Abstract
We have previously demonstrated that selective modulation of vestibular inputs, via sinusoidal galvanic vestibular stimulation (GVS) delivered at 0.5–0.8 Hz, can cause partial entrainment of muscle sympathetic nerve activity (MSNA). Given that we had seen interaction between the dynamic vestibular input and the normal cardiac-locked MSNA rhythm, we tested the hypothesis that frequencies of GVS remote from the cardiac frequency would cause a greater modulation of MSNA than those around the cardiac frequency. Bipolar binaural sinusoidal GVS (±2 mA, 200 cycles) was applied to the mastoid processes in 11 seated subjects at frequencies of 0.2, 0.5, 0.8, 1.1, 1.4, 1.7 and 2.0 Hz. In all subjects, the stimulation evoked robust vestibular illusions of “rocking in a boat” or “swinging from side to side.” Cross-correlation analysis revealed a cyclic modulation of MSNA at all frequencies, with the modulation index being similar between 1.1 Hz (78.5 ± 3.7%) and 2.0 Hz (77.0 ± 4.3%). However, vestibular modulation of MSNA was significantly stronger at 0.2 Hz (93.1 ± 1.7%) and significantly weaker at 0.8 Hz (67.2 ± 1.8%). The former suggests that low-frequency changes in vestibular input, such as those associated with postural changes, preferentially modulate MSNA; the latter suggests that vestibular inputs compete with the stronger baroreceptor inputs operating at the cardiac rhythm (~0.8 Hz), with vestibular modulation of MSNA being greater when this competition with the baroreceptors is reduced.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Changes in body posture induce hydrostatic challenges to the cardiovascular system, which must respond to these challenges in order to provide an adequate supply of blood to the brain. Rapid adjustments to the vascular system are mediated primarily by the autonomic nervous system and normally meet this challenge; failure of the cardiovascular system to respond to the effects of gravity on the vascular system can produce dizziness, light-headedness and fainting and can be debilitating in clinical postural hypotension (Mathias 1995) and the postural orthostatic tachycardia syndrome (POTS; Low et al. 1995). One of the primary determinants of blood pressure is the degree of constriction within muscle vascular beds, brought about by the activity of sympathetic muscle vasoconstrictor neurones. Direct recordings of muscle sympathetic nerve activity (MSNA) in awake human subjects have shown that MSNA occurs as bursts of impulses that, through the arterial baroreflex, are strongly coupled to the cardiac cycle. While baroreceptors provide the primary source of modulation of MSNA, other inputs also play a role. One of these is the vestibular system, for which the anatomical substrates (Yates et al. 1991, 1993; Yates and Miller 1994; Kerman et al. 2000) and physiological operation have been demonstrated in the cat: nose-up tilt increases blood pressure (Woodring et al. 1997) and section of vestibular afferents reduces the cardiovascular responses to changes in posture (Doba and Reis 1974; Jian et al. 1999).
Evidence for the operation of vestibulosympathetic reflexes has been found in humans: caloric stimulation of the ear canal causes a brief increase in MSNA to the legs (Cui et al. 1997), head-down neck flexion (but not extension) in the prone position causes a sustained increase in MSNA (Shortt and Ray 1997; Ray and Hume 1998; Hume and Ray 1999; Ray 2000) and off vertical-axis rotation in the seated position produces an increase in MSNA in phase with head-up tilt and a decrease during the phase corresponding to head-down tilt (Kaufmann et al. 2002), while linear sinusoidal acceleration in the horizontal plane has been shown to produce a decrease in MSNA (Cui et al. 1999, 2001). However, each of these stimuli also activates extra-vestibular receptors, bringing into question the specificity of the vestibular stimuli. To avoid this, we recently used a means of selective activation of the vestibular system to explore vestibulosympathetic reflexes (Bolton et al. 2004). Galvanic vestibular stimulation (GVS), in which a (usually) direct current is applied to one or both mastoid processes, has been used extensively to examine the vestibular contributions to posture and gait. It selectively activates the vestibular system by changing the firing of vestibular nerve afferents (Minor and Goldberg 1991) without acting on other, non-vestibular “graviceptors,” and has been used extensively to study postural and locomotor responses to vestibular inputs in human subjects (for review see Fitzpatrick and Day 2004 and Cathers et al. 2005). In our first study, we applied brief (1 s) 2-mA pulses to the mastoid processes of awake subjects at different times in the cardiac cycle: while brief static pulses (1 s) of GVS, time-locked to the R-wave of the ECG with different delays, did not produce any modulation of MSNA (Bolton et al. 2004), brief trains of ECG-locked GVS (30 ms, 333 Hz) did cause a significant increase in MSNA (Voustianiouk et al. 2006). The latter result suggested that a dynamic component of the stimulus is required to modulate MSNA. We then showed that bipolar sinusoidal GVS, which provides a continuous dynamic vestibular input, could cause partial entrainment of MSNA to the vestibular stimulus and even evoke de novo synthesis of muscle vasoconstrictor bursts (Bent et al. 2006). In that study, we used stimulation frequencies of 0.5 or 0.8 Hz, physiologically relevant to postural control (Petersen et al. 1994) yet close to the cardiac frequencies at which muscle vasoconstrictor neurones are entrained by baroreceptor inputs (~0.85–1.1 Hz). The primary frequency of upright postural sway in the antero-posterior direction is 0.30–0.45 Hz, though there are also higher frequency components around 0.60–0.75 and 1.05–1.20 Hz (Soames and Atha 1982). The purpose of the present study was to test the hypothesis that there is an optimal range of frequencies over which dynamic vestibular inputs can compete with the dominant cardiac (baroreflex) rhythm. Accordingly, we examined a larger range of frequencies (0.2–2.0 Hz) than those used previously, far removed from (but also including those around) the cardiac frequency, to assess potential interactions between these artificially induced vestibular inputs and physiologically generated baroreceptor inputs. We also used longer periods of stimulation (200 cycles) than in our previous study (60–100 cycles). We predicted that frequencies further away from the cardiac frequency would be less effective, or ineffective, at modulating muscle sympathetic nerve activity than those close to the cardiac rhythm.
Methods
Experiments were performed on 8 male and 6 female subjects (age 18–29), each of whom provided informed consent. The study was conducted with the approval of the Human Research Ethics Committee, University of Western Sydney, and satisfied the Declaration of Helsinki. Subjects were seated in a semi-reclined posture in a comfortable chair with the legs supported in the extended position. Muscle sympathetic nerve activity was recorded from fascicles of the common peroneal nerve supplying the ankle and toe extensor and foot everter muscles via tungsten microelectrodes (FHC, Bowdoinham, ME, USA) inserted percutaneously at the level of the fibular head. Oligounitary neural activity was amplified (gain 20 000, bandpass 0.3–5.0 kHz) using an isolated amplifier (NeuroAmp EX, ADInstruments, Sydney, Australia) and stored on computer (10-kHz sampling) using a computer-based data acquisition and analysis system (PowerLab 16SP hardware and Chart 5 software; ADInstruments, Sydney, Australia). ECG (0.3–1.0 kHz) was recorded with Ag–AgCl surface electrodes on the chest and sampled at 2 kHz. Respiration (DC-100 Hz) was recorded using a strain-gauge transducer (Pneumotrace, UFI, Morro Bay CA, USA) wrapped around the chest.
Sinusoidally modulated bipolar, binaural galvanic vestibular stimuli (GVS, −2 to 2 mA, 200 cycles) were applied at 0.2, 0.5, 0.8, 1.1, 1.4, 1.7 and 2.0 Hz in a quasi-random order to the mastoid processes via Ag–AgCl surface electrodes (anode on right mastoid). Given that the same number of cycles (200) was applied at each frequency, the stimulation time varied by frequency: the 0.2-Hz train lasted 16 min 30 s, the 0.5-Hz train 6 min 41 s, the 0.8-Hz train 4 min 5 s, the 1.1-Hz train 3 min 2 s, the 1.4-Hz train 2 min 22 s, the 1.7-Hz train 1 min 58 s and the 2.0-Hz train lasted 1 min 40 s. While 11 subjects received all frequencies, technical problems meant that three subjects only received the 0.8-Hz train. Subjects were instructed to relax with their eyes closed during the control and stimulation periods and to report their perceptions during GVS at the conclusion of each recording segment. Subjects were not informed of the start of the stimulation, which was delivered at unexpected times.
Muscle sympathetic nerve activity (MSNA) was displayed as an RMS-processed (root mean square, moving average time-constant 200 ms) signal but, as described previously (Bent et al. 2006), the analysis was conducted on the raw, negative-going, sympathetic spikes to avoid any contamination from spikes generated by positive-going myelinated axons (such as spontaneously active muscle spindles). Negative-going spikes in the neurogram (with a half-width of 0.2–0.5 ms), positive-going spikes in the ECG and the positive peaks of the sinusoidal stimulus were detected using window discriminator software (Spike Histogram for Macintosh v2.2, ADInstruments, Sydney, Australia); this same software was used to construct cross-correlation and autocorrelation histograms (correlograms). Discriminator levels were adjusted so that negative-going spikes exhibited a robust cardiac modulation, as revealed by cross-correlation between the neural activity and the ECG; the same discriminator settings were used for construction of cross-correlograms between MSNA and the positive peaks of the sinusoidal GVS. Quantification of the modulation of MSNA was performed from this cross-correlogram by measuring the difference in the number of spikes within the 50-ms bin at the peak of the modulation and the 50-ms bin at the trough, expressed as a percentage: modulation index = [(peak − trough)/peak] × 100. MSNA was also quantified according to standard time-domain analysis of the RMS-processed signal as burst frequency (bursts/min) and burst incidence (bursts/100 heart beats), measured immediately prior to the onset of sGVS and during the final 1 min of the stimulus). Total burst activity (mV) was computed as the cumulative sum of the burst amplitudes measured over 1 min. Analysis of variance, coupled with the Newman–Keuls multiple comparison test across different frequencies, was computed using statistical software (Prism 5.0 for Macintosh, GraphPad Software, USA). At each frequency, the modulation index was compared to that obtained at 1.1 Hz, which was used for convenience as it corresponds to the central frequency in the range from 0.2 to 2.0 Hz.
Results
As described previously (Bent et al. 2006), sinusoidal galvanic vestibular stimulation (sGVS) generated robust illusions that were consistently reported as having the character of either “rocking in a boat” or “swinging from side to side in a hammock.” The rate of perceived movement increased with increasing stimulation frequency, but the movement illusions were more often described as “pushing against the head” at 2.0 Hz. Although most subjects reported “tingling” at the electrodes, no subjects considered these to be painful and this sensation generally abated during the course of the stimulation. Sinusoidal GVS at the lowest frequencies (0.2 Hz) induced a degree of nausea in four subjects.
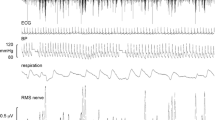
Experimental records from one subject, during application of sGVS at 1.4 Hz, are shown in Fig. 1. The negative-going sympathetic spikes have been discriminated and represented as standard pulses (spikes). In this subject the vestibular modulation was not overt, yet cross-correlation analysis of these spikes to the GVS revealed a cyclic modulation of MSNA that matched the frequency of the sinusoidal GVS. This is shown for three frequencies (0.2, 0.8 and 2.0 Hz) in the same subject in Fig. 2. It can be seen that the modulation of MSNA at 0.8 Hz (Fig. 2b) was weaker than that at 0.2 Hz (Fig. 2a) or 2.0 Hz (Fig. 2c). Data from another subject are shown in Fig. 3a during delivery of sGVS at 0.8 Hz. Here we have compressed the abscissa to illustrate, on the same time scale, the modulation of MSNA as a function of vestibular inputs (Fig. 3a), respiratory inputs (Fig. 3b) and baroreceptor inputs (Fig. 3c). It must be emphasised that the modulation of MSNA by vestibular or respiratory inputs was considerably weaker than the cardiac-related modulation.
Raw MSNA data during sGVS. Experimental records from one subject, a 19-year-old female. Spontaneous muscle sympathetic nerve activity was recorded from the peronei motor fascicle of the common peroneal nerve. Negative-going spikes, representing muscle sympathetic nerve activity (MSNA), were discriminated and are shown in the second trace from the top (spikes). Sinusoidal GVS was applied at 1.4 Hz
Vestibular modulation of MSNA at different frequencies of sGVS. Cross-correlation histograms between MSNA and GVS for the same subject shown in Fig. 1 during sinusoidal galvanic vestibular stimulation at 0.2 Hz (a), 0.8 Hz (b) and 2.0 Hz (c). In each panel the autocorrelation histogram (GVS–GVS) is shown below: the positive peaks correspond to the positive peaks of the sinusoid. The dashed vertical line indicates the peak of the GVS signal at time zero: earlier peaks in the stimulus train are shown to the left, later peaks to the right. n = numbers of counts comprising the histograms
Vestibular, respiratory and cardiac modulation of MSNA. Cross-correlation and autocorrelation histograms between MSNA and GVS at 0.8 Hz (a), MSNA and respiration (b) and MSNA and ECG (c). The dashed vertical line indicates the peak of the synchronizing peak (GVS, respiration or ECG) of the cross-correlogram or autocorrelogram at time zero: earlier peaks are shown to the left, later peaks to the right. Data from one subject. n = numbers of counts comprising the histograms
Cyclic modulation of MSNA was apparent at all frequencies of sGVS in all subjects. The mean modulation indices for the 11 subjects who received all frequencies are shown graphically in Fig. 4 and numerically in Table 1. Relative to the modulation index at 1.1 Hz there was no significant difference in the modulation at 1.4, 1.7 and 2.0 Hz. And while there was a clear tendency for the modulation to be greater at 0.5 Hz (Fig. 4), this failed to reach statistical significance. However, the vestibular modulation was significantly stronger at 0.2 Hz (93.1 ± 1.7%; P < 0.01). Moreover, as shown in Fig. 4, the modulation was significantly weaker at 0.8 Hz (67.2 ± 1.8%; P < 0.05). After incorporating data from the three subjects who had only received stimulation at 0.8 Hz, the mean modulation at this frequency was 66.5 ± 1.5%. There was no significant difference in the magnitude of the GVS-induced modulation of MSNA between the 8 males and 6 females (66.5 ± 2.5 vs 66.4 ± 1.8%) at this frequency.
Modulation index of MSNA as a function of GVS frequency. Magnitude of the modulation index, [(peak − trough)/peak] × 100, of MSNA during sGVS at different frequencies. Relative to the modulation index at 1.1 Hz, the modulation was significantly stronger at 0.2 Hz (P < 0.01) and significantly weaker at 0.8 Hz (P < 0.05), as represented by the black bars. Mean ± SE data from 11 subjects
When burst incidence, burst frequency and total burst activity were calculated from the RMS-processed MSNA signal there were no significant differences across stimulation frequency, and no differences from control levels (Table 2).
Discussion
The present investigation extends our recent work, in which we applied sinusoidal galvanic vestibular stimulation to examine vestibular modulation of muscle sympathetic nerve (Bent et al. 2006), by assessing a wider range of stimulation frequencies. We tested the hypothesis that there is an optimal range of frequencies over which dynamic vestibular inputs, induced by sGVS, operate in modulating muscle vasoconstrictor drive. We predicted that frequencies further away from the cardiac frequency would be less effective, or even ineffective, at modulating MSNA than those close to the cardiac rhythm because of the competing—and dominant—cardiac rhythm (mediated by the baroreflex). We have accepted the hypothesis that there does appear to be an optimal range of frequencies but our prediction was wrong: frequencies lower than the cardiac rhythm, closer to those associated with slow postural adjustments, were more effective. Importantly, we have shown that delivery of sGVS close to the cardiac rhythm is actually poorer at modulating MSNA.
Methodological considerations
As discussed previously (Bent et al. 2006), GVS selectively changes the inputs from the vestibular apparatus (although cutaneous afferents immediately under the electrodes are also stimulated), without changing other inputs that may affect the cardiovascular system. Sinusoidal GVS was delivered in a bipolar binaural manner, with the anode always applied over the right mastoid process and the cathode over the left; the sinusoidal fluctuations in current occurred bilaterally but in opposite polarity. It is known that depolarisation of vestibular afferents is generated at the cathode, with hyperpolarization occurring at the anode (for review see Fitzpatrick and Day 2004). Sinusoidal stimulation at frequencies ranging from 0.2 to 4.0 Hz have previously been shown to evoke a frequency-dependent postural sway in standing subjects (Petersen et al. 1994). In the present study, as in our previous work (Bent et al. 2006), the subjects were seated with the legs relaxed and supported horizontally. Despite this, the stimulus (−2 to 2 mA, 0.2–2.0 Hz) evoked robust and continuous illusions of postural sway from side to side. The illusions were clear in every subject, and all subjects were surprised at how strong the illusions were: no subject described the perceived motion as “weak,” though the rate of oscillation was directly related to the frequency of stimulation.
As in our previous study (Bent et al. 2006), we analysed the discriminated sympathetic spikes in the neurogram rather than the RMS-processed nerve signal. We believe this is a far more sensitive means of analysing multi-unit sympathetic nerve activity than quantifying nerve traffic as the number of bursts per minute (burst frequency) or per 100 heart beats (burst incidence). We know that human C-fibres generate negative-going action potentials and, given the tight cardiac rhythmicity exhibited in the discriminated spikes, we believe the activity we recorded represents the discharge of post-ganglionic muscle vasoconstrictor axons (for review see Macefield et al. 2002). We also know that GVS does not activate motor axons in relaxed leg muscles (Britton et al. 1993; Fitzpatrick et al. 1994) and we had confirmed this in our previous study by recording EMG over the pretibial flexors (Bent et al. 2006).
Vestibular modulation of MSNA
With the exception of caloric vestibular stimulation, which causes inconsistent effects on muscle sympathetic activity, the other experimental approaches previously used to modulate vestibular inputs are not specific. Head-down neck flexion (HDNF) changes the afferent balance from muscle (and other) receptors in the neck (see Bolton and Ray 2000), and linear sinusoidal acceleration (Cui et al. 2001) or off-vertical axis rotation (Kaufmann et al. 2002) also cause fluid shifts in the body. In addition, linear or off-vertical axis rotational acceleration exert different effects: MSNA decreases during anteroposterior or lateral displacement of the seated body (Cui et al. 2001) but increases during the phase of the off-vertical axis rotation cycle corresponding to head-up tilt and decreases during the phase corresponding to head-down tilt (Kaufmann et al. 2002). Conversely, GVS does not affect any other system that could potentially contribute to cardiovascular control. However, its limitation is that it activates the entire vestibule (Carter and Ray 2008), which may account for our observed lack of increase in total MSNA. Animal studies have documented direct changes in the firing of peripheral vestibular afferents (Minor and Goldberg 1991), and recent evidence suggests that afferents from both the otoliths and semicircular canals can contribute to the postural responses to GVS (Wardman and Fitzpatrick 2002; Cathers et al. 2005). Nevertheless, previous studies have shown that the semicircular canals do not contribute to the modulation of sympathetic outflow during vestibular activation, suggesting that modulation of sympathetic activity from the vestibular apparatus is otolithic in origin (Costa et al. 1995; Ray et al. 1998).
We previously showed that sinusoidal GVS does not entrain respiration, nor does it entrain the cardiac cycle (Bent et al. 2006). Moreover, we had also shown that GVS can result in the production of two peaks within a cardiac cycle, indicating that vestibular inputs can exert a potent excitation of muscle vasoconstrictor drive. Presumably, this acts through the rostral ventrolateral medulla (RVLM), the primary output nucleus for muscle vasoconstrictor neurones (Dampney et al. 2003a, b), as this nucleus has been shown to receive vestibular inputs, primarily from the otoliths (Yates et al. 1991, 1993). Accordingly, we believe that the frequency-dependent modulation of MSNA to sGVS reflects the operation of an independent input (vestibular) onto RVLM. However, given the tight coupling of muscle vasoconstrictor neurones to the cardiac cycle, the vestibular inputs must compete with baroreflex inputs, which project to the RVLM via the nucleus tractus solitarius (NTS) and caudal ventrolateral medulla (CVLM; Dampney et al. 2003a, b). Indeed, this may explain why the modulation at 0.8 Hz was significantly smaller than at other frequencies of sGVS: 0.8 Hz is close to the cardiac frequency, the frequency to which the baroreceptor afferents are entrained. Frequencies of sGVS remote from this cardiac rhythm could thereby exert stronger influences on muscle vasoconstrictor outflow because of the reduced competition between vestibular and baroreceptor afferents.
Conclusions
Using sinusoidal galvanic vestibular stimulation we have shown that vestibular inputs can modulate muscle sympathetic nerve activity in awake human subjects, and that the magnitude of this modulation depends on the frequency of stimulation: modulation is lowest when the stimulation is close to the cardiac frequency and highest when the vestibular inputs are slower than the cardiac frequency, i.e. the frequencies associated with slow postural changes. It may be that the vestibular system contributes to the normal cardiovascular behaviour of standing: MSNA to leg muscles increases linearly from the horizontal to vertical position (Burke et al. 1977; Iwase et al. 1987), but it is unclear whether this entrainment of muscle vasoconstrictor drive can be directly attributed to vestibular or baroreceptor inputs, or both (see Fu et al. 2001). Sinusoidal GVS has shown that the vestibular system can exert a potent modulation of muscle sympathetic outflow, such that disturbances in this vestibular control may underlie some of the pathophysiology associated with postural hypotension and postural orthostatic tachycardia syndrome.
References
Bent LR, Bolton PS, Macefield VG (2006) Modulation of muscle sympathetic bursts by sinusoidal galvanic vestibular stimulation in human subjects. Exp Brain Res 174:701–711
Bolton PS, Ray CA (2000) Neck afferent involvement in cardiovascular control during movement. Brain Res Bull 53:45–49
Bolton PS, Wardman DL, Macefield VG (2004) Absence of short-term vestibular modulation of muscle sympathetic outflow, assessed by brief galvanic vestibular stimulation in human subjects. Exp Brain Res 154:39–43
Britton TC, Day BL, Brown P, Rothwell JC, Thompson PD, Marsden CD (1993) Postural electromyographic responses in the arm and leg following galvanic vestibular stimulation in man. Exp Brain Res 94:143–151
Burke D, Sundlöf G, Wallin BG (1977) Postural effects on muscle nerve sympathetic activity in man. J Physiol 272:399–414
Carter JR, Ray CA (2008) Sympathetic responses to vestibular activation in humans. Am J Physiol Regul Integr Comp Physiol 294:R681–R688
Cathers I, Day BL, Fitzpatrick RC (2005) Otolith and canal reflexes in human standing. J Physiol 563:229–234
Costa F, Lavin P, Robertson D, Biaggioni I (1995) Effect of neurovestibular stimulation on autonomic regulation. Clin Auton Res 5:289–293
Cui J, Mukai C, Iwase S, Sawasaki N, Kitazawa H, Mano T, Sugiyama Y, Wada Y (1997) Response to vestibular stimulation of sympathetic outflow to muscle in humans. Response to vestibular stimulation of sympathetic outflow to muscle in humans. J Auton Nerv Syst 66:154–162
Cui J, Iwase S, Mano T, Katayama N, Mori S (1999) Muscle sympathetic nerve response to vestibular stimulation by sinusoidal linear acceleration in humans. Neurosci Lett 267:181–184
Cui J, Iwase S, Mano T, Katayama N, Mori S (2001) Muscle sympathetic outflow during horizontal linear acceleration in humans. Am J Physiol 281:R625–R634
Dampney RA, Horiuchi J, Tagawa T, Fontes MA, Potts PD, Polson JW (2003a) Medullary and supramedullary mechanisms regulating sympathetic vasomotor tone. Acta Physiol Scand 177:209–218
Dampney RA, Polson JW, Potts PD, Hirooka Y, Horiuchi J (2003b) Functional organization of brain pathways subserving the baroreceptor reflex: studies in conscious animals using immediate early gene expression. Cell Mol Neurobiol 23:597–616
Doba N, Reis DJ (1974) Role of the cerebellum and the vestibular apparatus in regulation of orthostatic reflexes in cats. Circ Res 40:9–18
Fitzpatrick RC, Day BL (2004) Probing the human vestibular system using galvanic stimulation. J Appl Physiol 96:2301–2316
Fitzpatrick R, Burke D, Gandevia SC (1994) Task-dependent reflex responses and movement illusions evoked by galvanic vestibular stimulation in standing humans. J Physiol 478:363–372
Fu Q, Iwase S, Niimi Y, Kamiya A, Kawanokuchi J, Cui J, Mano T, Suzumura A (2001) Effects of lower body positive pressure on muscle sympathetic nerve activity response to head-up tilt. Am J Physiol 281:R778–R785
Hume KM, Ray CA (1999) Sympathetic responses to head-down rotations in humans. J Appl Physiol 86:1971–1976
Iwase S, Mano T, Saito T (1987) Effects of graded head-up tilting on muscle sympathetic activities in man. Physiologist 30(Suppl 1):S62–S63
Jian BJ, Cotter LA, Emanuel BA, Cass SP, Yates BJ (1999) Effects of bilateral vestibular lesions on orthostatic intolerance in awake cats. J Appl Physiol 86:1552–1560
Kaufmann H, Biaggioni I, Voustianiouk A, Diedrich A, Costa F, Clarke R, Gizzi M, Raphan T, Cohen B (2002) Vestibular control of sympathetic activity. An otolith-sympathetic reflex in humans. Exp Brain Res 143:463–469
Kerman IA, Yates BJ, McAllen RM (2000) Anatomic patterning in the expression of vestibulosympathetic reflexes. Am J Physiol 279:R109–R117
Low PA, Opfer-Gehrking TL, Textor SC, Benarroch EE, Shen WK, Schondorf R, Suarez GA, Rummans TA (1995) Postural tachycardia syndrome (POTS). Neurology 45:S19–S25
Macefield VG, Elam M, Wallin BG (2002) Firing properties of single postganglionic sympathetic neurones recorded in awake human subjects. Auton Neurosci 95:146–159
Mathias CJ (1995) Orthostatic hypotension: causes, mechanisms, and influencing factors. Neurology 45:S6–S11
Minor LB, Goldberg JM (1991) Vestibular-nerve inputs to the vestibulo-ocular reflex: a functional ablation study in the squirrel monkey. J Neurosci 11:1636–1648
Petersen H, Magnusson M, Fransson PA, Johansson R (1994) Vestibular disturbance at frequencies above 1 Hz affects human postural control. Acta Otolaryngol 114:225–230
Ray CA (2000) Interaction of the vestibular system and baroreflexes on sympathetic nerve activity in humans. Am J Physiol 279:H2399–H2404
Ray CA, Hume KM (1998) Neck afferents and muscle sympathetic activity in humans: implications for the vestibulosympathetic reflex. J Appl Physiol 84:450–453
Ray CA, Hume KM, Steele SL (1998) Sympathetic nerve activity during natural stimulation of horizontal semicircular canals in humans. Am J Physiol Regul Integr Comp Physiol 275:R1274–R1278
Shortt TL, Ray CA (1997) Sympathetic and vascular responses to head-down neck flexion in humans. Am J Physiol 272:H1780–H1784
Soames RW, Atha J (1982) The spectral characteristics of postural sway behaviour. Eur J Appl Physiol Occup Physiol 49:169–177
Voustianiouk A, Kaufmann H, Diedrich A, Raphan T, Biaggioni I, MacDougall H, Ogorodnikov D, Cohen B (2006) Electrical activation of the human vestibulo-sympathetic reflex. Exp Brain Res 171:251–261
Wardman DL, Fitzpatrick RC (2002) What does galvanic vestibular stimulation stimulate? Adv Exp Biol Med 508:119–128
Woodring SF, Rossiter CD, Yates BJ (1997) Pressor response elicited by nose-up vestibular stimulation in cats. Exp Brain Res 113:165–168
Yates BJ, Miller AD (1994) Properties of sympathetic reflexes elicited by natural vestibular stimulation: implications for cardiovascular control. J Neurophysiol 71:2087–2092
Yates BJ, Yamagata Y, Bolton PS (1991) The ventrolateral medulla of the cat mediates vestibulosympathetic reflexes. Brain Res 552:265–272
Yates BJ, Goto T, Bolton PS (1993) Responses of neurons in the rostral ventrolateral medulla of the cat to natural vestibular stimulation. Brain Res 601:255–264
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grewal, T., James, C. & Macefield, V.G. Frequency-dependent modulation of muscle sympathetic nerve activity by sinusoidal galvanic vestibular stimulation in human subjects. Exp Brain Res 197, 379–386 (2009). https://doi.org/10.1007/s00221-009-1926-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-009-1926-y