training task procedures, and no side effects were reported. Age and hand differences in subject characteristics before training are displayed in Table 1. There was no difference between left and right hands in maximum thumb abduction force or APB EMG during MVC, TMS thresholds, and M-Waves before training. However, when both hands were combined, older adults had reduced APB EMG during MVC and M-Wave amplitude compared with young adults (Table 1). In addition, there were no differences in physical activity levels assessed by the IPAQ between young and old adults (Old = 3492 ± 777 MET-min; Young = 4117 ± 1296 MET-min, P = 0.67), although older adults were more strongly right handed than young adults (Old: median LQ = 0.92 ± 0.12; Young: median LQ = 0.82 ± 0.13, P < 0.04).
Effect of age and hand on motor learning
To quantify motor learning, the improvement in peak TAAcc for each 50 contractions was normalized to the first 10 movement trials for each session. For the first 10 movement trials (baseline), peak TAAcc was greater in the left compared with the right hand in all subjects (paired t-test, left = 21.15 ± 2.21 m s−2; right = 16.04 ± 1.50 m s−2, P = 0.02), but there was no difference between peak TAAcc in young and old adults (unpaired t-test, young = 17.86 ± 2.17 m s−2; old = 19.22 ± 1.76 m s−2, P = 0.63). Improvement in peak TAAcc in the left and right hand of young and old adults throughout training is shown in Fig. 1. A three-way repeated measures ANOVA indicated that normalized TAAcc improved over training blocks (Time effect, P < 0.01) and was greater for the right hand compared with the left hand (Hand effect, P = 0.03), but was not different between young and old adults (Age effects, P = 0.47). A significant hand × time × age interaction (P < 0.01) showed that the improvement in peak TAAcc for the last 100 movement trials (201–250 and 251–300) was ~40% greater in the right hand in young adults compared with the left hand in young adults (P < 0.04) and the right hand in old adults (P < 0.01). No difference between hands was observed in normalized TAAcc in old adults, and no age-related difference was observed in normalized TAAcc for the left hand.
Improvement in peak thumb abduction acceleration (TAAcc) for all 300 thumb movement trials for young and old adults in the left (a) and right (b) hand. Each symbol represents the mean of 50 movement trials that have been normalized to the first 10 movements in that session. *P < 0.05 compared with older adults. # P < 0.05 compared with the same time point in the left hand
For the mean rectified APB EMG throughout training, a three-way repeated measures ANOVA revealed that EMG was different between training blocks (Time effect, P = 0.01), and EMG was greater in young subjects (Age effect, P = 0.02), but there were no differences between left and right hands (Hand effect, P = 0.59). A time × age interaction (P < 0.01) and subsequent post hoc analysis showed that EMG was similar in young and old adults for training block 1 (P = 0.12), but was on average 32% greater in young subjects in training blocks 2–6 (all P values < 0.02). No significant differences in APB EMG were observed throughout training in young or old adults. Furthermore, the significant Age effect was removed (P = 0.8) when the EMG was normalized to the MVC EMG in each subject.
Effect of age and hand on training-dependent corticomotor excitability
The effect of motor training on the IO curves (assessed over TMS intensities of 100–180% RMT) of the relaxed APB in left and right hands of young and old adults are shown in Fig. 2. A four-way repeated measures ANOVA indicated that there was an increase in the size of the APB MEP amplitude with increasing stimulus intensity in both hands of all subjects (Intensity effect, P < 0.0001), MEP amplitude was greater following training (Time effect, P < 0.0001) and MEP amplitude was greater in the left compared with the right hand (Hand effect, P = 0.04). However, no difference between young and old adults (Age effect, P = 0.75) was observed. A hand × time × intensity interaction (P = 0.02) showed that MEP amplitude increased after training for the left hand at TMS intensities of 160% (P = 0.04) and 180% (P = 0.01) RMT for young and old adults combined. No differences in MEP amplitude were observed in the right hand after training at any TMS intensity (all comparisons, P > 0.28). For the ADM IO curve, one young and one old subject were excluded due to contamination by consistent pre-stimulus EMG activity. As for APB, MEP amplitude increased with increasing stimulator output for the control ADM muscle (Intensity effect, P < 0.0001). However, there was no significant Time (P = 0.64), Hand (P = 0.41), or Age (P = 0.30) effects, and no significant interactions (all interactions, P > 0.18). These data show that training did not influence MEPs in ADM and that the training-related increase in APB MEP amplitudes was specific to the muscles involved in the task.
Mean MEP amplitudes before and after training in the left (a, c) and right (b, d) APB muscle of young (a, b) and old (b, d) adults. Data are shown at increasing TMS intensities expressed relative to resting motor threshold (RMT). For young and old adults combined, MEP amplitude increased after training for the left hand at TMS intensities of 160% (P = 0.04) and 180% (P = 0.01) RMT. Inset shows MEP amplitudes before and after training that have been pooled across TMS intensities
M-wave amplitudes were 34% greater in young compared with old adults (P < 0.01). However, there were no differences in M-wave amplitude between hands (left = 13.94 ± 0.63 mV, right = 14.09 ± 0.74 mV, P = 0.72), no changes after training (before = 13.89 ± 0.68 mV, after = 14.14 ± 0.70 mV, P = 0.28), and no significant interactions (all interactions, P > 0.17).
Using linear regression of data from individual subjects, we examined whether the training-related change in APB MEP amplitude was associated with the improvement in peak TAAcc. There was no significant association between the change in APB MEP amplitude and the improvement in TAAcc for young (r 2 = 0.04, P = 0.38) or old (r 2 = 0.06, P = 0.23) adults and for left (r 2 = 0.05, P = 0.29) or right (r 2 = 0.12, P = 0.08) hands.
SICI was assessed using a paired-pulse paradigm that utilized an ~1 mV test pulse (before training) that was preceded by a subthreshold conditioning pulse (70, 80, or 90% AMT) at 3 ms. Data showing the changes in test-alone APB MEP amplitude and the extent of SICI in left and right hands of young and old adults are shown in Fig. 3. There was an increase of 41% in test APB MEP amplitude after training (Time effect, P < 0.0001), but no difference between left and right hands (Hand effect, P = 0.12) or young and old adults (Age effect, P = 0.62). A significant hand × time interaction (P = 0.04) and post hoc analysis indicated that test MEP amplitude after training for the left hand increased by 55% (P < 0.0001) and for the right hand increased by 25% (P = 0.05) compared with before training. In addition, test MEP amplitude was 21% greater in the left hand compared with the right hand after training (P = 0.04). This increase in MEP amplitude after training was specific to the muscle used in the task (APB), as there was no change in MEP amplitude (one young subject excluded due to pre-stimulus EMG activity) of the ADM in either left or right hands of young and old adults after training. For SICI, increasing the intensity of the conditioning stimulus increased the amount of SICI in APB for both hands in all groups (Intensity effect, P < 0.0001). However, there were no significant Time (P = 0.60), Hand (P = 0.69), or Age (P = 0.16) effects, and no significant interactions (all interactions, P > 0.16).
Mean test MEP amplitude (a, b) and the extent of SICI (c, d) in left and right hands of young and old adults. Test MEP amplitude increased after training for both the left (a) and right (b) hand, and this increase was greater in the left hand. The extent of SICI was influenced by conditioning TMS intensity, but was not different between left and right hands. *P < 0.05 compared with before training. # P < 0.05 compared with the same time point in the right hand
Discussion
The purpose of this study was to examine hemispheric differences in use-dependent corticomotor plasticity and motor learning following repetitive thumb abduction training in young and old adults. There were several new findings in this study. First, use-dependent corticomotor plasticity following repetitive thumb abduction training was greater in the left (non-dominant) hand, although there were no differences between young and old adults. Second, the extent of motor learning was greater for the right hand in young adults compared with the left hand in young adults and the right hand of old adults. Furthermore, SICI was not altered by training in either hand for young or old adults, suggesting that the increased use-dependent corticomotor plasticity in the right hemisphere (left hand) was not due to hemispheric differences in GABAergic intracortical inhibition in M1.
Increased corticomotor plasticity for control of the left hand
A number of studies using TMS have demonstrated corticomotor plasticity following motor learning, motor practice, or training. These studies have shown that increased MEP amplitude in the target muscle can last for up to an hour after the motor intervention (Muellbacher et al. 2001; Ziemann et al. 2001), although substantial variability in the response exists between studies. One factor that could be responsible for this variability is whether the dominant or non-dominant hand is used for the training. Similar to previous performance-based training intervention studies, we demonstrate that MEP amplitudes in the target muscle (APB) of both left and right hands were significantly facilitated following training, but the changes were larger when training was performed with the left (non-dominant) hand. This increased MEP following an intervention is believed to reflect use-dependent plasticity (Muellbacher et al. 2001; Ziemann et al. 2001), with pharmacological (Bütefisch et al. 2000; Sawaki et al. 2002) and physiological evidence (Muellbacher et al. 2002) suggesting that this occurs through LTP-like mechanisms. It is conceivable that the greater use-dependent plasticity in the right hemisphere (controlling the left hand) could take effect at cortical and/or spinal levels, but several lines of evidence suggest that at least some of the changes with this ballistic task are occurring at the cortical level. For example, there is a reduced change in spinal excitability as assessed with transcranial electrical stimulation (Classen et al. 1998), and there is impaired motor learning following administration of pharmacologically induced neuromodulators (Meintzschel and Ziemann 2006). Furthermore, learning of a brisk motor task is disrupted with repetitive TMS over M1, but not when administered over other brain areas (Muellbacher et al. 2002).
Despite known anatomical and physiological asymmetries between left and right motor regions of the brain (see Hammond 2002 for review), no previous studies have examined use-dependent plasticity for control of the left and right hands in older adults. Previous studies in young adults have found no differences in MEP facilitation between hands following the performance of complex sensorimotor tasks (Gallasch et al. 2009; Garry et al. 2004). However, greater training-related MEP facilitation has been observed for the right hemisphere after multiple training sessions, suggesting a more sustained MEP facilitation with goal-directed visuomotor tasks for the left hand (Gallasch et al. 2009). In support of this, we found a larger increase in MEPs following performance of a ballistic thumb-training task in the left hand. These findings suggest that the hand used for training is an important feature in determining the magnitude of use-dependent plasticity for simple ballistic tasks performed by hand muscles and that the factors responsible for the increased MEP with training are more effective in the right hemisphere. Alternatively, the time course of the training-induced MEP facilitation could be different between hands, with a more rapid facilitation in the skilled (right) hand followed by over learning of the task, which results in a return of MEP amplitude to baseline (Muellbacher et al. 2001; Rosenkranz et al. 2007a). Furthermore, the increased training-related MEP facilitation for control of the left hand may be due to the greater complexity of the task when performed with the non-dominant (unskilled) hand (Semmler and Nordstrom 1998), with an increased MEP evident during more demanding tasks (Datta et al. 1989).
The increased MEP amplitude in the left hand cannot simply be explained by greater motor learning, as the improvement in peak TAAcc was greater for the right hand of young adults, and there was no association between MEP facilitation and improvement in motor performance in individual subjects. These findings suggest that the extent of training-related MEP facilitation is not an important determinant of the magnitude of the behavioral improvement. It may be that factors other than the magnitude of motor learning, such as attentional focus (McNevin et al. 2000) are more important in mediating changes in corticomotor excitability. Nonetheless, differences in hand performance and learning between left and right sides are inconsistent and may depend on the details of the task performed. For example, some studies have shown that complex tasks involving the precise manipulation of objects are performed better with the right than the left hand (Bryden and Roy 1999; Garry et al. 2004). In contrast, the improvement in motor performance associated with learning new motor skills is achieved to a similar extent in both hands during complex motor tasks (Garry et al. 2004; Gallasch et al. 2009), but not for simple ballistic tasks (Ridding and Flavel 2006). In the present study, we found that baseline motor performance was greater in the left hand during the thumb abduction task, which may be a confounding factor in the reduced improvement in performance for this hand in young subjects.
Several studies have examined SICI in the left and right hemispheres in resting hand muscles, but the results have been inconsistent. For example, the left hemisphere can show increased (Smith et al. 2009), decreased (Ilic et al. 2004; Ridding and Flavel 2006) and no difference (Bäumer et al. 2007; Garry et al. 2004; Gallasch et al. 2009) in SICI compared with the right hemisphere, with these divergent findings likely due to the details of the experimental procedures used (muscle, stimulus parameters) and the subject population tested (extent of laterality, hand use, etc.). Furthermore, one important technical difference between many SICI studies involving training is whether the test stimulus is kept constant or whether it is adjusted so that the MEPs are matched before and after training. We tested SICI with a constant test TMS intensity, as previous studies have shown that measures of SICI are sensitive to test TMS intensity (Zoghi et al. 2003) and are unrelated to cortical excitability state and MEP size (Garry and Thomson 2009). When maintaining a constant test TMS intensity, we found no difference in resting SICI between hemispheres in young or old adults, suggesting that differences in resting SICI were not responsible for the differences in use-dependent plasticity between left and right sides.
Age-related changes in corticomotor plasticity and motor learning
Several previous studies involving TMS have shown that artificially induced and use-dependent plasticity are diminished in older adults (Sawaki et al. 2003; Tecchio et al. 2008; Rogasch et al. 2009). However, a reduction in neural plasticity in older adults is not always a consistent finding, as previous studies provide evidence that older adults are able to retain a high capacity for learning new motor skills (McNay and Willingham 1998; Smith et al. 1999; Voelcker-Rehage 2008; Wu and Hallett 2005). In support of this, we found that the extent of use-dependent plasticity was similar in young and older adults during ballistic thumb abduction training. This finding was unexpected, given that we had previously observed a lack of training-induced MEP facilitation in older adults during the same task when testing the left hemisphere during right hand performance (Rogasch et al. 2009). As similar experimental techniques were used in the two studies, it can only be assumed that differences in the subject population may have contributed to these disparate findings. For example, it is possible that the older subjects tested in the present study were more physically active than those in our previous study. In general, physical activity levels are reduced with increasing age (Ravussin and Bogardus 1989), but there were no differences in physical activity levels (assessed by questionnaire) between young and old adults in the present study, although this was not measured in our previous study (Rogasch et al. 2009). It is now well accepted that regular physical activity and exercise provides neuroprotective and neuroplastic benefits to the aging brain and may serve to reduce biological senescence in humans (see Cotman and Berchtold 2002). We have recently shown that artificially induced motor cortex plasticity is greater in physically active individuals (Cirillo et al. 2009), and participation in regular exercise in older adults may have contributed to the similar extent of use-dependent plasticity observed in the present study. Other factors specific to the subject population that could contribute to alterations in corticomotor excitability and plasticity include the extent of skilled hand use (Rosenkranz et al. 2007b), prior history of synaptic activity (see Ridding and Ziemann 2010), attentional focus (McNevin et al. 2000) and emotional state of the subjects (Tormos et al. 1997).
Age-related changes in the central and peripheral neuromotor system are believed to be responsible for reduced motor function with advancing age. Recent studies on central nervous system changes with aging have attributed the decline in motor function to reduced brain volume and decreased cerebral gray and white matter in older adults (Courchesne et al. 2000; Jernigan et al. 2001). Furthermore, there are substantial neuromuscular changes with advancing age (see Vandervoort 2002), including a decline in the proportion of muscle occupied by fast twitch (type II) fibers (Klein et al. 2003), which is likely to influence performance on ballistic tasks. However, we found diminished motor learning in older adults compared to young adults, but only for the right (dominant) hand. These findings suggest an age-related decline in asymmetry of motor learning. One possible reason for an age-related reduction in motor learning in the right hand is a reduced need to learn new motor skills with advancing age, as shown by a more balanced use of left and right hands in everyday tasks performed by older adults (Kalisch et al. 2006), resulting in an age-related modification of the mechanisms important for use-dependent plasticity. Furthermore, several neuroimaging studies have shown increased recruitment of cortical and subcortical areas during movement tasks in older adults (Mattay et al. 2002), including increased bilateral cortical activation (Naccarato et al. 2006), which are usually interpreted as compensatory changes in the aging brain (Ward 2006). The possibility exists that these compensatory mechanisms lead to less cortical lateralization in older adults, which result in similar motor learning capabilities for both hands in the elderly (Kalisch et al. 2006).
Several studies have shown that modulation of SICI plays an important role in skilled hand movement (Stinear and Byblow 2003; Zoghi et al. 2003) and removal of SICI plays a critical role in use-dependent plasticity (Ziemann et al. 2001). It is therefore possible that age-related differences in intracortical inhibition may contribute to impaired hand performance (see Sale and Semmler 2005) and reduced M1 plasticity (Sawaki et al. 2003; Rogasch et al. 2009) that is generally observed in older adults. Some studies have shown a decrease (Peinemann et al. 2001) or an increase (Kossev et al. 2002; Smith et al. 2009) in SICI in older adults, but a larger number of studies have reported no age-related change (Wassermann 2002; Oliviero et al. 2006; Rogasch et al. 2009), which was supported by the present study. Furthermore, as reported previously (Rogasch et al. 2009), there was no change in SICI in young or old adults after training with the ballistic thumb abduction task, suggesting that the modulation of SICI that sometimes accompanies training may require a more demanding task involving selective muscle activation (e.g., Liepert et al. 1998).
In conclusion, we have examined corticomotor plasticity and motor learning following repetitive thumb abduction training in left and right hands of young and old adults. Despite greater task improvement for the right (dominant) hand, there was increased use-dependent corticomotor plasticity in the right hemisphere controlling the left (non-dominant) hand, suggesting that the factors responsible for the increased MEP with training are more effective in the right hemisphere. Although an age-related decline in motor learning occurred for the right (dominant) hand, use-dependent corticomotor plasticity was not altered with advancing age during this task. We therefore suggest that some older adults are able to retain similar use-dependent plasticity for both hands during a ballistic motor training task. The factors that promote this age-related maintenance in use-dependent plasticity remain to be determined.
References
Amunts K, Schlaug G, Schleicher A, Steinmetz H, Dabringhaus A, Roland PE, Zilles K (1996) Asymmetry in the human motor cortex and handedness. Neuroimage 4:216–222
Bäumer T, Dammann E, Bock F, Kloppel S, Siebner HR, Munchau A (2007) Laterality of interhemispheric inhibition depends on handedness. Exp Brain Res 180:195–203
Boyke J, Driemeyer J, Gaser C, Buchel C, May A (2008) Training-induced brain structure changes in the elderly. J Neurosci 28:7031–7035
Bryden PJ, Roy EA (1999) Spatial task demands affect the extent of manual asymmetries. Laterality 4:27–37
Bütefisch CM, Davis BC, Wise SP, Sawaki L, Kopylev L, Classen J, Cohen LG (2000) Mechanisms of use-dependent plasticity in the human motor cortex. Proc Natl Acad Sci USA 97:3661–3665
Carroll TJ, Lee M, Hsu M, Sayde J (2008) Unilateral practice of a ballistic movement causes bilateral increases in performance and corticospinal excitability. J Appl Physiol 104:1656–1664
Cirillo J, Lavender AP, Ridding MC, Semmler JG (2009) Motor cortex plasticity induced by paired associative stimulation is enhanced in physically active individuals. J Physiol 587:5831–5842
Classen J, Liepert J, Wise SP, Hallett M, Cohen LG (1998) Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol 79:1117–1123
Cotman CW, Berchtold NC (2002) Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci 25:295–301
Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA (2000) Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology 216:672–682
Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P (2003) International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35:1381–1395
Datta AK, Harrison LM, Stephens JA (1989) Task-dependent changes in the size of response to magnetic brain stimulation in human first dorsal interosseous muscle. J Physiol 418:13–23
Fogelholm M, Malmberg J, Suni J, Santtila M, Kyrolainen H, Mantysaari M, Oja P (2006) International physical activity questionnaire: validity against fitness. Med Sci Sports Exerc 38:753–760
Gallasch E, Christova M, Krenn M, Kossev A, Rafolt D (2009) Changes in motor cortex excitability following training of a novel goal-directed motor task. Eur J Appl Physiol 105:47–54
Garry MI, Thomson RH (2009) The effect of test TMS intensity on short-interval intracortical inhibition in different excitability states. Exp Brain Res 193:267–274
Garry MI, Kamen G, Nordstrom MA (2004) Hemispheric differences in the relationship between corticomotor excitability changes following a fine-motor task and motor learning. J Neurophysiol 91:1570–1578
Guye M, Parker GJ, Symms M, Boulby P, Wheeler-Kingshott CA, Salek-Haddadi A, Barker GJ, Duncan JS (2003) Combined functional MRI and tractography to demonstrate the connectivity of the human primary motor cortex in vivo. Neuroimage 19:1349–1360
Hammond G (2002) Correlates of human handedness in primary motor cortex: a review and hypothesis. Neurosci Biobehav Rev 26:285–292
Ilic TV, Jung P, Ziemann U (2004) Subtle hemispheric asymmetry of motor cortical inhibitory tone. Clin Neurophysiol 115:330–340
Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR (2001) Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging 22:581–594
Kalisch T, Wilimzig C, Kleibel N, Tegenthoff M, Dinse HR (2006) Age-related attenuation of dominant hand superiority. PLoS ONE 1:e90
Klein CS, Marsh GD, Petrella RJ, Rice CL (2003) Muscle fiber number in the biceps brachii muscle of young and old men. Muscle Nerve 28:62–68
Kossev AR, Schrader C, Dauper J, Dengler R, Rollnik JD (2002) Increased intracortical inhibition in middle-aged humans; a study using paired-pulse transcranial magnetic stimulation. Neurosci Lett 333:83–86
Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD (1993) Corticocortical inhibition in human motor cortex. J Physiol 471:501–519
Liepert J, Classen J, Cohen LG, Hallett M (1998) Task-dependent changes of intracortical inhibition. Exp Brain Res 118:421–426
Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, Weinberger DR (2002) Neurophysiological correlates of age-related changes in human motor function. Neurology 58:630–635
McNay EC, Willingham DB (1998) Deficit in learning of a motor skill requiring strategy, but not of perceptuomotor recalibration, with aging. Learn Mem 4:411–420
McNevin NH, Wulf G, Carlson C (2000) Effects of attentional focus, self-control, and dyad training on motor learning: implications for physical rehabilitation. Phys Ther 80:373–385
Meintzschel F, Ziemann U (2006) Modification of practice-dependent plasticity in human motor cortex by neuromodulators. Cereb Cortex 16:1106–1115
Muellbacher W, Ziemann U, Boroojerdi B, Cohen L, Hallett M (2001) Role of the human motor cortex in rapid motor learning. Exp Brain Res 136:431–438
Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, Boroojerdi B, Poewe W, Hallett M (2002) Early consolidation in human primary motor cortex. Nature 415:640–644
Naccarato M, Calautti C, Jones PS, Day DJ, Carpenter TA, Baron JC (2006) Does healthy aging affect the hemispheric activation balance during paced index-to-thumb opposition task? An fMRI study. Neuroimage 32:1250–1256
Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM (1996) Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci 16:785–807
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Oliviero A, Profice P, Tonali PA, Pilato F, Saturno E, Dileone M, Ranieri F, Di Lazzaro V (2006) Effects of aging on motor cortex excitability. Neurosci Res 55:74–77
Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M (1995) Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol 74:1037–1045
Peinemann A, Lehner C, Conrad B, Siebner HR (2001) Age-related decrease in paired-pulse intracortical inhibition in the human primary motor cortex. Neurosci Lett 313:33–36
Ravussin E, Bogardus C (1989) Relationship of genetics, age, and physical fitness to daily energy expenditure and fuel utilization. Am J Clin Nutr 49:968–975
Ridding MC, Flavel SC (2006) Induction of plasticity in the dominant and non-dominant motor cortices of humans. Exp Brain Res 171:551–557
Ridding MC, Ziemann U (2010) Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J Physiol. doi:10.1113/jphysiol.2010.190314 (in press)
Rogasch NC, Dartnall TJ, Cirillo J, Nordstrom MA, Semmler JG (2009) Corticomotor plasticity and learning of a ballistic thumb training task are diminished in older adults. J Appl Physiol 107:1874–1883
Rosenkranz K, Kacar A, Rothwell JC (2007a) Differential modulation of motor cortical plasticity and excitability in early and late phases of human motor learning. J Neurosci 27:12058–12066
Rosenkranz K, Williamon A, Rothwell JC (2007b) Motorcortical excitability and synaptic plasticity is enhanced in professional musicians. J Neurosci 27:5200–5206
Sale MV, Semmler JG (2005) Age-related differences in corticospinal control during functional isometric contractions in left and right hands. J Appl Physiol 99:1483–1493
Sanes JN, Donoghue JP (2000) Plasticity and primary motor cortex. Annu Rev Neurosci 23:393–415
Sawaki L, Boroojerdi B, Kaelin-Lang A, Burstein AH, Bütefisch CM, Kopylev L, Davis B, Cohen LG (2002) Cholinergic influences on use-dependent plasticity. J Neurophysiol 87:166–171
Sawaki L, Yaseen Z, Kopylev L, Cohen LG (2003) Age-dependent changes in the ability to encode a novel elementary motor memory. Ann Neurol 53:521–524
Semmler JG, Nordstrom MA (1998) Hemispheric differences in motor cortex excitability during a simple index finger abduction task in humans. J Neurophysiol 79:1246–1254
Smith CD, Umberger GH, Manning EL, Slevin JT, Wekstein DR, Schmitt FA, Markesbery WR, Zhang Z, Gerhardt GA, Kryscio RJ, Gash DM (1999) Critical decline in fine motor hand movements in human aging. Neurology 53:1458–1461
Smith AE, Ridding MC, Higgins RD, Wittert GA, Pitcher JB (2009) Age-related changes in short-latency motor cortex inhibition. Exp Brain Res 198:489–500
Stefan K, Wycislo M, Gentner R, Schramm A, Naumann M, Reiners K, Classen J (2006) Temporary occlusion of associative motor cortical plasticity by prior dynamic motor training. Cereb Cortex 16:376–385
Stinear CM, Byblow WD (2003) Role of intracortical inhibition in selective hand muscle activation. J Neurophysiol 89:2014–2020
Tecchio F, Zappasodi F, Pasqualetti P, De Gennaro L, Pellicciari MC, Ercolani M, Squitti R, Rossini PM (2008) Age dependence of primary motor cortex plasticity induced by paired associative stimulation. Clin Neurophysiol 119:675–682
Tormos JM, Canete C, Tarazona F, Catala MD, Pascual-Leone Pascual A, Pascual-Leone A (1997) Lateralized effects of self-induced sadness and happiness on corticospinal excitability. Neurology 49:487–491
Triggs WJ, Calvanio R, Levine M (1997) Transcranial magnetic stimulation reveals a hemispheric asymmetry correlate of intermanual differences in motor performance. Neuropsychologia 35:1355–1363
Vandervoort AA (2002) Aging of the human neuromuscular system. Muscle Nerve 25:17–25
Voelcker-Rehage C (2008) Motor-skill learning in older adults—a review of studies on age-related differences. Eur Rev Aging Phys Act 5:5–16
Volkmann J, Schnitzler A, Witte OW, Freund H (1998) Handedness and asymmetry of hand representation in human motor cortex. J Neurophysiol 79:2149–2154
Ward NS (2006) Compensatory mechanisms in the aging motor system. Ageing Res Rev 5:239–254
Wassermann EM (2002) Variation in the response to transcranial magnetic brain stimulation in the general population. Clin Neurophysiol 113:1165–1171
Wu T, Hallett M (2005) The influence of normal human ageing on automatic movements. J Physiol 562:605–615
Ziemann U, Muellbacher W, Hallett M, Cohen LG (2001) Modulation of practice-dependent plasticity in human motor cortex. Brain 124:1171–1181
Ziemann U, Ilic TV, Pauli C, Meintzschel F, Ruge D (2004) Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J Neurosci 24:1666–1672
Zoghi M, Pearce SL, Nordstrom MA (2003) Differential modulation of intracortical inhibition in human motor cortex during selective activation of an intrinsic hand muscle. J Physiol 550:933–946
Acknowledgments
A grant from the National Health and Medical Research (NHMRC) of Australia supported this work. This study forms part of the PhD of John Cirillo, who is supported by the University of Adelaide Postgraduate Research Scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cirillo, J., Rogasch, N.C. & Semmler, J.G. Hemispheric differences in use-dependent corticomotor plasticity in young and old adults. Exp Brain Res 205, 57–68 (2010). https://doi.org/10.1007/s00221-010-2332-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-010-2332-1