Abstract
Purpose
To test whether long-term cortical adaptations occur bilaterally following chronic unilateral training with a simple motor task.
Methods
Participants (n = 34) were randomly allocated to a training or control groups. Only the former completed a 4-week maximal-intensity isometric training of the right first dorsal interosseus muscle through key pinching. Maximal strength was assessed bilaterally in four different movements progressively less similar to the training task: key, tip and tripod pinches, and handgrip. Transcranial magnetic stimulation was used to probe, in the left and right primary hand motor cortices, a number of standard tests of cortical excitability, including thresholds, intra-cortical inhibition and facilitation, transcallosal inhibition, and sensory-motor integration.
Results
Training increased strength in the trained hand, but only for the tasks specifically involving the trained muscle (key +8.5 %; p < 0.0005; tip +7.2 %; p = 0.02). However, the effect size was small and below the cutoff for meaningful change. Handgrip and tripod pinch were instead unaffected. There was a similar improvement in strength in the untrained hand, i.e., a cross-education effect (key +6.4 %; p = 0.02; tip +4.7 %; p = 0.007). Despite these changes in strength, no significant variation was observed in any of the neurophysiological parameters describing cortico-spinal and intra-cortical excitability, inter-hemispheric inhibition, and cortical sensory-motor integration.
Conclusions
A 4-week maximal-intensity unilateral training induced bilaterally spatial- and task-specific strength gains, which were not associated to direct or crossed cortical adaptations. The observed long-term stability of neurophysiological parameters might result from homeostatic plasticity phenomena, aimed at restoring the physiological inter-hemispheric balance of neural activity levels perturbed by the exercise.
Trial registration number
ClinicalTrials.gov identifier NCT02010398.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Strength training is well known to induce structural alterations in the skeletal muscle, which contribute to significant increases in muscle strength and size (Abernethy et al. 1994). Gains in strength following resistance training are detected not only in those muscles specifically trained, but also in the contra-lateral homologous muscles that were not targeted with exercise, portraying a phenomenon known as “cross education” (CE) (Munn et al. 2004). Since morphological or enzymatic changes in the untrained muscles proved undetected in the initial phases of training (Narici et al. 1989), neural mechanisms have been accounted for the CE effect, with no consensus, however, on a dominant mechanism or site of adaptation (Enoka 1997; Hortobagyi 2005; Carroll et al. 2006; Fimland et al. 2009; Kidgell et al. 2011).
Most studies examining the mechanism of CE effects have been performed on the upper limb due to the ease with which a range of common electrophysiological techniques can be applied, particularly those involving transcranial magnetic stimulation (TMS). In general, these studies have shown that, following a single session of strength training employing a simple motor task, there is a transient increase in excitability of cortico-spinal projections in the “trained” hemisphere as well as a smaller increase in the “untrained” hemisphere (Yue and Cole 1992; Cramer et al. 1999; Muellbacher et al. 2000; Perez et al. 2004; Farthing et al. 2005; Carroll et al. 2006; Perez and Cohen 2008; Kidgell et al. 2011; Liang et al. 2014; Leung et al. 2015). The latter has been interpreted as one possible mechanism underlying the CE effect.
However, the data are less clear in experiments testing the effects of longer term training protocols. Some studies report no changes in cortico-spinal excitability in either hemisphere, whereas others find persisting increases in excitability in both hemispheres (Classen et al. 1999; Lee et al. 2009; Hortobagyi et al. 2011; Kidgell et al. 2015). Changes in intra-cortical excitability as measured by short-interval intra-cortical inhibition (SICI) and inter-hemispheric inhibition (IHI) have also been debated both in acute (Warbrooke and Byblow 2004; Duque et al. 2008; Vercauteren et al. 2008) and chronic studies (Hortobagyi et al. 2011; Kidgell et al. 2015). However, direct comparisons between these studies are difficult, because of the differences in muscle studied and training provided. Therefore, the question remains whether chronic training involving a simple resistance motor task is capable to cause significant and meaningful contra-lateral strength gains and whether they are paralleled by noteworthy supraspinal neural adaptations. From a functional perspective, we hypothesize that the neurophysiological changes associated to acute training are unlike to persist following chronic exercise, since they would engage motor and learning circuits, otherwise available for new tasks. Therefore, the aim of this study was to explore a very wide range of sensory-motor pathways after chronic CE to test whether long-term changes occur following a 4-week maximal-intensity unilateral isometric training of the first dorsal interosseus (FDI) muscle.
Methods
Study design and participants
This was a parallel-group case–control study (ClinicalTrials.gov identifier: NCT02010398) in a randomized 1:1 allocation ratio. Thirty-four healthy individuals volunteered for the study (M:F = 23:11; mean age 25.5 ± 6.0 years; weight 67.6 ± 11.1 kg; height 170.3 ± 9.4 cm). All subjects were right handed according to the Edinburgh Handiness Inventory (laterality quotient >85 %). Participants were recruited from the university student population and staff. At the clinical examination (E.R.D.N.), none of the participants had a history of neurological and/or psychiatric diseases and/or upper limb musculoskeletal disorders, neither presented contraindications to undergo TMS and/or surface electrical stimulation procedures. After baseline evaluation, 34 opaque envelopes were numbered consecutively (A.M.) and randomly assigned (M.P.C.) to an intervention (training; n = 17) or to a no-intervention (control; n = 17) group, with a blocking procedure employing the Research Randomizer 3.0 software. Testing procedures were performed before (PRE) and after (POST) the experimental period by the same operator at the same time of the day. Both outcome assessors and statistician were blinded to the allocation group. All subjects gave written informed consent to the experimental procedure, which was approved by the local ethics committee. The study was performed in accordance with the Declaration of Helsinki. Data were collected and stored at the Department of Biomedical Sciences, University of Sassari (January–July 2015).
Strength assessment
Participants were seated in front of a computer screen with feet flat on the floor, back straight, and supported by a backrest, shoulders adducted and neutrally rotated, elbows at 90°, and forearms in a neutral position supported by the chair armrests. The wrist was positioned between 0° and 30° of dorsiflexion and between 0° and 15° of ulnar deviation (Pryce 1980). Prior to the baseline assessment, all participants attended a preparatory session to familiarize with positioning and testing/training procedures. A light warm-up consisting of two trials performed at sub-maximal intensity (5 s duration each) preceded the testing procedures. Subjects were then required to exert three 5 s maximal voluntary isometric contractions (MVIC) for each motor task performed, with 1 min of inter-trial rest.
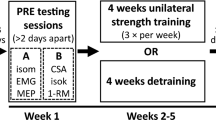
To assess the strength of the FDI muscle, the third, fourth, and fifth fingers were extended and restrained in a cast with Velcro straps, as well as the wrist. For the criterion test, the subject was asked to hold a force transducer (P200 Biometrics Ltd, Newport, UK) between the pad of the thumb and the lateral aspect of the middle phalanx of index finger (key or lateral pinch) and between the tips of the thumb and index fingers (fingertip precision grip or tip pinch). Due to its insertion onto the tubercle of proximal phalanx, the strongest activity of the FDI is in key pinch; the FDI is also highly active in tip pinch, where it mainly acts as a flexor at the metacarpophalangeal joint (Schreuders et al. 2007). To control for the specificity of the training-induced changes, the pinches between the thumb pad and the pads of index and middle fingers (tripod or palmar pinch) were also tested. To be maintained in the horizontal plane, the wrist and the fingers pinching the transducer rested on a Plexiglas support base affixed to the chair’s armrest with a Velcro strap. The force transducer was connected to a data acquisition system (DataLOG MWX8, Biometrics Ltd, Newport, UK). After FDI, strength testing was completed, a 10-min rest was allowed for full recovery, and then, the handgrip strength was also assessed with an electronic handgrip dynamometer (G200 Biometrics Ltd, Newport, UK) to verify spatial specificity of the contra-lateral transfer of strength. The dominant hand was tested first. A 5-min rest was allowed between the testing of the two sides. For each strength test, the scores of three successive trials were recorded for each hand. The highest of the three strength scores recorded for each test was taken as the participant’s MVIC. The plan of measurements with the timeline of the study is summarized in Fig. 1.
Contra-lateral transfer of muscle strength
To control and estimate the familiarization/learning-effect and obtain net changes in muscle strength following the intervention, the equation by Carroll et al. (2006) was employed. For each side, the mean strength change recorded in the controls group was, therefore, subtracted from the mean change of the Training group.
Neurophysiological assessments
Subjects sat in a comfortable chair with the neck supported and were asked to keep their eyes open and to stay relaxed, but alert during data collection.
Electromyography (EMG)
TMS-evoked motor potentials (MEP) at rest and EMG recruitment, at the MVIC, were bilaterally recorded (D360 amplifier; Digitimer Ltd, Welwyn Garden City, UK) from the FDI muscle using 9-mm-diameter Ag–AgCl electrodes in a belly tendon montage. EMG signals were amplified (×1000), filtered (bandpass 3–3000 Hz) and sampled (6 kHz) using a CED 1401 (Cambridge Electronic Design, Cambridge, UK) power analog-to-digital converter. Raw EMG recordings were rectified for off-line measurements.
TMS
Magnetic stimuli were generated by Magstim 200 stimulators connected in a Bistim module (Magstim Co., Whitland, Dyfed, UK) and delivered in separate sessions to the hand contra-lateral and ipsi-lateral primary motor cortex (cM1 and iM1, respectively) through a figure-of-eight coil (external loop diameter of 9 cm) with the handle pointing backwards and laterally at 45° away from the midline. The optimal stimulation site for the activation of the contra-lateral FDI was marked on the scalp with a soft tip pen to ensure that the coil remained in the same place throughout the experiments. Motor threshold was expressed as a percentage of the maximum stimulator output. The test stimulus intensity was the intensity sufficient to evoke a 1-mV MEP in the relaxed FDI, and, in all trials, frequency of TMS was randomized to 0.2 ± 10 % Hz (one pulse every 4.5–5.5 s).
Electrical stimulations
To test sensorimotor integration, the median nerve ipsi-lateral to the recorded FDI was electrically stimulated at the wrist (Digitimer DS7A constant current stimulator) through single square-wave pulses (0.2 ms, 0.25 Hz) at an intensity nearly 2–3 times the perceptual threshold, just above the motor threshold for evoking a visible twitch of the thenar muscles.
Neurophysiological protocols
Neurophysiological protocols were applied to the hand iM1 and cM1 according to the most acknowledged guidelines (Rossini et al. 2015). Resting motor threshold (RMT) was determined, as the minimum stimulus intensity required to produce MEPs >50 μV peak-to-peak amplitude in at least five out of ten consecutive trials in the relaxed muscle. Active motor threshold (AMT) was established as the minimum stimulus intensity able to evoke MEPs >200 μV peak-to-peak amplitude in at least five out of ten consecutive trials during isometric contraction of the tested muscle at 10 % of MVIC. Central motor conduction time (CMCT) was calculated subtracting the mean latency of the 1 mV MEP (TMStest) induced by TMS of the cervical spinal root at C7 level from the mean latency of the TMStest elicited cortical TMS. The recruitment curve (RC) was constructed plotting peak-to-peak amplitudes of mean MEPs induced by a single-pulse TMS delivered at intensities from 90 to 150 % of RMT. Seven blocks of ten stimuli each were collected. SICI and intra-cortical facilitation (ICF) were assessed with a conditioning stimulus of 80 % RMT preceding a test stimulus of TMStest by 3 and 10 ms, respectively. Short-interval intra-cortical facilitation (SICF) was tested pairing stimuli of TMStest and 80 % RMT intensities at an inter-stimulus interval (ISI) of 1.5 ms. Long-interval intra-cortical inhibition (LICI) was evaluated pairing stimuli of TMStest at ISIs of 100 and 200 ms. Short- and long-latency inter-hemispheric inhibition (SIHI and LIHI, respectively) were tested using 7-cm double coils (model D702) and delivering a conditioning stimulus of TMStest to the hand M1 of one side, 10 and 40 ms before the administration of a test stimulus of TMStest to the cM1, respectively. EMG recruitment was also assessed as an index of cortico-spinal excitability by measuring the root mean square in the rectified raw EMG recorded during 1-s MVIC of the FDI (5 trials for each muscle).
Cortical sensorimotor integration was bilaterally assessed measuring short- and long-afferent intra-cortical inhibition (SAI and LAI, respectively). SAI and LAI were tested coupling the ES of the median nerve with TMS of the cM1 at ISIs of 20 and 200 ms, respectively. For each trial of the above protocols, 10 MEPs (test MEP or conditioned MEPs at any ISI) were randomly recorded and averaged. Peak-to-peak amplitude was measured for each MEP and that of conditioned MEPs was expressed as a ratio of the averaged test MEP.
Intervention
A maximal-intensity isometric training of the right dominant FDI was administered to the training group. The participants were seated in the same chair employed for the testing procedure and consistently positioned. The training protocol consisted of a 4-week unilateral training, 3 days/week on non-consecutive days, with a 20-min duration per session. After a light warm-up (see above), subjects performed five sets of ten isometric key or lateral pinch contractions holding the same force transducer employed for the assessments between the pad of the thumb and the lateral aspect of the middle phalanx of index finger. The duration of the contraction and the inter-contraction rest were both 5 s long. To avoid the onset of fatigue, a 3-min recovery was allowed among sets. During exercise, participants were provided with a visual feedback displaying the real-time level of strength reached to motivate the achievement of maximal performance. To minimize any mirror activity in the untrained FDI, subjects were instructed to keep this muscle completely relaxed and were helped in achieving this goal by an auditory feedback of left FDI EMG activity. Volunteers assigned to the control group attended the laboratory for the same number of sessions and placed in the same standardized position for the 20-min session duration without performing any exercise.
Statistical analysis
Data analysis was performed using the SPSS 18 software (SPSS Inc., Chicago, IL, USA). An a priori power analysis conservatively assuming an expected effect size (Cohen’s d) of 0.5 and a statistical power of 0.80 at a 0.05 alpha level was performed, resulting in a sample size of 17 subjects per group. The Shapiro–Wilk test was used to assess normality. Homogeneity of variances between groups at baseline for dynamometric variables was evaluated by Levene’s test. All the analyses were performed by original assigned groups. Demographic and anthropometric variables (age, weight, height, and gender) were analyzed at baseline with one-way ANOVA or Chi-Square test, when appropriate.
Dynamometric variables
PRE-to-POST changes in the dynamometric parameters were analyzed with a repeated-measures analysis of variance (ANOVA) with GROUP (training, control), TIME (PRE, POST), and SIDE (dominant, non-dominant) as factors. When significant F values were observed, paired t tests with Bonferroni adjustment were used. Cohen’s d effect size magnitudes (small ≤0.5; moderate 0.51–0.79; large ≥0.8) (Cohen 1988) were also used to quantify differences in the data after intervention. Only for dynamometric variables, test–retest relative reliability between the two baseline testing sessions (Sessions 1 and 2) on non-consecutive days was assessed by two-way random intra-class correlation coefficient for single measures (ICC2,1). The ICC coefficients were calculated taking a value <0.4 as an index of poor reliability, 0.41–0.75 fair to good reliability, and >0.75 excellent reliability (Shrout and Fleiss 1979). Absolute reliability was estimated with the coefficient of variation (CV%) and with the standard error of measurement (SEM) (Weir 2005). The responsiveness of strength measurements to training was estimated by calculating the individual smallest real difference (SRDi), which serves as a cut-off threshold for meaningful change (Lexell and Downham 2005).
Neurophysiological variables
Repeated-measures ANOVA was used to assess the effect of TIME (PRE, POST), SIDE (dominant, non-dominant), and GROUP (training, control) for RMT, AMT, TMStest, CMTC, and EMG. The effect of TMS intensity was evaluated for RC (90–150 % of RMT). For paired-pulse TMS–TMS and ES–TMS protocols, the effect of training (PRE, POST), SIDE (dominant, non-dominant), GROUP (training and control), and ISI (SICI 3 ms; ICF 10 ms; SICF 1.5 ms; LICI 100 and 200 ms; SAI 20 ms; LAI 200 ms, IHI 10 and 40 ms) on the amplitude of the conditioned MEP (expressed as conditioned amplitude/unconditioned amplitude) was assessed by a repeated-measures ANOVA. In the case of significant differences, paired t tests were performed applying the Bonferroni correction for multiple comparisons.
Compound symmetry of data was evaluated with Mauchly’s sphericity test. The Greenhouse–Geisser correction was used to compensate for non-spherical data.
For all variables, a p value <0.05 was considered significant. Unless otherwise stated, values are expressed as mean ± standard deviation (SD).
Results
All participants (n = 34) completed all the testing and training sessions with no adverse effects reported. Demographic and anthropometric characteristics of the sample are detailed by group in Table 1.
Reproducibility and responsiveness of strength measurements
Relative reproducibility of the strength measurements as calculated by ICC coefficients proved very high (all coefficients >0.8). The analysis of the absolute reproducibility between the two testing sessions at baseline revealed a low variability as assessed by CV% (range 2.9–6.4 %) and SEM (range 0.92–1.26 units). The calculation of the individual smallest real difference in absolute values (SRDi) for each motor task allowed the definition of a cutoff to be exceeded to consider the training-induced changes as meaningful in both the right trained (key pinch +3.6 N; tip pinch +4.2 N; tripod pinch +4.9 N; handgrip +4.3 N) and in the left untrained hand (key pinch +3.9 N; tip pinch +4.9 N; tripod pinch +4.7 N; handgrip +4.1 N). All the reproducibility and responsiveness results are detailed by side and by motor task in Table 2.
Maximal voluntary strength
There was no difference between groups at baseline in the levels of strength of the dominant right and non-dominant left hands as tested by tip, key, and tripod pinches and by the whole handgrip test (Table 3). Gross and net training-induced changes are displayed in Fig. 2, while Table 3 details raw strength data by type of motor task and by side, with the accompanying ANOVA results in Table 4. The mirror EMG activity in the left FDI, expressed as percent of the MVIC activity, was less than 1 % in all the motor tasks performed.
Spatial and task specificity of training-induced changes in maximal voluntary strength. a, b Percent increases in strength are shown by motor task performed for the training group (black columns) undergoing a 4-week period of maximal isometric strength training of the first dorsal interosseous muscle (FDI) of the dominant hand and for the control group (white columns) undergoing a 4-week period of no-intervention. c, d Between-group differences in strength gains (obtained subtracting the PRE–POST changes exhibited by the control group from those exhibited by the training group) are shown for each task in the trained and untrained hand (cross-education effect). The FDI is specifically engaged during the key and the tip pinches, only marginally involved by the tripod pinch and not involved during the handgrip. *p < 0.05; **p < 0.01
The increase in strength was significantly greater in the trained than in the control (untrained) group for the key pinch and tip pinch tasks, but not for the tripod or handgrip tasks (significant GROUP × TIME interaction for the first two tasks only). It should be noted that, although the effect was greater in the trained group, even the control group improved in strength in the first two tasks, perhaps, due to familiarity with the task. The additional increment in strength due to training over controls was relatively small (8.5 and 7.2 % for key pinch and tip pinch in the trained hand; 6.4 and 4.7 % in the untrained hand), well below the SRDi thresholds and associated to low effect sizes (Cohen’s d 0.2–0.4). The increase in strength was not significantly different on the trained and untrained sides (i.e., none of the TIME × SIDE interactions were significant).
Neurophysiological results
Cortico-spinal and intra-cortical excitability
Repeated-measures ANOVA showed that RMT and TMStest were not significantly different by TIME and by GROUP, while a significant GROUP × TIME interaction was detected for CMCT. A significant main effect of TIME was detected for AMT, but no significant GROUP and GROUP × TIME interaction were found (Table 5).
Repeated-measures ANOVA for the RC showed a significant effect (p < 0.0001) of TMS intensity (90, 100, 110, 120, 130, 140, and 150 % of RMT), but no main effects of TIME, GROUP, and no interactions among factors (Table 5 and Fig. 3). Repeated-measures ANOVA revealed no main effects of TIME and GROUP in the paired-pulse TMS–TMS protocols testing SICF, SICI, ICF, LICI, SIHI, and LIHI. A significant main effect of ISI (at ISIs of 1.5, 3, 10, 100, and 200 ms, respectively, and 10 and 40 ms for SIHI and LIHI) was detected (all p values <0.001), but no significant interactions among factors (Table 5 and Fig. 4).
Recruitment curves obtained from both the training and control groups are reported for each first dorsal interosseous muscle (FDI). Recruitment curves acquired at baseline (PRE, black line) and after 4-week maximal-intensity isometric training of the dominant FDI in the training group or after a 4-week period of no intervention in the control group (POST, grey line) are superimposed. Vertical axes indicate mean MEP amplitude, and horizontal axes indicate transcranial magnetic stimulation (TMS) intensities (in % of the resting motor threshold, RMT). Error bars represent the standard error
Effects of 4-week maximal isometric strength training and of no-intervention on intra-cortical and inter-hemispheric excitability and on sensory-motor integration at cortical level. Histograms report short-interval intra-cortical inhibition (SICI), intra-cortical facilitation (ICF), short-interval intra-cortical facilitation (SICF), long-interval intra-cortical inhibition at 100 and 200 ms inter-stimulus interval (LICI 100 and LICI 200, respectively), short- and long-interval inter-hemispheric inhibition (SIHI and LIHI, respectively), and short- and long-afferent inhibition (SAI and LAI, respectively) measured bilaterally from the FDI muscle before (white columns) and after (black columns) a 4-week period of maximal isometric strength training of the dominant FDI (training group) or no intervention (control group). Vertical axes indicate MEP amplitude, expressed as the mean ± SEM ratio between conditioned MEP and unconditioned MEP, induced by single-pulse TMS, taken as 1.0 (dotted horizontal line). In both the training and control groups, all parameters of intra- and inter-cortical excitability and of cortical sensory-motor integration appeared substantially unchanged after the training and the no-intervention period, respectively
Sensorimotor integration at the cortical level
Repeated-measures ANOVA revealed no main effects of TIME and GROUP in the paired-pulse ES–TMS protocols testing SAI and LAI. A significant effect of ISI (at ISIs of 20 and 200 ms, for SAI and LAI, respectively) was observed (all p < 0.001), but no significant interactions among factors (Table 5 and Fig. 4).
Overall, no main effect of SIDE was observed for any of the neurophysiological variables analyzed. Following the intervention, no main effects of TIME (F 1,64 = 0.090; p = 0.76), GROUP (F 1,64 = 1.238; p = 0.27), or TIME × GROUP interaction (F 1,64 = 0.053; p = 0.82) were detected in EMG activity during a MVIC.
Discussion
This study explored a wide range of neurophysiological parameters describing cortico-spinal and intra-cortical excitability, inter-hemispheric inhibition, and cortical sensory-motor integration to investigate neural adaptations following chronic unilateral strength training of the dominant hand. The main finding of the study is that the significant gains in strength detected in both the trained and untrained hands were not paralleled by any changes in the ipsi- or contra-lateral primary hand motor cortices.
Spatial specificity
A chronic training of the dominant hand with a key lateral pinch motor task induced significant improvements in maximal strength not only in the FDI muscle targeted with exercise, but also in the homologous contra-lateral untrained muscle, depicting a CE effect. Strength adaptations in response to training showed a high spatial specificity depending on the biomechanical features of the motor task. In fact, this phenomenon was observed in the two tasks, specifically engaging the FDI, but not in those involving only marginally or not involving this muscle, according to the well-established specificity of the response to unilateral training (refs. in Farthing et al. 2005, 2009).
Training-induced changes in strength
Following the intervention, MVIC increased to a similar extent in both the trained and untrained FDI, with the net contra-lateral strength gains ranging 4.7–6.4 %, in line with CE magnitudes outlined for the upper limb (Munn et al. 2004; Carroll et al. 2006). A greater extent of CE (40–70 %) has been previously reported, but several factors, such as heterogeneity in the training regimen, upper limb muscle, or study design, can account for this discrepancy (Farthing Farthing 2009; Hortobagyi et al. 2011). When assessing the extent of the CE effect, a between-subject design, like that chosen in this study, is considered mandatory to control for familiarization and to weigh the training-induced net change (Carroll et al. 2006). In the trained and untrained FDI, we estimated a familiarization/learning-effect among the four different tasks of 0.1–5.5 and 1.4–4.8 %, respectively, which, although generally considered small (Carroll et al. 2006), represents a quite relevant fraction of the gross strength gains measured in the ipsi-lateral trained (7.3–14 %) and in the contra-lateral untrained (7.8–9.5 %) muscles and needs, therefore, to be taken into proper account, since testing itself can improve performance due to habituation of subjects to the test procedure (Gleeson and Mercer 1996; Manca et al. 2015).
The PRE-to-POST differences in strength exhibited by both the trained and untrained FDI muscles during the key and tip pinches, despite being significant, appeared not relevant. In fact, although strength levels were higher than baseline, they were associated to between-subject and between-side very small/small effect sizes (Cohen’s d = 0.2–0.4).
The net strength improvements observed in the trained and untrained FDI muscles did not exceed their thresholds for relevant change as estimated by the SRDi. Taken together, this data suggest that, in a functional perspective, the isometric training is not capable to meaningfully impact on the level of strength, even if conducted at maximal intensity.
Neural adaptations following a long-lasting training
As reviewed in the “Introduction”, there is some debate over the presence and nature of changes in excitability of central motor circuits after chronic strength training (Classen et al. 1999; Lee et al. 2009; Hortobagyi et al. 2011; Kidgell et al. 2015). These discrepancies have been partly explained by factors, such as the different nature of the intervention, the body territory, and the duration of the training provided. In this study, no changes in any of the neurophysiological parameters describing cortico-spinal and intra-cortical excitability, IHI, and cortical sensory-motor integration were observed in both iM1 and cM1, after 4 weeks of maximal-intensity isometric training. While our findings of an unchanged cortico-spinal and intra-cortical excitability are in agreement with those reported by Hortobagyi et al. (2011) in the same muscle following a training protocol similar to ours, but with a longer duration (8 versus 4 weeks, respectively), they are in disagreement with the significant reduction in IHI observed in that study. Interestingly, in both studies, any mirror activity in the resting hand was intentionally suppressed, which might have contributed to the evidence of unchanged cortico-spinal excitability (Sohn et al. 2003). Although further research is required to weigh the role played by the voluntary suppression of mirror movements on CE, compelling evidence has been provided on the apparently paradoxical role played by IHI reduction in preventing mirror EMG activity while chronically mediating CE (Hortobagyi et al. 2011). In fact, it is assumed that during the exercise of one hand, IHI’s function is to eliminate unwanted mirror activity in the resting contra-lateral hand to strengthen inter-limb independence (Hoy et al. 2004; Leocani et al. 2000) and facilitate unimanual movements (Vercauteren et al. 2008). In this perspective, a reduction in IHI from the trained to the untrained hemisphere would make the two limbs less independent, potentially inducing a greater tendency to make mirror movements or impair bimanual coordination. In the absence of any mirror activity, Hortobagyi et al. (2011) found a significant reduction in IHI, whereas we could not observe any change. Our finding of an unchanged IHI from the trained to the untrained hemisphere after a 4-week training (12 sessions) is in disagreement with the reduced IHI found by Hortobagyi et al. (2011) at mid-term (−21 % at the 10th training session) and final (−30.9 % at the 20th training session) assessments. A couple of differences between Hortobagyi’s and our study should be considered: one is the baseline level of SIHI (75 versus 35–40 %) and the other is the intensity of the exercise (sub-maximal versus maximal). Hortobagyi et al. (2011) might have found it easier to reduce IHI, since they began with more IHI; in addition, while emphasizing the need to maintain the non-trained hand relaxed, we used maximal contraction. This could have paradoxically increased IHI (at least during the training) from trained hemisphere to untrained hemisphere, counteracting any reduction that might have occurred otherwise.
We also detected no significant physiological changes at the level of a wide range of intra-cortical circuits regulating cortical excitability and sensory-motor integration either in the resting iM1, as it would have been anticipated with CE paradigms (for reviews: Munn et al. 2004; Carroll et al. 2006), or in the directly “trained” cM1, which is a generally expected outcome in strength conditioning literature (Enoka 1997). These findings are difficult to interpret in light of the available evidence, which reports that isometric training protocols are able to induce changes in inter-hemispheric plasticity (Hortobagyi et al. 2011) and that the voluntary isometric tonic contraction of hand muscles is capable of inducing bidirectional changes in plasticity between homologous areas of the iM1 and cM1 (Gentner et al. 2008; Hortobagyi et al. 2011). Furthermore, the afferent feedback from a muscle targeted with exercise has been reported to induce a reorganization of motor cortex outputs (Lucas and Fetz 2013). The stability of neurophysiological parameters that we found after a long-lasting period of training might be viewed as the result of homeostatic plasticity phenomena which may have occurred to ensure the maintenance of stable levels of neural activity over time (Müller-Dahlhaus and Ziemann 2015). This type of metaplasticity operates in both excitatory and inhibitory intra-cortical circuits (Murakami et al. 2012) and can last for days (Abraham et al. 2001) and even weeks (Buschler and Manahan-Vaughan 2012). We hypothesize that the acute effects, revealed by TMS protocols probing easily modulated motor circuits, may be needed to initiate the CE, while the persisting effects may be consolidated in circuits other than these, to leave them available for other functions and/or learning tasks. In this perspective, the intervention of homeostatic plasticity could counteract the imbalance between the two hemispheres induced by the training protocol, restoring the physiological balance of baseline conditions. The functional role of homeostatic plasticity in long-term training of the small muscles of the hand should be viewed in the context of the biomechanical and functional properties of hand muscles. Hand function is based on the combination of two basic grips: power grip (mainly performed by extrinsic muscles) and precision grip (mainly performed by intrinsic muscles, such as the FDI). While power grip requires firm control of holding an object during activity, which demands a relatively high amount of muscle strength, precision grip basically needs a perfectly coordinated interplay of the small muscles of the hand rather than the exertion of high levels of strength.
An alternative possible interpretation for the lack of neural adaptations in FDI M1 might reside in the relationship between intrinsic and extrinsic muscles during motor tasks specifically involving the FDI. It might be possible that the observed increases in maximal strength of the key pinch are related to an increased CNS ability to fine-tune the recruitment of those extrinsic muscles that stabilize the wrist, rather than an increased capability of M1 to recruit the FDI, so that a better stabilization would have allowed producing a stronger pinch. Considering that several extrinsic muscles are capable of exerting a maximal force higher than that needed to stabilize the wrist during a maximal key pinch, fine-tuning the wrist stabilization should not need a strength training of extrinsic muscles, but just some experience of the pinch task. Such experience could have been obtained by both the training and control groups during the PRE sessions of strength testing, especially because these tests involved also other pinch/grip tasks, all requiring a proper wrist stabilization. This would be in agreement with the significant main effects of TIME found on the strength outcomes of all the four pinch/grip tasks, as well as on the significantly lowered AMT. This would suggest that after having learnt to stabilize the wrist, the voluntary FDI recruitment might be associated to a properly tuned activation of extrinsic muscles, and, in turn, this might enhance the excitability of the FDI representation in M1, so that a stronger pinch may be exerted. The hypothesized linkage between wrist stabilization and maximal pinch strength, which might have contributed to the observed strength improvements also in the control group, needs to be addressed in future studies, by applying the various tests of cortical and inter-hemispheric excitability used in this study while recording MEPs in some of the above extrinsic muscles.
The experimental conditions here chosen, which focused on supraspinal rather than spinal mechanisms, do not allow to exclude as possible contributors any adaptations in spinal excitability, which have been previously shown to mediate CE (Hortobagyi 2005; Fimland et al. 2009). Furthermore, remote cortical regions, such as supplementary cortical areas, parietal cortices, basal ganglia, cerebellum, and brainstem, cannot be ruled out (Carroll et al. 2006; Koch et al. 2009; Sehm et al. 2010). Finally, the present findings should be cautiously generalized to body districts other than the hand muscles.
Neural adaptation following a chronic simple motor task
Although simple motor tasks have been portrayed as capable of inducing a consistent CE effect along with direct and crossed neural adaptations (for reviews: Munn et al. 2004; Carroll et al. 2006), it has been proposed that motor skill acquisition, or motor learning, is a prerequisite factor in driving representational plasticity in M1 (Plautz et al. 2000) and, accordingly, the repetitive execution of a simple movement has been found to induce no functional reorganization of cortical maps in the M1 of monkeys (Plautz et al. 2000) nor considerable M1 chronic adaptations in humans, not even when maximally increasing the force required to execute the task (Carroll et al. 2002). In light of these reports, we argue that besides the temporal factor, the low-demanding nature of the isometric finger pinching task employed in this study might have played a crucial role in the minor extent of cortico-cortical and/or cortico-spinal adaptations and, indirectly, of the CE effect here observed. In this sense, dynamic training may be superior to isometric training (Morrissey et al. 1995) and, compared to the latter, isotonic contractions result in greater neural adaptations (Schmitz and Westwood 2001; Yahagi et al. 2003; Kidgell et al. 2015). These heterogeneous findings call for fresh research specifically aimed at comparing the effects of different regimens of muscle contractions (i.e., isometric versus isotonic versus isokinetic) at different intensities (i.e., maximal versus sub-maximal) and with different durations (i.e., acute versus chronic) on the magnitude of contra-lateral gains in strength and on their neurophysiological correlates.
Conclusions
In this study, a 4-week maximal-intensity unilateral isometric training increased strength in both the trained and untrained FDI muscles, depicting a CE effect, with high spatial and task specificity. The increases in strength, although significant, occurred at trivial effect sizes and were below the cutoffs for meaningful change. Notably, strength gains were not associated to evidence of direct or crossed cortical or cortico-spinal adaptations in response to chronic exercise.
Abbreviations
- ANOVA:
-
Analysis of variance
- CE:
-
Cross education
- cM1:
-
Contra-lateral primary motor cortex
- CMCT:
-
Central motor conduction time
- CV:
-
Coefficient of variation
- EMG:
-
Electromyography
- FDI:
-
First dorsal interosseus
- ICC:
-
Intra-class correlation coefficient
- ICF:
-
Intra-cortical facilitation
- IHI:
-
Inter-hemispheric inhibition
- iM1:
-
Ipsi-lateral primary motor cortex
- ISI:
-
Inter-stimulus interval
- LAI:
-
Long-latency afferent inhibition
- LICI:
-
Long-interval intra-cortical inhibition
- LIHI:
-
Long-interval inter-hemispheric inhibition
- MEP:
-
Motor-evoked potential
- mV:
-
Millivolt
- MVIC:
-
Maximum voluntary isometric contraction
- RC:
-
Recruitment curve
- RMT:
-
Resting motor threshold
- SAI:
-
Short-latency afferent inhibition
- SD:
-
Standard deviation
- SEM:
-
Standard error of measurement
- SICF:
-
Short-interval intra-cortical facilitation
- SICI:
-
Short-interval intra-cortical inhibition
- SIHI:
-
Short-interval inter-hemispheric inhibition
- SRDi:
-
Individual smallest real difference
- TMS:
-
Transcranial magnetic stimulation
- TMStest:
-
1 mV MEP
References
Abernethy PJ, Jürimäe J, Logan PA, Taylor AW, Thayer RE (1994) Acute and chronic response of skeletal muscle to resistance exercise. Sports Med 17:22–38
Abraham WC, Mason-Parker SE, Bear MF, Webb S, Tate WP (2001) Heterosynaptic metaplasticity in the hippocampus in vivo: a BCM-like modifiable threshold for LTP. Proc Natl Acad Sci 11:10924–10929
Buschler A, Manahan-Vaughan D (2012) Brief environmental enrichment elicits metaplasticity of hippocampal synaptic potentiation in vivo. Front Behav Neurosci 14:85
Carroll TJ, Riek S, Carson RG (2002) The sites of neural adaptation induced by resistance training in humans. J Physiol 15:641–652
Carroll TJ, Herbert RD, Munn J, Lee M, Gandevia SC (2006) Contralateral effects of unilateral strength training: evidence and possible mechanisms. J Appl Physiol 1985(101):1514–1522
Classen J, Liepert J, Hallett M, Cohen L (1999) Plasticity of movement representation in the human motor cortex. Electroencephalogr Clin Neurophysiol Suppl 51:162–173
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Lawrence Earlbaum Associates, Hillsdale
Cramer SC, Finklestein SP, Schaechter JD, Bush G, Rosen BR (1999) Activation of distinct motor cortex regions during ipsilateral and contralateral finger movements. J Neurophysiol 81:383–387
Duque J, Mazzocchio R, Stefan K, Hummel F, Olivier E, Cohen LG (2008) Memory formation in the motor cortex ipsilateral to a training hand. Cereb Cortex 18:1395–1406
Enoka RM (1997) Neural adaptations with chronic physical activity. J Biomech 30:447–455
Farthing JP (2009) Cross-education of strength depends on limb dominance: implications for theory and application. Exerc Sport Sci Rev 37:179–187
Farthing JP, Chilibeck PD, Binsted G (2005) Cross-education of arm muscular strength is unidirectional in right-handed individuals. Med Sci Sports Exerc 37:1594–1600
Fimland MS, Helgerud J, Solstad GM, Iversen VM, Leivseth G, Hoff J (2009) Neural adaptations underlying cross-education after unilateral strength training. Eur J Appl Physiol 107:723–730
Gentner R, Wankerl K, Reinsberger C, Zeller D, Classen J (2008) Depression of human corticospinal excitability induced by magnetic theta-burst stimulation: evidence of rapid polarity-reversing metaplasticity. Cereb Cortex 18:2046–2053
Gleeson NP, Mercer TH (1996) The utility of isokinetic dynamometry in the assessment of human muscle function. Sports Med 21:18–34
Hortobágyi T (2005) Cross education and the human central nervous system. IEEE Eng Med Biol Mag 24:22–28
Hortobágyi T, Richardson SP, Lomarev M, Shamim E, Meunier S, Russman H, Dang N, Hallett M (2011) Interhemispheric plasticity in humans. Med Sci Sports Exerc 43:1188–1199
Hoy KE, Fitzgerald PB, Bradshaw JL, Armatas CA, Georgiou-Karistianis N (2004) Investigating the cortical origins of motor overflow. Brain Res Rev 46:315–327
Kidgell DJ, Stokes MA, Pearce AJ (2011) Strength training of one limb increases corticomotor excitability projecting to the contralateral homologous limb. Mot Control 15:247–266
Kidgell DJ, Frazer AK, Daly RM, Rantalainen T, Ruotsalainen I, Ahtiainen J, Avela J, Howatson G (2015) Increased cross-education of muscle strength and reduced corticospinal inhibition following eccentric strength training. Neuroscience 6:566–575
Koch G, Ruge D, Cheeran B, Fernandez Del Olmo M, Pecchioli C, Marconi B, Versace V, Lo Gerfo E, Torriero S, Oliveri M, Caltagirone C, Rothwell JC (2009) TMS activation of interhemispheric pathways between the posterior parietal cortex and the contralateral motor cortex. J Physiol 1:4281–4292
Lee M, Gandevia SC, Carroll TJ (2009) Unilateral strength training increases voluntary activation of the opposite untrained limb. Clin Neurophysiol 120:802–808
Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M (2000) Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain 123:1161–1173
Leung M, Rantalainen T, Teo WP, Kidgell D (2015) Motor cortex excitability is not differentially modulated following skill and strength training. Neuroscience 1:99–108
Lexell JE, Downham DY (2005) How to assess the reliability of measurements in rehabilitation. Am J Phys Med Rehabil 84:719–723
Liang N, Funase K, Takahashi M, Matsukawa K, Kasai T (2014) Unilateral imagined movement increases interhemispheric inhibition from the contralateral to ipsilateral motor cortex. Exp Brain Res 232:1823–1832
Lucas TH, Fetz EE (2013) Myo-cortical crossed feedback reorganizes primate motor cortex output. J Neurosci 20:5261–5274
Manca A, Pisanu F, Ortu E, De Natale ER, Ginatempo F, Dragone D, Tolu E, Deriu F (2015) A comprehensive assessment of the cross-training effect in ankle dorsiflexors of healthy subjects: a randomized controlled study. Gait Posture 42:1–6
Morrissey MC, Harman EA, Johnson M (1995) Resistance training modes: specificity and effectiveness. Med Sci Sports Exerc 27:648–660
Muellbacher W, Facchini S, Boroojerdi B, Hallett M (2000) Changes in motor cortex excitability during ipsilateral hand muscle activation in humans. Clin Neurophysiol 111:344–349
Müller-Dahlhaus F, Ziemann U (2015) Metaplasticity in human cortex. Neuroscientist 21:185–202
Munn J, Herbert RD, Gandevia SC (2004) Contralateral effects of unilateral resistance training: a meta-analysis. J Appl Physiol 1985(96):1861–1866
Murakami T, Müller-Dahlhaus F, Lu MK, Ziemann U (2012) Homeostatic metaplasticity of corticospinal excitatory and intracortical inhibitory neural circuits in human motor cortex. J Physiol 15:5765–5781
Narici MV, Roi GS, Landoni L, Minetti AE, Cerretelli P (1989) Changes in force, cross-sectional area and neural activation during strength training and detraining of the human quadriceps. Eur J Appl Physiol Occup Physiol 59:310–319
Perez MA, Cohen LG (2008) Mechanisms underlying functional changes in the primary motor cortex ipsilateral to an active hand. J Neurosci 28:5631–5640
Perez MA, Lungholt BK, Nyborg K, Nielsen JB (2004) Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Exp Brain Res 159:197–205
Plautz EJ, Milliken GW, Nudo RJ (2000) Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use versus learning. Neurobiol Learn Mem 74:27–55
Pryce JC (1980) The wrist position between neutral and ulnar deviation that facilitates the maximum power grip strength. J Biomech 13:505–511
Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, Di Lazzaro V, Ferreri F, Fitzgerald PB, George MS, Hallett M, Lefaucheur JP, Langguth B, Matsumoto H, Miniussi C, Nitsche MA et al (2015) Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol 126:1071–1107
Schmitz RJ, Westwood KC (2001) Knee extensor electromyographic activity-to-work ratio is greater with isotonic than isokinetic contractions. J Athl Train 36:384–387
Schreuders TAR, Brandsma JW, Stam HJ (2007) The intrinsic muscles of the hand. Function, assessment and principles for therapeutic intervention. Phys Med Rehab Kuror 17:20–27
Sehm B, Perez MA, Xu B, Hidler J, Cohen LG (2010) Functional neuroanatomy of mirroring during a unimanual force generation task. Cereb Cortex 20:34–45
Shrout PE, Fleiss JL (1979) Intraclass correlations: uses in assessing rater reliability. Psychol Bull 86:420–428
Sohn YH, Jung HY, Kaelin-Lang A, Hallett M (2003) Excitability of the ipsilateral motor cortex during phasic voluntary hand movement. Exp Brain Res 148:176–185
Vercauteren K, Pleysier T, Van Belle L, Swinnen SP, Wenderoth N (2008) Unimanual muscle activation increases interhemispheric inhibition from the active to the resting hemisphere. Neurosci Lett 21:209–213
Warbrooke SA, Byblow WD (2004) Modulation of interhemispheric inhibition during passive movement of the upper limb reflects changes in motor cortical excitability. Exp Brain Res 156:11–19
Weir JP (2005) Quantifying test–retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res 19:231–240
Yahagi S, Ni Z, Takahashi M, Takeda Y, Tsuji T, Kasai T (2003) Excitability changes of motor evoked potentials dependent on muscle properties and contraction modes. Mot Control 7:328–345
Yue G, Cole KJ (1992) Strength increases from the motor program: comparison of training with maximal voluntary and imagined muscle contractions. J Neurophysiol 67:1114–1123
Acknowledgments
This study was partly supported by Fondazione Italiana Sclerosi Multipla (FISM Grant 2013/R/11) and by Fondazione Banco di Sardegna (FBS Grant 2014/0190).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no actual or potential conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Communicated by Guido Ferretti.
A. Manca and F. Ginatempo have equally contributed to this work.
Rights and permissions
About this article
Cite this article
Manca, A., Ginatempo, F., Cabboi, M.P. et al. No evidence of neural adaptations following chronic unilateral isometric training of the intrinsic muscles of the hand: a randomized controlled study. Eur J Appl Physiol 116, 1993–2005 (2016). https://doi.org/10.1007/s00421-016-3451-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-016-3451-6