Abstract
This study investigates the early development of postural adjustments during external perturbations in two different standing positions: standing with support and standing without support. The aim of the study was to assess a group of 13 infants four times during the period in life when independent standing is achieved; at 8, 10, 12 and 14 months. However, longitudinal data could be achieved only in four infants. Muscle activations of the neck, hip and ankle were recorded using surface electromyography. Based on earlier studies and controversies, three main issues were addressed: (1) Is direction specificity present before independent standing is established? (2) How do postural adjustments change with increasing age (8–14 months)? (3) Are postural adjustments task-specific in the young child? The results showed that our small sample of infants aged 8 and 10 months, who were not yet able to stand independently, exhibited direction-specific postural adjustments both during standing with and without support, though not consistently during all trials and at all body levels. Therefore, we argue that direction specificity might constitute a prerequisite for the development of independent standing. We also found that the development of postural adjustments in standing with support resembles that of sitting, i.e. great variation in the postural adjustments at early age, and fine-tuning to the situation with increasing age and experience. This, we find that this is in agreement with the proposal that postural control develops through a selection process of the most suitable postural adjustments for the situation from a repertoire of direction-specific postural adjustments. The development of postural adjustments during standing without support is discussed. Additionally, differences in response rates were noted between the two standing positions, indicating that even before independent standing is established, sophisticated sensorimotor integration enables task-specific postural adjustments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Independent standing, one of the major milestones of gross motor development, is reached around the age of 12 months (Piper and Darrah 1994). To achieve this, the infant needs to maintain equilibrium in the upright position based on the small support surface provided by both feet.
Over the last decade, it has been discussed whether postural adjustments develop as a result of genetically based neural connectivity or by experience. Woollacott et al. have previously argued in favour of the development of postural adjustments based on experience (Woollacott et al. 1987, 2005; Sundermier and Woollacott 1998), while our group suggested that basic postural adjustments primarily have an innate origin (Hirschfeld and Forssberg 1994; Hadders-Algra et al. 1996; Forssberg 1999; Hadders-Algra 2000). This was recently supported by the findings of Hedberg et al. (2004, 2005) who demonstrated that basic direction-specific postural adjustments during sitting are present already by the age of 1 month, i.e. long before the infant can sit by itself. Direction-specific postural adjustments are organized to maintain equilibrium of the body, which means that the ventral muscles are activated when the body sways backward (bw) and dorsal muscles during forward (fw) sway of the body to prevent from further falling in the direction of the sway. The first question we addressed in the present study was whether direction-specific postural adjustments are present before independent standing develops.
Postural adjustments have been proposed to develop with the selection of a particular direction-specific synergy from a variety of possible muscle activation patterns (Hadders-Algra et al. 1996; Forssberg 1999). Since the proposal is based on data achieved in the sitting position (Hadders-Algra et al. 1996), we do not know whether experience-based selection of the most effective muscle activation pattern is a general principle also present, e.g. in the standing position. Apart from the development of specific combinations of muscle contractions, changes in recruitment order and latencies have been reported for the sitting position with increasing age (Hadders-Algra et al. 1996). A dominant cranial to distal recruitment order gets replaced with a distal to cranial recruitment order. However, during early standing, Woollacott et al. reported that the muscles closest to the surface of support are primarily engaged in the muscle response, i.e. a reverse recruitment order from that during early sitting. They concluded that with increasing experience and age, more proximal muscles are included in the postural adjustments (Woollacott et al. 1987; Sundermier and Woollacott 1998; Woollacott and Burtner 1996). Since the recruitment order of postural adjustments during early standing was reported to differ from that in sitting, we wanted to investigate the latencies to response onset in the various muscles included in the postural adjustments during the establishment of independent standing. We wondered whether Woollacott et al. caught the first stages of postural adjustments during standing, as they assessed the youngest infants only during standing with support. Or, could it be, that postural adjustments in sitting and standing develop differently.
Standing with and without support provide different postural challenges. Biomechanically, during standing without support, the translation of the surface of support will result in an inverted pendulum movement of the body, i.e. the whole body will sway around the ankle and the torque around the ankle therefore has to be controlled. When a person is holding on to a support, the biomechanical condition is different and the hands can be used to provide postural stability. The nervous system in adults reacts differently to these two situations. When an adult person is standing with hand support, postural muscle activity will primarily be found in the arms and the muscle activity of the legs will be low. When an adult person is standing without support, the postural adjustments will instead mainly be found in the muscles controlling the ankle torque (Cordo and Nashner 1982; Jeka and Lackner 1994). This task-dependent gain control of the postural adjustments of the lower limbs has great impact on the interpretation of the postural muscle activity in children achieving independent standing. If the infant is not able to stand without support and is being assessed with support, absence of postural muscle activation in the legs could have two possible explanations. Either the infant is not able to produce adequate postural muscle activity in the lower limb muscles or there is a reduced gain of the postural leg muscle activity due to the extra arm support. By comparing the postural adjustments in standing with support and standing without support we addressed the question if infants are able to adapt the postural adjustment to the situation.
In summary, in our studies on how postural adjustments develop during the period in life when independent standing is achieved, we addressed four problems: (1) Is direction specificity present prior to the establishment of independent standing? (2) How do postural adjustments change over the age period of 8–14 months? (3) Are postural adjustments task-specific in the young child, i.e. can they change the gain of the postural adjustment in different conditions?
Methods
Participants
A total of 13 typically developing infants with no medical history participated in the study. They were recruited from a local children’s health care nurse. All parents gave informed consent and the procedures were approved by the Medical Ethical Committee of the Karolinska Hospital. The study included four serial assessments, which were conducted at the ages of 8, 10, 12 and 14 months. The time window for each assessment varied to a maximum of 2 weeks, i.e. from 1 week prior to the age and to 1 week after having reached the specific age. Only four infants could be assessed on all four occasions. Table 1 gives an overview of the number of assessed infants at each age, the range and median number of trials included in the analysis, and the standing capacity of the infants. At the age of 8 months, none of the infants could stand independently. At 10 months, two out of nine infants had achieved the function of independent standing. By the age of 12 months, 7 out of 11 had achieved this function and at 14 months independent standing was established in all 10 infants assessed at this age.
Protocol
Assessment of standing function
The infants ability to stand was assessed with the stand subscale of the Alberta Infant Motor Scale (AIMS, Piper and Darrah 1994). Independent standing was attributed as a function to the infant when the item ‘stands alone’ was achieved.
Assessment of postural adjustments
The infants stood on a movable platform that produced horizontal forward or backward translations with an amplitude of 60 mm. A set of trials consisted of 16 slow perturbations (fw—60 mm/s, 1 s duration; bw—60 mm/s, 1 s duration), followed by 16 fast ones (fw—120 mm/s, 500 ms duration; bw—120 mm/s, 500 ms duration). The direction of the translations (fw and bw) were presented in a random order. The infants were tested during two standing conditions: first, standing with support when the infant held on to a horizontal bar attached to the movable platform at about the level of the shoulders, and second, standing without support. During the independent standing position, infants who could not yet stand independently were given postural support by the experimenter until 1–3 s before the platform started moving. Immediately after the trial, support was re-established. The parent or caregiver of the infant was sitting in front of the platform, ready to encourage and comfort the infant. The following criteria for position standardization were set: head in the mid-line, arms in a neutral position, weight bearing on both feet and feet in parallel. Arms had to be in a neutral position and not occupied with large or fast movements such as waving. In the supported condition, either both hands or the right hand had to be holding on to the bar. Additionally, the infants should be alert and non-crying. The entire assessment was video-recorded.
Bipolar surface electrodes with an interelectrode distance of 15 mm and an in-built 2,000× preamplification (MYO 115, Liberty Technology, Hopkinton, MA, USA) were placed over the surface of the ankle plantarflexor (gastrocnemius; GA), ankle dorsiflexor (tibialis anterior; TA), hip flexor (rectus femoris; RF), hip extensor (hamstrings; HA), lumbar extensor (LE, 1 cm paravertebral at the L3-4 level), the neck flexor (NF, sternocleidomastoideus) and neck extensor (NE, at the C5-6 level) muscles and on biceps brachii (BB), on the right side of the body.
Data analysis
Only trials meeting the aforementioned criteria for standing position and behavioural state were analysed. These were selected on the basis of the video-recordings. The signals from the platform and the electromyography (EMG) were sampled at 800 Hz, and stored in SC/SZOOM, a dedicated signal analysis computer system (Department of Physiology, Umeå University, Sweden). A graphics terminal was used to visually define EMG events for each trial. EMG recordings with artefacts were excluded from the analysis. EMG baseline activity was defined as the mean activity recorded 500 ms before trial onset. EMG events occurring within 30 ms after trial onset were excluded from the analysis. EMG bursts were defined when the muscle activity exceeded the baseline activity by 2 SD for a duration of at least 30 ms. EMG events starting later than 500 ms after platform onset were not included in the analysis. The first step in the analysis consisted of the documentation of the muscle activation patterns by describing the presence of bursts and inhibition in the recorded muscles. The response rates for each child and each condition were calculated by dividing the number of trials with a response with the total number of trials for that specific child during the present condition. The next step consisted of the analysis of EMG latencies. Latency was defined as the time interval between the onset of platform movement and the onset of an EMG response. For each infant, the mean latency to response onset was calculated for each muscle and condition. Direction-specific adjustments were defined as adjustments during which agonist muscle activation or antagonist inhibition preceded antagonist muscle activation. Muscle activity in ventral muscles during fw translations and in dorsal muscles during bw translations were considered to be direction-specific. Consequently, the agonists during fw translations were TA, RF and NF, and during bw translations were GA, HA and NE. Additionally, TA, RF and NF were defined as flexors and GA, HA, NE and LE as extensors. For example, ‘extensor inhibition’ (INH) and ‘flexor inhibition’ (INH) refer to this definition. Co-activity was considered to be present when muscle activation of the antagonist occurred within 100 ms after the onset of agonist activation. On group level, the median values are presented.
Statistical analysis
Throughout the analyses, the Wilcoxon matched pairs test (Statistica 6.0) was used to recognize differences within ages between the conditions with and without support or between fw and bw translations, as well as between ages within conditions. Throughout the analyses, differences with a P-value <0.05 were considered to be statistically significant. Subjects were excluded from the analysis if less than three adequate trials for the specific condition and direction was obtained. The assessment during standing without support in children who were not yet capable of standing independently turned out to be difficult. Eventually, assessment of at least three trials was attained in three non-standing infants at the age of 8 months and in four non-standing infants at 10 months (Table 1). Due to the limited data, these results are presented only descriptively.
Results
Postural adjustments during unsupported standing in non-standing infants
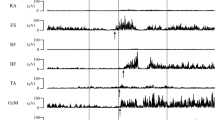
To test our first hypothesis, i.e. that direction-specific postural adjustments develop before the establishment of independent standing, muscle activity in three non-standing infants aged 8 months and four aged 10 months was examined. Two of the infants (A and B) were tested at both 8 and 10 months. The data showed that although these infants could not stand independently, all infants showed direction-specific postural adjustments on at least one level: neck, upper leg or lower leg (Fig. 1; Table 2). It should be noted that direction- specific activity was not consistently present in each trial at all recorded levels.
Electromyography examples of one infant aged 8 months during six trials, standing without support. Upper row showing EMG traces during fw translations and lower row during bw translations. Horizontal lines denote activity in direction-specific muscles, dotted lines denote muscle activity in antagonistic muscles and activity in the arm. For abbreviations see Sect. ’Methods’
Development of the postural adjustments
We wanted to investigate how postural adjustments change, as the ability to stand independently emerges. We hypothesized that direction-specific postural adjustments would be more frequent with increasing age. We also expected to find changes in latencies to response onset for individual muscles as the infants got older.
By analysing the data in four various conditions at the four ages (see below), three developmental trends were revealed, although variation in the postural adjustments at all ages was noted: (1) At a younger age, there was a predominantly direction-specific agonist/antagonist activation in the proximal muscles. With age, a more pronounced direction- specific activation of agonists/antagonists was developed in all the muscle groups. (2) At a younger age, proximal and distal muscles were activated to the same degree. With age, the distal muscles were more frequently activated, while proximal muscle activity remained (Fig. 2). (3) At a younger age, proximal and distal muscles were activated with similar latencies. With age, latencies in distal muscles decreased (Fig. 3).
Direction-specific muscle activation rates in neck (filled square; NF or NE) and ankle (open square; TA or GA), during a standing with support, fw translations; b standing with support, bw translations; c standing without support, fw translations and d standing without support, bw translations. Significant difference in ankle activation rate between 10 and 12 months is indicated in a. The asterisk denotes significant differences between neck and ankle activation rates within indicated age group. Boxes show 25–75% of the data, whiskers show non-outlier ranges. The stretch of the boxes and the lack of whiskers for some of the muscle activation rates are due to the variation and skewness of the data
Standing with support, fw translations
At 8 months, neck and upper leg direction-specific postural adjustments were present at the neck and hip levels (Table 3); with increasing age, direction-specific distal muscles were also activated. Due to a significant increase in TA activity from 28% at 10 months to 53%, direction specificity was, for example, found in the ankle muscles at 12 months (Fig. 2a, P < 0.05). A three-muscle synergy was significantly more common at 12 months, as compared to 8 months, whereas the frequency of trials not resulting in any direction-specific response decreased significantly from 35% at 8 months to 8% at 14 months (P < 0.05, respectively).
The direction-specific NF was significantly faster to respond to the perturbation than its antagonist at 8 months (latency to onset; NF 212 ms, NE 297 ms, P < 0.05), and continued to be faster throughout the tested ages (10 months; NF 160 ms, NE 330 ms, 12 months; NF 250 ms, NE 285 ms, P < 0.05 respectively, 14 months; NF 197 ms, NE 420 ms; P = 0.06). In general, the direction-specific hip and ankle muscles were not recruited significantly earlier than the antagonistic hip and ankle muscles. Exceptions to the rule were at 12 months, when the direction-specific ankle muscle was significantly faster to respond than the antagonist (latency to onset; TA 140 ms, GA 290 ms) and at 14 months, when the direction-specific hip muscle was faster than its antagonist (RF 152 ms, HA 337 ms, P < 0.05, respectively).
Standing with support, bw translations
During bw translations, a less clear direction-specific organization of the muscle activity at an early age was noted, as direction specificity was not seen at all at 8 months (Table 3). In contrast to the development of postural adjustments during fw translations, the postural responses developed in a distal to proximal order. At 10 months, direction-specific postural adjustments were found at the ankle level, at 12 months at the ankle and hip and finally at 14 months in the neck muscles, i.e. at all three levels (P < 0.05, respectively).
Direction-specific temporal organization during bw translations, standing with support, was only noted at 8 months at the neck level (latency to onset; NE 244 ms, NF 330 ms, P < 0.05) and at 12 months in the hip muscles (HA 180 ms, RF 290 ms, P < 0.05).
Standing without support, fw and bw translations
During standing without support, direction specificity was seen during both fw and bw translations at all levels at 12 months, with only one exception (Table 4). Note that 8 and 10 months are not included in the statistical analysis during standing without support. Again, developmental changes were observed with more muscles activated with increasing age. At 14 months, the three-muscle combination was more common than at the 12-month fw translations (P < 0.05). During standing without support, the distal synergies of RF + TA during fw translations and HA + GA during bw translations were significantly more often activated than the proximal synergies, i.e. NF + RF during fw, NE + LE during bw translations (P < 0.05, respectively). RF + TA was activated in 21% of the trials at 12 months and in 44% at 14 months. HA + GA was activated in 24% of the trials at 12 months and in 26% at 14 months, whereas the proximal synergies were activated in <4% at both ages.
Temporal fine-tuning of the dorsal muscles with increasing age was observed during standing without support, during both fw and bw translations. A significant difference between agonistic and antagonistic muscle activity of the ankle appeared as a result of a decrease in latency to onset in the dorsiflexor, from 250 ms at 10 months to 100 ms at 14 months, during fw translations and in the plantar flexor, from 200 ms at 10 months to almost 100 ms at 14 months of age during bw translations (Fig. 3). Similar trends were observed for the hip muscles, but for the neck muscles, the latencies remained constant.
Task specificity
We hypothesized that the postural adjustments would be task-specific, i.e. there would be differences in postural adjustments between standing with support and standing without support, although the perturbation of the support surface was the same.
Figure 4 shows a tendency for more muscles being activated when children were standing without support (open bars). A three-muscle combination was significantly more often found at 14 months during standing without support as compared to standing with support (P < 0.05), while activation of only a single muscle or no muscle at all was significantly more common during standing with support. Note also that a similar pattern was already present at 8 months of age. However, due to the small number, we did not perform any statistical analysis at this age.
Number of direction-specific muscles at 8 and 14 months, activated during the postural adjustments, presented in % (median values). Direction-specific muscles during fw translations; NF, RF and TA during bw translations; NE, LE, HA, GA. Filled square standing with support; open square standing without support. Significant differences in activation rates between the two conditions are indicated by asterisks
Discussion
Methodological considerations
To begin with we would like to highlight two methodological problems in the present study. First, to have non-standing infants to stand without support is truly a challenge. This was accomplished as the experimenter helped the infant to position body segments and to acquire equilibrium. However, the inability to control for any influence by the initial position or additional voluntary motor activity on the response pattern was a clear limitation. Additional kinematic analysis could have been beneficial. In order to reduce the pre-perturbation position effect, all trials where the infants had started to fall before platform acceleration were excluded from the analysis. The older and more stable the infants got, more trials could be included in the unsupported condition.
Second, we were faced with small data samples in this study. Therefore, the results of non-standing infants in the unsupported standing condition should be considered as preliminary. However, the data is unique and complies with our previous results from the sitting condition (which is more stable). Future studies that exclusively focus on postural adjustments in non-standing infants during unsupported standing are needed to deepen our understanding for the development of postural adjustments during the accomplishment of independent standing.
Neurodevelopmental considerations
The present study focuses on the development of postural adjustments during the period in life when independent standing is established. The first question we addressed was if postural adjustments during standing without support are direction-specific, before the emergence of independent standing. If the postural adjustments, in this novel situation, were direction- specific, this could indicate that direction specificity constitutes a prerequisite for mastering the achievement of independent standing. We managed to capture the direction-specific properties of the postural adjustments in non-standing infants during standing without support. The infants had no prior experience of this unstable standing position, but still produced direction-specific postural muscle activity. Although the data should be interpreted with caution due to the small sample size, it could be surmised that direction specificity might be required for the development of independent standing. The same principle has been proposed for the development of independent sitting (Forssberg and Hirschfeld 1994; Hadders-Algra et al. 1996; Hedberg et al. 2004, 2005). Our results therefore oppose the idea that the development of direction specificity would emerge as a result of self organization during exposure to the novel position (Sveistrup and Woollacott 1997; Smith and Thelen 2003). One could argue that previous experience of antigravity positions such as sitting would influence the process of mastering postural control in standing. From a local biomechanical perspective on forces applied to the neck for example, the two situations may seem perfectly similar. However, the two positions also differ substantially from each other biomechanically, e.g. in the size of the support surface, in the number of joints to control, the postural muscles to be activated as well as conceptually. Our data indicate that, starting from an epigenetically based origin, the direction-specific character of postural adjustments improves with increasing age and experience. This finding underlines the continual intricate interaction of genetic background and experience in motor development (Forssberg 1999; Hadders-Algra 2000).
The second question we asked was how do postural adjustments change with increasing age? The present study indicates that at early age postural activity is characterized by a large variation of muscle activation patterns. From the vast repertoire, the most appropriate adjustment, i.e. the three-muscle adjustment, is strengthened through a selection process. This means that the selection process is not only present during sitting, but also during standing and thus might be regarded a general developmental principle (Hadders-Algra et al. 1996; Forssberg 1999; Hadders-Algra 2000). Additional developmental changes could be observed in direction specificity, muscle recruitment order and latencies to response onset. In the present study, direction specificity was first noted in the neck and hip muscles. With increasing age, direction-specific ankle muscle activity increased. Not only were the ankle muscles more often included in the response with increasing age, but also they were recruited with shorter latencies. At the same time, the activation of the neck muscles stayed approximately the same. These findings are in line with the proximal to distal developmental sequence reported for infants learning to cruise (Haehl et al. 2000) and for the postural adjustments during the development of sitting (Hedberg et al. 2004, 2005). However, it is in contradiction to other studies of the development of postural control in standing. Woollacott et al. have argued for the reverse developmental sequence, i.e. distal to proximal. They have reported that young infants who stand with support more often recruit ankle muscles than hip muscles (Woollacott et al. 1987; Roncesvalles et al. 2004a, b). In fact, we found the same during bw perturbations at an early age in the condition with support (Fig. 3). Since we found that the postural adjustments are direction-specific prior to the emergence of independent standing but not primarily at the level of the ankle, we propose that the emergence of ankle control is the result of adjusting the postural muscle activity to the constraints of the situation. The development of postural adjustments in standing will continue for many years. It is not until the age of around 10 years that postural adjustments begin to show similar consistency as in adults (Massion et al. 1998; Forssberg and Nashner 1982; Berger et al. 1987; Woollacott et al. 1987; Roncesvalles et al. 2004a, b).
Finally, to test if the infants could adjust the postural muscle activity to different situations, i.e. if the postural adjustments were task-specific during the period when independent standing emerges, we compared postural adjustments during two conditions: standing with and without support. These two situations constitute different biomechanical and sensorimotor conditions (Cordo and Nashner 1982), as well as different constraints for developing the interface between perception and action (Massion et al. 2004). Our results indicate that the infants rapidly grasp the contextual differences between the two conditions; standing with and without support, and adjust the postural muscle activity accordingly. This supports the results of Metcalfe et al. (2004) who found that infants integrate haptic cues into postural sway during quite stance. Although we found differences in postural adjustment strategies between the two standing positions at an early age, the differences were not as sophisticated as seen in adults. In infants, more muscles were included in the response, as the demands were higher, i.e. during standing without support. In adults, lowered gain of ankle muscle activity is found as a result of providing postural support (Cordo and Nashner 1982), and during quite stance even a light fingertip contact to a postural support reduces body sway (Jeka and Lackner 1994). The present study indicates that infants begin to build internal representation of the body early and explore its interaction with the external world as they are exposed to a new standing condition. According to Massion et al. (2004), postural control has two main functions: antigravity control and controlling the relationship between perception and action. Together they shape the internal representation of the body’s configuration or body scheme (Gurfinkel and Levik 1993), and its relationships to the external world (Massion et al. 1998, 2004). We want to emphasize that, although the infants can distinguish and adjust to the two different standing positions before they can stand independently, the refinement of the postural adjustments and the building of internal representations of the body and the environment will continue for years to come (Forssberg and Nashner 1982; Massion et al. 1998; Roncesvalles et al. 2004a, b).
Concluding remarks
The present study indicated that the basic level of postural control due to external perturbations during stance is already functionally active prior to the ability to stand independently. In addition, the study revealed that postural adjustments in standing involves substantial fine-tuning of activity at the first and second level of control. This information can be relevant for people working in the field of physical therapy in children with neuromotor dysfunction, since it is important to evaluate the different motor components responsible for the motor dysfunction of the child. Is the motor dysfunction due to the inability to voluntarily control movements, or is the dysfunction due to lack of postural control, which in turn does not enable a position, stable enough, to perform the movements in? The finding that both temporal and spatial organization of postural adjustments changes with increasing age could be of interest when evaluating neuropediatric physical therapy. Could changes in temporal and spatial organization also be found with physical therapy treatment, reflecting rewiring of sensorimotor integration networks in children with neuromotor deficits? Recently, Woollacott et al. have shown in a pilot study that this could in fact be the case (Woollacott et al. 2005). Children with spastic CP, both hemiplegia and diplegia, were trained in reactional postural control. The improvements resembled the developmental sequence found in our data for temporal organization. Although the study of Woollacott et al. only included six children and the results need to be confirmed in a larger population, it is conceivable that a temporal reorganization of basic response patterns can be achieved with training.
References
Berger W, Quintern J, Dietz V (1987) Afferent and efferent control of stance gait: developmental changes in children. Electroencephalogr Clin Neurophysiol 66:244–252
Cordo PJ, Nashner LM (1982) Properties of postural adjustments associated with rapid arm movements. J Neurophysiol 47:287–302
Forssberg H (1999) Neural control of human motor development. Curr Opin Neurobiol 9:676–682
Forssberg H, Hirschfeld H (1994) Postural adjustments in sitting humans following external pertubations: muscle activity and kinematics. Exp Brain Res 97:515–527
Forssberg H, Nashner LM (1982) Ontogenetic development of postural control in man: adapting to altered support and visual conditions during stance. J Neurosci 2:545–552
Gurfinkel VS, Levik YS (1993) The suppression of cervico-ocular response by the haptokinetic information about the contact with a rigid, immobile object. Exp Brain Res 140–145
Hadders-Algra M (2000) The neuronal group selection theory: an attractive framework to explain variation in normal motor development. Dev Med Child Neurol 42:566–572
Hadders-Algra M, Brogren E, Forssberg H (1996) Ontogeny of postural adjustments during sitting in infancy: variation, selection and modulation. J Physiol 493:273–288
Haehl V, Vardaxis V, Ulrich B (2000) Learning to cruise: Bernstein’s theory applied to skill acquisition during infancy. Hum Mov Sci 19:685–715
Hedberg Å, Forssberg H, Hadders-Algra M (2004) Postural adjustments due to external perturbations during sitting in one-month-old infants: evidence for the innate origin of direction specificity. Exp Brain Res 157:10–17
Hedberg Å, Brogren Carlberg E, Forssberg H, Hadders-Algra M (2005) Development of postural adjustments in sitting position during the first half year of life. Dev Med Child Neurol 47:312–320
Hirschfeld H, Forssberg H (1994) Epigenetic development of postural responses for sitting during infancy. Exp Brain Res 97:528–540
Jeka JJ, Lackner JR (1994) Fingertip contact influences human postural control. Exp Brain Res 100(3):495–502
Massion J, Amblard B, Assaiante C, Mouchnino L, Vernazza S (1998) Body orientation and control of coordinated movements in microgravity. Brain Res Rev 28:83–91
Massion J, Alexandrov A, Frolov A (2004) Why and how are posture and movement coordinated? Prog Brain Res 143:13–25
Metcalfe JS, McDowell K, Chang T-Y, Chen L-C, Jeka JJ, Clark JE (2004) Development of somatosensory-motor integration: an event-related analysis of infant posture in the first year of independent walking. Dev Psychobiol 46:19–35
Piper MC, Darrah J (1994) Motor assessment of the developing infant. W.B. Saunders Company, Philadelphia
Roncesvalles MNC, Woollacott M, Brown N, Jensen JL (2004a) An emerging postural response: is control of the hip possible in the newly walking child? J Mot Behav 36:147–159
Roncesvalles MNC, Woollacott M, Brown N, Jensen JL (2004b) An emerging postural response: is control of the hip possible in the newly walking child? J Mot Behav 2:147–159
Smith L, Thelen E (2003) Development as a dynamic system. Trends Cogn Sci 7:343–348
Sundermier L, Woollacott M (1998) The influence of vision on the automatic postural muscle responses of newly standing and newly walking infants. Exp Brain Res 120:537–540
Sveistrup H, Woollacott M (1996) Longitudinal development of the automatic postural response in infants. J Mot Behav 28(1):58–70
Woollacott M, Burtner P (1996) Neural and musculoskeletal contributions to the development of stance balance control in typical children and in children with cerebral palsy. Acta Paediatr Suppl 416:58–62
Woollacott M, Debû B, Mowatt M (1987) Neuromuscular control of posture in the infant and child: is vision dominant? J Mot Behav 19:167–186
Woollacott M, Shumway-Cook A, Hutchinson S, Ciol M, Price R, Kartin K (2005) Effect of balance training on muscle activity used in recovery of stability in children with cerebral palsy: a pilot study. Dev Med Child Neurol 47:455–461
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hedberg, Å., Schmitz, C., Forssberg, H. et al. Early development of postural adjustments in standing with and without support. Exp Brain Res 178, 439–449 (2007). https://doi.org/10.1007/s00221-006-0754-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-006-0754-6