Abstract
There is evidence in experimental animals for a potent vestibulosympathetic reflex, but its existence in humans is controversial. Static head-down neck flexion and off-vertical axis rotation have been shown to increase muscle sympathetic nerve activity (MSNA), but not skin sympathetic nerve activity (SSNA), whereas horizontal linear acceleration decreases MSNA in humans. However, both forms of stimuli also activate other receptors. To examine the effects of a pure vestibular stimulus on MSNA and SSNA, and its potential interaction with the baroreceptors, we used galvanic vestibular stimulation (GVS) in 12 healthy seated subjects. MSNA was recorded in ten subjects via a percutaneous microelectrode in the peroneal nerve; ECG, blood pressure, respiration, skin blood flow and sweating were also recorded. GVS (2 mA, 1 s pulse) was delivered via surface electrodes over the mastoid processes at unexpected times, triggered from the R-wave with a delay of 0, 200, 400 or 600 ms. In addition to causing robust postural illusions, GVS caused cutaneous vasoconstriction and sweat release in all subjects (due to a short-latency increase in SSNA, three subjects), but no significant change in MSNA. The failure of GVS to elicit a change in muscle sympathetic nerve activity, as documented by averaging, suggests that the vestibular system is not engaged in short-term modulation of muscle sympathetic activity. Conversely, phasic vestibular inputs do excite cutaneous sympathetic neurones, consistent with the observation that motion sickness is accompanied by pallor and sweating.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is convincing evidence from studies in the cat that the vestibular system plays an important role in the control of blood pressure via direct actions of the vestibular apparatus on the sympathetic nervous system (for review see Yates 1996). For example, the control of blood pressure in response to postural challenges is compromised following bilateral section of the vestibular nerves (Doba and Reis 1974; Jian et al. 1999), and sympathetic neural activity can be modified by electrical or natural stimulation of vestibular afferents (Yates and Miller 1994; Kerman et al. 2000). Moreover, the rostroventrolateral medulla (RVLM), the major source of sympathetic drive to the preganglionic neurones, has been shown to receive vestibular inputs, primarily from the otoliths (Yates et al. 1991, 1993). However, it can be difficult to extrapolate results from a small quadruped—an animal which deals with relatively small postural challenges to blood pressure control—to human subjects, who have adapted to cope with the ongoing cardiovascular challenges of an upright (standing or seated) posture.
In humans the prevention of venous pooling in the legs and postural hypotension on standing is dependent on adequate vasoconstriction within the legs, and direct recordings of muscle sympathetic nerve activity (MSNA) to the leg have shown that muscle vasoconstrictor activity increases linearly from the horizontal to vertical position (Burke et al. 1977; Iwase et al. 1987). But whether this is mediated by vestibular inputs or by the detection of fluid shifts by the baroreceptors (or by other receptors) has yet to be clarified in humans, though there are arguments in favour of both. For instance, while head-up tilt increases MSNA, application of lower-body positive pressure (which offsets the fall in pressure) attenuates the response, suggesting that the height of the hydrostatic pressure column, as detected by the baroreceptors, could explain the changes in muscle vasoconstrictor activity (Fu et al. 2001). Caloric stimulation of the external auditory meatus in human subjects—a potent vestibular stimulus—has been reported to increase (Cui et al. 1997) or to have no effect on muscle sympathetic outflow in supine subjects (Costa et al. 1995). Sinusoidal acceleration of seated subjects in the horizontal (anteroposterior or lateral plane) decreases MSNA to the legs (Cui et al. 2001), whereas off-vertical axis rotation causes an increase during the phase of the rotation corresponding to head-up tilt (Kaufmann et al. 2002). Static head-down neck flexion (HDNF), but not extension, in prone subjects causes a sustained increase in MSNA to the legs (Shortt and Ray 1997; Ray and Hume 1998; Hume and Ray 1999). Each of these studies attributes the sympathetic responses to activation of otoliths, yet each experimental paradigm uses a stimulus that also stimulates other receptors in the body (e.g. "graviceptors").
We have reassessed the potential contribution of the vestibular system to the sympathetic control of the cardiovascular system in humans, with particular reference to short-term influences, by the use of galvanic vestibular stimulation (GVS), which allows application of a precisely controlled change in the activity of vestibular nerve afferents from all vestibular organs without any of the potentially confounding changes in non-vestibular inputs associated with changes in head or whole-body position (Wardman and Fitzpatrick 2002). We modelled our protocol on that used to address the effect of transcranial magnetic stimulation of the cortex (Macefield et al. 1998), or sensory stimulation (Donadio et al. 2002), on MSNA: stimuli were delivered at fixed delays with respect to the R-wave of the ECG and averaged and compared with the averaged responses to dummy stimuli. A preliminary report of this work has been presented (Bolton et al. 2002).
Materials and methods
Experiments were performed on six male and six female human volunteers (age 20–55 years), each of whom provided informed written consent. The study was conducted with the approval of the Committee for Experimental Procedures Involving Human Subjects, University of New South Wales. Surface Ag-AgCl electrodes were placed over the left and right mastoid process and, in some cases, in the midline over the inion. Stimuli (2 mA DC pulse, duration 1 s) were applied across the mastoid processes while the subject stood unassisted with the feet together and eyes closed. In all subjects a clear postural sway towards the anode was evoked (Day et al. 1997), demonstrating the effectiveness of the galvanic vestibular stimulation (GVS). In addition to the postural illusions, all subjects reported perceiving the GVS as an innocuous pinprick on the surface of the skin under the electrodes. Subjects were seated in a semi-reclining (n=6) or reclining (n=6) posture in a comfortable chair with the legs supported in the extended position. Muscle or cutaneous sympathetic activity was recorded from the common peroneal nerve via a percutaneous tungsten microelectrode, along with ECG, non-invasive blood pressure (CBM-7000, Colin Corp, Japan), respiration, cutaneous blood flow and sweating (skin potential) from the foot, as described previously (Macefield et al. 1998). Neural activity (gain 10,000–20,000, bandwidth 0.3–5.0 kHz) was recorded on computer at 12.8 kHz with ECG (3 Hz–1 kHz), blood pressure, respiration (Pneumotrace), skin blood flow and sweating. ECG and skin potentials were digitized at 3.2 and 0.8 kHz, respectively, and arterial pressure, cutaneous blood flow and respiration at 0.4 kHz. Stimuli were triggered from the R-wave of the ECG (0, 200, 400 or 600 ms delay) and delivered at unexpected times during waves of sympathetic activity. Ipsilateral or contralateral anodal stimuli were given while the subjects maintained their gaze in the forward position. Subjects were not constrained to fixate on a target; some subjects chose to close their eyes during the stimulation periods. The root mean square (RMS) was computed (time constant 100 ms) from the filtered neurogram. All signals were averaged (16–32 sweeps) in 8-s epochs centered around the R-wave associated with the real or dummy stimulus. ANOVA was used to compare the normalized burst amplitudes in the two conditions.
Results
In all subjects galvanic vestibular stimulation (GVS) was shown to be effective in causing appropriate postural responses (sway towards the anode) when standing, and occulomotor responses when seated (Britton et al. 1993; Fitzpatrick et al. 1994; Day et al. 1997). In the latter condition subjects reported an illusion of displacement of the head away from the anode, regardless of whether the eyes were open or closed.
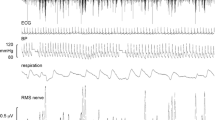
To examine whether short-lasting verstibular inputs have any effects on muscle sympathetic nerve activity (MSNA), GVS was delivered during a wave of muscle sympathetic bursts, usually 200 ms following an ECG trigger appearing in the first half of the wave. Control runs, in which no stimuli were given, were also triggered from the ECG in a similar manner. In four subjects GVS was delivered during an inspiratory-capacity apnoea, a manoeuvre known to cause a sustained increase in MSNA (Macefield and Wallin 1995b), thereby allowing any potential inhibitory response to GVS to be more readily observed. Figure 1 shows experimental data from one supine subject, with a high level of spontaneous MSNA at rest. Single bipolar stimuli (2 mA, 1 s) were given at unexpected times over the mastoid processes (anode on right, cathode on left), causing cutaneous vasoconstriction and sweat release from the hand and foot. Averaging (16–32 sweeps) revealed inhibition of one sympathetic burst in this and one other subject, but given that this also occurred when electrical stimuli were delivered to the earlobe (at comparable intensities), it is likely that this inhibition was related to the arousal component of the stimulus (see Discussion).
Recording of spontaneous muscle sympathetic activity from a fascicle of the common peroneal nerve supplying the foot everter muscles (peronei). A single galvanic stimulus (2 mA, 1 s) was delivered across the mastoid processes during a wave of sympathetic bursts (triggered with a delay of 200 ms from the ECG). While there was no overt change in burst amplitude in single trials, averaging did reveal inhibition of a muscle sympathetic burst in this subject
In the remaining eight subjects, intermittent GVS caused no change in average MSNA. This was true regardless of whether the stimulation was bilateral-bipolar (mastoid-mastoid, eight subjects), unipolar (mastoid-inion, four subjects) or whether anodal stimulation was delivered to the right mastoid process or to the left. Figure 2A shows the group (n=8) data, which includes data from the two subjects in whom inhibition was observed, but excludes data from two subjects in whom insufficient bursts were recorded during the stimulation. Averaged records from one subject are shown in Fig. 2B. Analysis (ANOVA) of the difference in averaged burst amplitude for latency matched averages triggered from the R-wave during control (R-wave) and GVS trials showed there was no significant difference (P>0.1).
In four subjects, changing the stimulus delay from 200 ms to 0, 400 or 600 ms following the R-wave trigger had no effect on average burst amplitude. Experimental records from the same subject as in Fig. 1, but with a stimulus delay of 600 ms, are shown in Fig. 3A.
Recording of spontaneous muscle sympathetic activity from the same subject shown in Fig. 1, during delivery of a single galvanic stimulus (2 mA, 1 s) across the mastoid processes (triggered with a delay of 600 ms from the ECG). Note the absence of a change in burst amplitude in this trial. B Oligounitary recording of cutaneous sympathetic activity from a fascicle of the common peroneal nerve supplying the dorsum of the foot during delivery of a single galvanic vestibular stimulus (triggered with a 0 ms delay from the ECG). Note the short-latency (~650 ms) sympathetic burst. Skin potential was recorded between the sole and the dorsum of the foot
In three subjects, recordings of skin sympathetic nerve activity (SSNA) were made during GVS. As shown in Fig. 3B, this caused a short-latency burst of cutaneous sympathetic activity (average latencies 654, 685 and 689 ms for each subject). Given that in all subjects unexpected GVS caused cutaneous vasoconstriction and release of sweat, these sympathetic bursts were composed of vasoconstrictor and sudomotor impulses. In one subject skin sympathetic activity was also recorded during stimulation of the ear lobe (1 s pulse 1 mA), which caused distinct cutaneous sensations at the stimulation site and cutaneous vasoconstriction and release of sweat in the feet and hands. In this subject the average burst latency of the cutaneous sympathetic burst was 640 ms.
Discussion
Galvanic vestibular stimulation (GVS) is used widely to study postural responses to vestibular inputs (e.g. Britton et al. 1993; Fitzpatrick et al. 1994; Day et al. 1997). The present investigation is the first to apply this technique to the human cardiovascular system. Given the capacity of GVS to activate vestibular afferents selectively (although cutaneous afferents immediately under the electrodes are also stimulated), this allows one to assess responses to brief vestibular perturbations without changing the balance of other inputs that may affect the cardiovascular system. Indeed, with the exception of caloric vestibular stimulation—which, as noted above, causes inconsistent effects on MSNA—other means of stimulating vestibular inputs also alter other systems. For instance, head-down neck flexion (HDNF), which causes a sustained increase in MSNA, could potentially cause venous congestion in the head and/or change transmural pressure at the carotid sinus. It also changes the afferent balance from muscle (and other) receptors in the neck (see Bolton and Ray 2000), although experiments have been performed that address both of these issues (Ray and Hume 1998; Hume and Ray 1999). Linear sinusoidal acceleration (Cui et al. 2001) or off-vertical axis rotation (Kaufmann et al. 2002) also cause fluid shifts in the body and entrain respiration to the stimulation cycle; it is known that MSNA burst amplitude waxes and wanes with respiration (Macefield and Wallin 1995a). Moreover, the responses to these two forms of acceleration are different: an overall decrease in MSNA during anteroposterior or lateral displacement of the seated body (Cui et al. 2001), yet an increase during the phase of the off-vertical axis rotation cycle corresponding to head-up tilt and a decrease during the phase corresponding to head-down tilt (Kaufmann et al. 2002). And while the latter study could be considered to be the cleanest in terms of activation of otolith receptors in the vestibular apparatus, the authors concede that other "graviceptors," located elsewhere in the body, could play a role in mediating the vestibulosympathetic responses. Accordingly, there remains controversy in defining what constitutes a vestibulosympathetic reflex in human subjects, hence our desire to use galvanic vestibular stimulation to address this issue.
For the majority of subjects studied in the present investigation, brief (1 s) pulses of current (2 mA) applied bilaterally across the mastoid processes, or unilaterally between one mastoid process and the inion, failed to modulate MSNA. This is despite the clear perceptual illusions of vestibular stimulation. Delivery of the stimuli during a sustained increase in MSNA (inspiratory-capacity apnoea) failed to reveal inhibition, and delivery of a stimulus in individuals with little spontaneous MSNA failed to reveal an increase. In two subjects, however, inhibition of one sympathetic burst did occur, in a manner similar to that observed with transcranial stimulation of the cortex (Macefield et al. 1998) or with unexpected sensory stimuli (Donadio et al. 2002). One explanation is that the inhibition we observed in these two subjects may simply be related to the arousal produced by a sudden stimulus, as documented by unexpected electrical stimulation of a finger or a sudden visual flash (Donadio et al. 2002). Moreover, application of the same stimuli to an earlobe caused a similar inhibition. In all subjects unexpected GVS did cause signs of increases in cutaneous sympathetic activity (decreases in skin blood flow and increases in sweat release), confirmed in three subjects by direct recordings of short-latency (654–689 ms) increases in skin sympathetic nerve activity (SSNA). Magnetic stimulation of the motor cortex has also been shown to cause short-latency (mean 739–895 ms) increases in SSNA, responses that the authors attribute to a direct activation of sympathetic neurones rather than to a consequence of arousal or movement-related feedback (Silber et al. 2000). The fact that our latencies were shorter than those reported by Silber et al. (2000), and are too short to be mediated indirectly (Fagius and Wallin 1980), fits with the more caudal point of stimulation with GVS (over the mastoid processes) than transcranial magnetic stimulation (6 cm lateral to the vertex of the head), and lends further support to a direct (vestibular) activation of cutaneous sympathetic pathways.
Conclusions
The absence of short-latency excitation, or inhibition, of muscle vasoconstrictor neurones in this study indicates that short-duration vestibular stimuli have no demonstrable effect on muscle sympathetic outflow. If vestibular inputs do contribute to "vestibulo-sympathetic reflexes" in humans, activation of specific vestibular end-organs—or long duration (>1 s) stimuli—may be required. However, it is clear that short-duration vestibular stimuli do excite cutaneous vasoconstrictor and sudomotor neurones at latencies consistent with a direct activation of medullary output neurones via vestibular-nerve afferents, rather than an indirect, arousal-related increase. Indeed, direct vestibular stimulation of cutaneous sympathetic activity is presumably responsible for the pallor and sweating associated with motion sickness (Benson 1999).
References
Benson AJ (1999) Motion sickness. In: Ernsting J, Nicholson AN, Rainford DJ (eds) Aviation Medicine. Butterworth Heinemann, Oxford, pp 455–471
Bolton PS, Ray CA (2000) Neck afferent involvement in cardiovascular control during movement. Brain Res Bull 53:45–49
Bolton PS, Wardman DL, Macefield VG (2002) Effects of galvanic stimulation on sympathetic outflow in awake human subjects. Proc Aust Neurosci Soc 13:96
Britton TC, Day BL, Brown P, Rothwell JC, Thompson PD, Marsden CD (1993) Postural electromyographic responses in the arm and leg following galvanic vestibular stimulation in man. Exp Brain Res 94:143–151
Burke D, Sundlof G, Wallin BG (1977) Postural effects on muscle nerve sympathetic activity in man. J Physiol 272:399–414
Costa F, Lavin P, Robertson D, Biaggioni I (1995) Effect of neurovestibular stimulation on autonomic regulation. Clin Auton Res 5:289–293
Cui J, Mukai C, Iwase S, Sawasaki N, Kitazawa H, Mano T, Sugiyama Y, Wada Y (1997) Response to vestibular stimulation of sympathetic outflow to muscle in humans. J Autonom Nerv Sys 66:154–162
Cui J, Iwase S, Mano T, Katayama N, Mori S (2001) Muscle sympathetic outflow during horizontal linear acceleration in humans. Am J Physiol Regul Integr Comp Physiol 281:R625–R634
Day BL, Severac-Cauquil A, Bartolomei L, Pastor MA, Lyon IN (1997) Human body-segment tilts induced by galvanic stimulation: a vestibularly driven balance protection mechanism. J Physiol 500:661–672
Doba N, Reis DJ (1974) Role of the cerebellum and the vestibular apparatus in regulation of orthostatic reflexes in cats. Circ Res 40:9–18
Donadio V, Kallio M, Karlsson T, Nordin M, Wallin BG (2002) Inhibition of muscle sympathetic activity by sensory stimulation. J Physiol 544:285–292
Fagius J, Wallin (1980) Sympathetic reflex latencies and conduction velocities in normal man. J Neurol Sci 47:433–448
Fitzpatrick R, Burke D, Gandevia SC (1994) Task-dependent reflex responses and movement illusions evoked by galvanic vestibular stimulation in standing humans. J Physiol 478:363–372
Fu Q, Iwase S, Niimi Y, Kamiya A, Kawanokuchi J, Cui J, Mano T, Suzumura A (2001) Effects of lower body positive pressure on muscle sympathetic nerve activity response to head-up tilt. Am J Physiol Regul Integr Comp Physiol 281:R778–785
Hume KM, Ray CA (1999) Sympathetic responses to head-down rotations in humans. J Appl Physiol 86:1971–1976
Iwase S, Mano T, Saito M (1987) Effects of graded head-up tilting on muscle sympathetic activities in man. Physiologist 30[Suppl 1]):S62–63
Jian BJ, Cotter LA, Emanuel BA, Cass SP, Yates BJ (1999) Effects of bilateral vestibular lesions on orthostatic intolerance in awake cats. J Appl Physiol 86:1552–1560
Kaufmann H, Biaggioni I, Voustianiouk A, Diedrich A, Costa F, Clarke R, Gizzi M, Raphan T, Cohen B (2002) Vestibular control of sympathetic activity. An otolith-sympathetic reflex in humans. Exp Brain Res 143:463–469
Kerman IA, Yates BJ, McAllen RM (2000) Anatomic patterning in the expression of vestibulosympathetic reflexes. Am J Physiol Regul Integr Comp Physiol 279:R109–R117
Macefield VG, Wallin BG (1995a) Modulation of muscle sympathetic activity during spontaneous and artificial ventilation and apnoea in humans. J Autonon Nerv Syst 53:137–147
Macefield VG, Wallin BG (1995b) Effects of static lung inflation on sympathetic activity in human muscle nerves at rest and during asphyxia. J Autonon Nerv Syst 53:148–156
Macefield VG, Taylor JL, Wallin BG (1998) Inhibition of muscle sympathetic outflow following transcranial cortical stimulation. J Autonon Nerv Syst 68:49–57
Ray CA, Hume KM (1998) Neck afferents and muscle sympathetic activity in humans: implications for the vestibulosympathetic reflex. J Appl Physiol 84:450–453
Shortt TL, Ray CA (1997) Sympathetic and vascular responses to head-down neck flexion in humans. Am J Physiol Regul Integr Comp Physiol 272:H1780–H1784
Silber DH, Sinoway LI, Leuenberger UA, Amassian VE (2000) Magnetic stimulation of the human motor cortex evokes skin sympathetic nerve activity. J Appl Physiol 88:126–134
Wardman DL, Fitzpatrick RC (2002) What does galvanic vestibular stimulation stimulate? Adv Exp Biol Med 508:119–128
Yates BJ (1996) Vestibular influences on the autonomic nervous system. Ann NY Acad Sci 781:458–473
Yates BJ, Miller AD (1994) Properties of sympathetic reflexes elicited by natural vestibular stimulation: implications for cardiovascular control. J Neurophysiology 71:2087–2092
Yates BJ, Yamagata Y, Bolton PS (1991) The ventrolateral medulla of the cat mediates vestibulosympathetic reflexes. Brain Res 552:265–272
Yates BJ, Goto T, Bolton PS (1993) Responses of neurons in the rostral ventrolateral medulla of the cat to natural vestibular stimulation. Brain Res 601:255–264
Acknowledgments
VGM is a Senior Research Fellow of the National Health and Medical Research Council of Australia. DLW was supported by a grant from the National Health and Medical Research Foundation. PSB is supported in part by a grant to the Hunter Medical Research Institute from the NSW Dept of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bolton, P.S., Wardman, D.L. & Macefield, V.G. Absence of short-term vestibular modulation of muscle sympathetic outflow, assessed by brief galvanic vestibular stimulation in awake human subjects. Exp Brain Res 154, 39–43 (2004). https://doi.org/10.1007/s00221-003-1631-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-003-1631-1