Abstract
Young children rely heavily on vision for postural control during the transition to walking. Although by 10 years of age, children have automatic postural responses similar to adults, it is not clear when the integration of sensory inputs becomes fully developed. The purpose of this study was to examine this transition in the sensory integration process in children aged 7–12 years. Healthy children and adults stood on a fixed or sway-referenced support surface while viewing full-field optic flow scenes that moved sinusoidally (0.1 and 0.25 Hz) in an anterior–posterior direction. Center of pressure was recorded, and measures of sway amplitude and phase were calculated at each stimulus frequency. Children and adults had significant postural responses during approximately two-thirds of the trials. In adults, there was a 90% decrease in sway on the fixed surface compared with the sway-referenced surface, but only a 50% decrease in children. The phase between the optic flow stimulus and postural response in children led that of adults by 52° at 0.1 Hz and by 15° at 0.25 Hz. Adults and children aged 7–12 years have similar ability to use dynamic visual cues for postural control. However, 7–12-year-old children do not utilize somatosensory cues to stabilize posture to the same extent as adults when visual and somatosensory cues are conflicting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The integration of visual, somatosensory, and vestibular inputs for the control of balance develops throughout childhood (Forssberg and Nashner 1982; Shumway-Cook and Woollacott 1985). Of these sensory modalities, vision has been studied the most. Two main experimental paradigms have been used to assess the contribution of vision to postural control in children. First, “moving room” or optic flow stimuli have been used to understand how subjects use dynamic visual cues to control posture (Lee and Aronson 1974; Butterworth and Hicks 1977; Bertenthal et al. 1997; Barela et al. 2000). Second, “sensory organization tests” have been used to investigate how posture is stabilized when eyes are closed and when the visual field is referenced to body sway (Forssberg and Nashner 1982). Of the two paradigms, the moving room provides a stimulus that is more similar to that experienced in the real world. Both paradigms can reveal interesting aspects of the development of postural control.
Optic flow studies have shown age-related changes in the use of dynamic visual cues for controlling posture. Infants demonstrate postural adjustments that are scaled to the magnitude of constant velocity anterior–posterior optic flow as early as 3 days after birth (Jouen et al. 2000). In studies involving infants aged 5–10 months, the magnitude and correlation of seated postural adjustments to moving visual environments generally increase as a function of age (Bertenthal and Bai 1989; Higgins et al. 1996; Bertenthal et al. 1997). Older infants (7–18 months) who can stand independently and walk, show large standing postural responses to moving visual environments (Lee and Aronson 1974; Butterworth and Hicks 1977; Bertenthal and Bai 1989; Delorme et al. 1989; Foster et al. 1996). The magnitude of the response is possibly related to the acquisition of a new motor skill, such as independent standing or walking (Delorme et al. 1989; Foster et al. 1996). After 2 years of age, when walking has been well-practiced, postural adjustments to moving visual stimuli are reduced (Stoffregen et al. 1987; Foster et al. 1996).
The contribution of vision to sensory organization has been tested by comparing performance of subjects during eye closure with performance when eyes are open. Amplitude of sway increases with age when both eyes are open and in closed conditions (Forssberg and Nashner 1982; Shumway-Cook and Woollacott 1985; Riach and Hayes 1987; Foudriat et al. 1993; Portfors-Yeomans and Riach 1995; Nougier et al. 1998). Below age 4, it is difficult for children to stand with eyes closed, perhaps indicating increased reliance on visual cues for stabilizing posture (Forssberg and Nashner 1982; Riach and Hayes 1987). Another interpretation is that children are unable to use the available vestibular and somatosensory information afforded to them (Forssberg and Nashner 1982; Shumway-Cook and Woollacott 1985). Between ages 4–6, the amount of sway during eyes closed conditions remains greater in children than in adults (Forssberg and Nashner 1982; Shumway-Cook and Woollacott 1985). However, these levels are similar to adult levels by age 7 (Shumway-Cook and Woollacott 1985; Peterka and Black 1990b; Cherng et al. 2001). For children greater than 3 years, the use of somatosensory cues starts becoming more important, because more sway is generated during sway-referenced surface conditions compared with eyes closed or visual sway-referencing (Forssberg and Nashner 1982; Shumway-Cook and Woollacott 1985; Peterka and Black 1990b; Foudriat et al. 1993). Under conditions relying primarily on vestibular control of posture, increased sway is found as late as 20 years of age (Peterka and Black 1990b; Cherng et al. 2001).
Dynamical systems theory proposes that execution of a motor task is dependent upon the development and interaction among many component systems, including motor, sensory, perceptual, biomechanical, environmental, and others (Thelen et al. 1987; Kamm et al. 1990). Inability to produce a behavior may be due to constraints imposed by any of the components. Furthermore, development of a skill occurs through a continuous process of stable and unstable transitional periods. For example, the development of postural responses seems to occur with periods of increased and decreased variability. Transitional periods appear to occur when children are achieving independent walking (Delorme et al. 1989; Foster et al. 1996), and between 4–6 years of age (Forssberg and Nashner 1982; Shumway-Cook and Woollacott 1985; Kirshenbaum et al. 2001). However, by the time children reach the ages of 7–10, they are assumed to make postural adjustments similar to adults. For example, several groups have shown that children aged 7–10 years have the same muscle activation onset latency and distal-to-proximal muscle activation patterns to platform perturbations as adults (Forssberg and Nashner 1982; Shumway-Cook and Woollacott 1985; Peterka and Black 1990a). While these automatic postural responses appear to be similar, it is not clear whether the selection of strategies during conditions of conflicting sensory inputs is also the same in 7–12-year-old children and adults.
The primary purpose of this study was to examine the sensory integration process in children aged 7–12 years. In particular, we examined the influence of dynamic visual cues on postural control while subjects viewed sinusoidal optic flow stimuli on a fixed and sway-referenced platform. The findings suggest that both children and adults are able to extract the dynamic visual cues equally well. However, during fixed platform conditions, children respond with greater magnitude of sway, suggesting that they are unable to use somatosensory input in order to limit the body sway generated by dynamic visual cues to the same extent as adults. Finally, the phase of the postural response in children leads that of adults at low frequencies.
Methods
Subjects
Nineteen healthy children aged 7–12 years (mean 9.8 ± 1.5 years, 10 females) and 20 healthy adults aged 21–30 years (mean 23.8 ± 2.9 years, 11 females) participated after providing consent was obtained. Heights ranged from 1.25–1.68 m (mean 1.45 ± 0.12 m) in children and 1.55–1.86 m (1.71 ± 0.09 m) in adults. This study was approved by the local institutional review boards.
Experimental design
A mixed-factor repeated measures design was used to examine the effects of age group (AGE, children and adults), surface support (SURF, fixed and sway-referenced), and frequency of optic flow (FREQ, 0.1 and 0.25 Hz) on postural sway. We further examined the effects of SURF and FREQ (0.1, 0.25, 0.4, and 0.7 Hz) on postural sway in children only.
Equipment
Subjects stood on a modified Neurotest platform (Neurocom, Inc., Clackamas, OR) that was surrounded on three sides by back-projected screens displaying a full-field (180° horizontal × 70° vertical) optic flow environment (Fig. 1). The platform contained vertical force transducers that allowed computation of center of pressure. In addition, the platform could rotate about an axis collinear with the ankle joint, permitting ankle plantarflexion and dorsiflexion movements. In addition, subjects wore a harness suspended from the ceiling that did not restrict motion.
Schematic of experimental set-up. Subjects stood upright on force platform that was able to pitch up and down in proportion to body sway. The optic flow environment consisted of alternating black and white squares on the side walls (>30° from midline) and alternating black and white concentric rings on the front wall
The visual surround was a custom-developed optic flow environment designed to simulate movements of the “moving room” devices employed in past research. In the default position, the approximate distance between the subject and the front screen was 1.55 m and between the subject and the side screen was 1.14 m. On the front screen (30° field of view from midline), a pattern of black and white concentric rings (increasing radii of 5°) was displayed that provided radial flow to the central field of view. The vertical location of the center of the central ring was adjusted to the eye height of each subject. On the side screens (30–90° field of view from midline), a pattern of alternating black and white squares was displayed that produced lamellar flow to the peripheral field of view. The side screens were not perpendicular to the front screen; they opened out so that the included angle between the front and side screens was 100° . The squares were approximately 15 × 15 cm at the point perpendicular to the subjects‘ line of sight. The squares appeared smaller at a distance due to applied perspective correction. The structure of the optic flow was designed so that the visuo-postural system was maximally responsive (Stoffregen 1985). All elements in the environment (i.e., central rings and peripheral squares) translated the same apparent distance in the anterior–posterior direction. The luminance of the black and white portions of the patterns was 1 and 170 cd/m2, respectively. The images were displayed using Epson 810p PowerLite LCD projectors, with a pixel resolution (vertical × horizontal) of 1024 × 768 for each 2 × 1.5 m screen. Each projector was connected to the video output of a Pentium III PC (550 Mhz). The movement of the images across the three screens was synchronized and controlled by a fourth computer via a local area network.
A six degrees-of-freedom electromagnetic tracking device (Polhemus Fastrak, Colchester VT) was placed on the top of the head and on the back of the pelvis at the level of the iliac crest. These trackers were used to monitor head sway and approximate center of mass; the latter measure provided the signal used for sway-referencing the platform at 20 Hz. Sway-referencing was used to reduce the amount of reliable somatosensory cues that provide orientation to the fixed world by keeping the angle of the ankle relatively constant (Nashner 1971). After observing that the body behaved as an inverted pendulum for most stimuli that we studied, the anterior–posterior position of the pelvis marker was used to compute the ankle angle for sway-referencing. The optic flow images moved independently from the sway-referenced surface.
Experimental procedure
Subjects stood on the force platform without shoes, with their feet placed approximately shoulder-width apart and with their ankles aligned with the rotational axis of the platform. Subjects were instructed to cross their arms over the chest and look directly at the central ring of the target pattern. The children performed eight trials consisting of a random combination of two platform conditions (fixed and sway-referenced surface) and four optic flow conditions (16 cm peak-to-peak sinusoidal A–P translation at 0.1, 0.25, 0.4, and 0.7 Hz). The range of frequencies was chosen to be consistent with the range that has been tested in previous studies (Bertenthal et al. 1997; Barela et al. 2000). Each trial started with 30 s while the visual environment was stationary, followed by 90 (0.1 Hz), 60 (0.25 Hz), or 30 s (0.4 and 0.7 Hz) of optic flow. The length of the trials for the children was specified so that at least nine cycles of optic flow would occur (e.g., 0.1 Hz trials are 90 s in duration), yet the trial length would be minimized because of children’s propensity for losing interest and wanting to move around. Between trials, the children were allowed to move around for approximately 1 min before getting set for the next trial, taking care to place their feet in the same position. After the fourth trial, the children were permitted to sit for a period of 2 min.
The data reported for the adult subjects were collected as a part of a larger experiment examining the influence of optic flow presented in the central and peripheral fields on postural sway (Jasko et al. 2003). The procedure for adult subjects was identical to that for the children with the following exceptions: (1) Only two frequencies were examined: 0.1 and 0.25 Hz, due to a number of other conditions: (2) Instead of having a 30-s period without optic flow at the beginning of each trial, optic flow started approximately 3 s after viewing a stationary scene, and 30 s baseline conditions without optic flow were performed as separate trials, and (3) the trial length for the 0.25 Hz condition was 90 s for adults rather than 60 s for children.
Data analysis
Identification of significant postural responses
A statistics-based method for identifying the presence of significant signal power embedded in noise was performed to determine if there was movement at the stimulus frequency over the length of the trial (Percival 1994; Sparto et al. 2004). Briefly, this method involves computing an F-statistic that is the ratio of the response power at the stimulus frequency to the average response power at the nonstimulus frequencies. The statistical method used to identify the entrained responses is designed to distinguish isolated peaks in the frequency spectrum. This is accomplished by prewhitening the data so that the overall frequency spectrum is flat. For example, unperturbed sway has greater signal power at low frequencies; this trend is removed before computing the F-statistic. Nonparametric statistics (χ 2) delineated differences in the number of significant responses due to the main effects of AGE, SURF, and FREQ.
Magnitude of postural responses
Platform forces and data from the tracking devices were digitized at 100 and 20 Hz, respectively. Anterior–posterior center of pressure (COP) was computed from the platform forces and the anterior–posterior translation of the head and pelvis sway was extracted from the tracking devices. These data were digitally bandpass filtered (fourth-order Butterworth, 0 phase shift, pass band = stimulus frequency ± 0.05 Hz). The root-mean-square (RMS) of these signals were computed for the baseline and optic flow periods, after de-trending each component. In order to account for differences due to subject height and the size of base of support, all responses were normalized to subject height. Because children had a greater amount of baseline sway, the RMS was adjusted by subtracting the frequency-specific RMS value of the sway obtained from the baseline period. Mixed-factor repeated measures analysis of variance was used to examine the main effects of AGE, SURF, and FREQ, as well as the two-way and three-way interactions. The significance level was set at 0.05. A logarithmic transformation of the RMS data was used to satisfy the assumption of normality and stabilize the variance. Multiple comparisons were accounted for by using a Bonferroni adjustment.
Phase
Average phase between the optic flow stimulus and the postural response was determined using linear systems analysis. The cross-spectral density function was computed using Welch’s averaged modified periodogram method (frequency resolution 0.025 Hz) in Matlab. The phase was determined from the real (Re) and imaginary (Im) parts of the cross-spectral density function (C x,y) between the stimulus (x) and the response (y):
Because average phase is not stable when there is not significant power at the frequency of interest, average phase was determined for all significant responses across all subjects. Differences in phase due to the main effects of AGE, SURF, and FREQ were examined using directional statistics (Mardia and Jupp 2000; Sparto and Schor 2004). With directional statistics, there is no model similar to the three-way ANOVA. Furthermore, we expect based on prior literature that the phase will be dependent on frequency of optic flow. Hence a priori we performed six statistical tests: (1) Effect of frequency for children only, (2) Effect of frequency in adults only, (3) Effect of age group at 0.1 Hz, (4) Effect of age group at 0.25 Hz, (5) Effect of surface at 0.1 Hz, and (6) Effect of surface at 0.25 Hz. Consequently, we used a Bonferroni correction to the significance level to adjust for multiple tests: α = 0.05/6 = 0.0083.
Results
Responses to the optic flow stimuli as a function of surface support and frequency are shown for one child (Fig. 2). A noticeable postural response at the stimulus frequency was seen in six of the eight trials (excluding 0.1 Hz fixed surface and 0.4 Hz sway-referenced surface). An increase in the magnitude of the response can also be observed in the sway-referenced surface conditions compared to the fixed surface conditions. The pattern of responses obtained from anterior–posterior COP, pelvis translation, and head translation was similar for both children and adults at 0.1 and 0.25 Hz. Consequently, the comparison of responses between adults and children will be shown for COP only.
Optic flow stimuli (dashed lines) and anterior–posterior center of pressure (A–P COP, solid lines) recordings from a 12-year-old female, who was a consistent responder, as a function of SURF (columns) and FREQ (rows). Asterisks designate trials in which a significant response was detected. Vertical scales are the same for each plot, while time scales are different according to FREQ, in order to show detail
Baseline sway with stationary visual surround
The amount of RMS sway obtained from unperturbed baseline stance was computed at each stimulus frequency. Averaging across 0.1 and 0.25 Hz conditions, children had approximately 1.8 times as much unperturbed sway as adults on the fixed surface (0.13 cm vs 0.07 cm), and 2.0 times as much sway as adults on the sway-referenced surface (0.47 cm vs 0.23 cm). Overall, the ratio of the magnitude of sway obtained from sway-referenced surface conditions compared with fixed surface conditions was similar in children (3.6) and adults (3.3).
Identification of significant responses in children and adults—0.1 and 0.25 Hz stimuli
Not all subjects responded well to optic flow stimuli. Furthermore, subjects who generally responded well did not respond to all conditions. The number of significant responses are tabulated as a function of AGE, SURF, and FREQ in Table 1. Approximately the same proportion of significant postural responses were obtained in children and adults (62% vs 67%), indicating no difference due to AGE. However, there were differences in the number of significant postural responses due to SURF (p = .003) and FREQ (p < .001). Approximately three-fourths of the postural responses on the sway-referenced surface were significant, compared with only one-half on the fixed surface. Furthermore, the 0.25 Hz stimuli elicited more significant responses compared with the number elicited during 0.1 Hz stimuli (79% vs 49%).
Response magnitude in children and adults—0.1 and 0.25 Hz stimuli
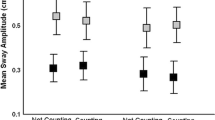
The magnitude (RMS) of anterior–posterior COP elicited by the 0.1 and 0.25 Hz stimuli for children and adults is displayed in Fig. 3. Both adults and children had greater amount of sway during optic flow stimulation. The ratio of frequency-specific sway obtained during the optic flow stimulation to sway obtained during baseline was 1.43, 1.59 in children and adults, respectively. Analysis of variance revealed significant main effects of AGE (p = .003), SURF (p < .001), and FREQ (p < .001). By far the largest effect was due to the surface condition, in which the sway-referenced surface trials elicited approximately three times as much sway compared to the fixed surface trials. Furthermore, there was a significant AGE×SURF interaction (p = .006). In adults, there was a 90% decrease in sway in the fixed compared to sway-referenced conditions, but only a 50% decrease in children. No other two-or three-way interactions were significant.
Identification of significant responses in children—0.1, 0.25, 0.4, and 0.7 Hz stimuli
In order to quantify visually-induced postural responses in children over a wider range of frequencies, children also viewed stimuli at 0.4 and 0.7 Hz. Significant COP responses are tabulated as a function of SURF and FREQ (Table 2). Across all frequencies, there was not a statistically significant effect of SURF. Significant responses were found during 59 and 72% of the fixed and sway-referenced trials, respectively. The number of significant responses did depend on FREQ, however. Qualitatively, there were less responses at 0.1 Hz compared with the other frequencies.
Response magnitude in children—0.1, 0.25, 0.4, and 0.7 Hz stimuli
The magnitude of sway elicited at the stimulus frequency is displayed in Fig. 4 for COP, head, and pelvis sway. First observe the same trend between COP, pelvis, and head responses at 0.1, 0.25, and 0.4 Hz. At 0.7 Hz, there is some divergence in the responses obtained from the head, pelvis, and COP. On the fixed platform, the increase in COP sway at 0.7 Hz was somewhat larger than the increase for the head and pelvis compared with at 0.4 Hz. On the sway-referenced platform, an increase in COP sway was observed at 0.7 Hz compared with the COP sway at 0.4 Hz, whereas the magnitude of the head and pelvis sway was reduced at 0.7 Hz. For all three measures, there were significant SURF effects (p < 0.004). The FREQ effect was significant only for the COP (p < 0.001). In this case, the sway at 0.1 Hz was significantly less than the sway at 0.25 and 0.7 Hz. There were no significant SURF×FREQ interactions.
Phase
Average phase values were tabulated for each trial in which there was a significant response (Fig. 5). In 4% of the cases, the value was 180° out of phase from the typical response; these data were treated as outliers and removed. There was no difference in phase values due to SURF at 0.1 and 0.25 Hz. Therefore, values were averaged across surface conditions. There was a strong effect of FREQ in both children (p < .0001) and adults (p = 0.002). In children, the phase of the response transitions from a lead to lag at approximately 0.25 Hz. In adults, there is a lead at 0.1 Hz and a lag at 0.25 Hz. The postural responses of children led the responses of adults at 0.1 Hz (p < 0.001), but not at 0.25 Hz (p = 0.14).
Discussion
The primary findings in this study are the following: (1) there is no age difference in the number of significant postural responses to the optic flow stimuli, (2) children have a less reduction in sway compared with adults on the fixed surface relative to the sway-referenced surface, and (3) the postural responses of children show greater phase lead than the responses of adults at 0.1 Hz.
When interpreting the findings, it is helpful to consider the relative use of visual, vestibular, and somatosensory cues during various aspects of the testing protocol. In this protocol, the optic flow is designed to elicit postural responses by inducing a sense of vection, or self-movement. When standing on a fixed platform, adult subjects are able to rely on both vestibular and somatosensory cues in order to limit body sway generated by perception of movement in the optic flow field. When standing on the sway-referenced platform, which effectively attenuates the feedback contribution of proprioception, adult subjects primarily use vestibular cues to limit body sway (Peterka and Benolken 1995; Peterka 2002). However, it is possible that other somatosensory modalities, that is, pressure receptors in the sole of the foot and force feedback from the golgi tendon organs, can be used for postural correction (Magnusson et al. 1990a, 1990b; Dietz et al. 1992). If a person does not have a significant postural response at the stimulus frequency, then we surmise that the visual cues are being ignored, or that the combination of vestibular and/or somatosensory cues are limiting the amount of sway to negligible levels.
Identification of significant responses in children and adults—0.1 and 0.25 Hz stimuli
Similar numbers of children and young adults were influenced by dynamic visual cues. During the 0.1 and 0.25 Hz conditions, significant responses occurred in 62% of the trials performed by children and 67% of the trials performed by adults. It is important to note that the statistical method used for identifying significant postural responses incorporates a prewhitening filter that removes low-pass trends in the data that are characteristic of spontaneous sway. Therefore, the influence of how people sway during unperturbed conditions is minimized, and we have greater confidence that responses are true responses and not an artifact of spontaneous sway. Subsequent analysis of the coherence between the optic flow stimulus and postural response (data not shown) reflected the findings obtained from our statistical method. Overall, the response rates are comparable with previous studies, which have documented significant responses in 60–80% of adults (Lee and Lishman 1977; Lestienne et al. 1977; Kay and Warren 2001). Studies of postural responses in children have reported a broad range of response rates of 17–100%, with response rates often coinciding with developmental ability (Lee and Aronson 1974; Butterworth and Hicks 1977; Stoffregen et al. 1987; Bertenthal and Bai 1989; Delorme et al. 1989; Foster et al. 1996; Bertenthal et al. 1997). For example Bertenthal et al. (1997) found greater response rates to optic flow in infants with greater experience in independent sitting. Delorme et al. (1989) examined children from the period of supported standing (mean age 7 months) to experienced walking (mean age 4 years). The greatest responses were in those subjects who could stand but not walk independently. Later, Foster et al. (1996) found that newly experienced walkers had the greatest postural responses.
The frequency of the optic flow stimulus had a large effect on the number of significant responses. There were significant responses during 79% of the trials at 0.25 Hz versus 49% of the trials conducted at 0.1 Hz. Furthermore, in children, the greatest number of significant responses was at 0.25 Hz. These findings suggest that the ability to use dynamic cues for postural control is frequency-dependent, and is consistent with recent experimental efforts that have examined the coupling of sway to optic flow. These studies demonstrate that peak coupling occurs in the range of 0.2–0.3 Hz (Schoner 1991; Dijkstra et al. 1994; Giese et al. 1996). Other evidence supports the assertion that coupling between vision and posture may be velocity-dependent (Oie et al. 2002), which is consistent with a velocity-dependent coupling between somatosensory drive and sway (Jeka et al. 1997). Since the optic flow stimuli used in this study had constant amplitude, the velocity of the stimulus varied with frequency. Therefore, we are not able to discern whether the frequency dependence of the responses is due in part to a velocity dependence.
In our sample, 10 of 19 children and 12 of 20 adults had a significant response in 75% of the trials. Consistent responses to optic flow may relate to visual field dependence and thus involve an intrinsic perceptual style that each person develops according to experience (Isableu et al. 1998). However, within-subject variability in responses seen here, demonstrated by the observation that subjects do not have significant responses during all trials (see Fig. 2), and by others suggest that other factors, for example, fatigue and attention, may also play a role. Furthermore, in light of recent models of the sensory control of posture that include individually weighted gain factors for each of the sensory channels (Peterka 2002), it is possible that subjects who consistently have a significant postural response weight the visual channel to a greater extent compared with the other subjects. Conversely, subjects who do not respond well may have relatively low weighting of the visual input, resulting in negligible visually induced sway.
Response magnitude in children and adults—0.1 and 0.25 Hz stimuli
The amplitude of COP sway at the stimulus frequency demonstrated that children aged 7–12 years had greater sway than young adults (Fig. 3). The most compelling finding was the interaction between AGE and SURF. Averaging over frequency conditions, we found that on the sway-referenced surface, children swayed 1.3 times the amount of adults. This finding suggests that when proprioceptive cues are degraded, 7–12-year-old children can use their vestibular system and/or other somatosensory modalities to limit sway almost as well as adults. On the contrary, while standing on a fixed surface, children had 6.3 times the amount of sway as adults. Therefore, when somatosensory cues are fully available but conflicting with the visual cues, 7–12-year-old children were unable to utilize somatosensation to limit sway to the same extent as adults. Furthermore, the sixfold increase in sway in children relative to adults on fixed platform cannot be explained by larger sway overall, because during the baseline condition, that is, with subjects viewing a stationary visual surround, children had only 1.8 times as much sway as adults.
Although several studies provide evidence that children aged 7–10 have similar automatic postural responses when compared with adults (Forssberg and Nashner 1982; Shumway-Cook and Woollacott 1985; Peterka and Black 1990a), our study supports substantial differences in the sensory integration of posture in this age group compared with adults. Several other groups have put forth a similar hypothesis that children are unable to select the appropriate strategy for limiting body sway when sensory information is conflicting (Forssberg and Nashner 1982; Shumway-Cook and Woollacott 1985; Cherng et al. 2001; Barela et al. 2003).
Current theories of adult sensory integration include an adaptive sensory reweighting of the visual, vestibular, and somatosensory channels based on the reliability of information coming from each sensory channel and environmental factors (van der Kooij et al. 2001; Peterka 2002). One particular model (Peterka 2002) proposes that when somatosensory inputs are unreliable, for example, stance on sway-referenced platform, greater weight is given to the visual and vestibular systems, resulting in greater coupling between the optic flow and postural adjustments. Our data (Fig. 3) show that children and adults have greater sway during sway-referenced platform conditions. Furthermore, the 30% increase in sway that children have compared with adults in the sway-referenced condition suggests that relative weighting of visual to vestibular feedback is slightly greater in children. When somatosensory cues to the fixed world are available, adults increase the somatosensory feedback weighting to a much greater extent compared with children.
It has been suggested that young children (less than 3 years) assign greater weight to vision in order to calibrate postural responses when acquiring new motor skills such as independent stance and walking (Lee and Aronson 1974; Butterworth and Hicks 1977; Delorme et al. 1989; Foster et al. 1996). After they have mastered these skills and have achieved a stable base, older children can use somatosensation to further refine their responses (Shumway-Cook and Woollacott 1985). Kirshenbaum et al. (2001) suggest that ballistic postural responses used by younger children to correct postural movements give way to the use of sensory feedback at around 6 years of age, and that a period of calibration ensues as children learn to use this feedback. Consequently, in children, the weighting of somatosensory and vestibular cues theoretically increases with age. Our work supports the hypothesis that the weighting of somatosensation is not up to adult levels by age 7–12. One potential area for future work is to examine how these weights adaptively change as a function of age.
Response magnitude in children—0.1, 0.25, 0.4, and 0.7 Hz stimuli
The magnitude of head and pelvis responses was similar to the COP response at 0.1, 0.25, and 0.4 Hz, validating the use of COP as a measure of sway at these frequencies. Both head and pelvis sway have peaks at 0.25 Hz, whereas the COP sway is variable between 0.25 and 0.7 Hz. Other studies that have examined the frequency response of sway in younger children found peaks in sway amplitude in the range of 0.2–0.6 Hz in 5–13-month-old seated infants (Bertenthal et al. 1997, 2000; Barela et al. 2000), and 0.2–0.4 Hz in 3–6-year-old standing children (Schmuckler 1997). These studies are comparable to those performed with adults demonstrating that the peak amplitude of postural sway generated in optic flow environments occurs in the range of 0.2–0.5 Hz (Peterka and Benolken 1995; Peterka 2002) There are several possible reasons for the COP to show an increase at 0.7 Hz. It is possible that the motor system is able to generate torque at these frequencies (as revealed by the COP), but the body is unable to respond with large magnitude due to the inertial properties as revealed by the head sway. In addition, a biomechanical model of spontaneous sway predicts that the difference between the COP and center of mass is proportional to the acceleration of the center of mass (Winter et al. 1998). If we assume that the movement of the pelvis is reflective of the movement of the center of mass, then the greater acceleration of the pelvis that occurs at higher frequencies would result in greater COP excursion. As seen in Fig. 4, the difference between magnitude of COP and pelvis sway (i.e., center of mass) increases as a function of frequency.
Phase
The phase relationship between the postural response and the optic flow varied as a function of frequency for children, who demonstrated a lead of 70° at 0.1 Hz, nearly 0° phase at 0.25 Hz, and lags at 0.4 and 0.7 Hz (Fig. 5). The phase of the postural response in adults also shows a decrease at higher frequencies. Experimental studies with children (Bertenthal et al. 1997, 2000; Schmuckler 1997; Barela et al. 2000), and adults (Dijkstra et al. 1994; Peterka 2002) and models of postural sway based on the coupling between the head and the optic flow stimulus demonstrate similar patterns (Schoner 1991; Giese et al. 1996). In these reports, phase values consistently have a zero crossing between 0.1 and 0.3 Hz, which is consistent with our data. Our data also showed that the phase of children led that of adults at 0.1 Hz. These differences could be explained by changes in the body size or by central nervous system processing. For example, if the body is modeled as an inverted pendulum, the smaller body size results in a higher natural frequency for children compared with adults. An increase in natural frequency would result in an increasing lead in children relative to adults at 0.1 Hz.
Limitations
The computer-generated optic flow was designed to simulate the moving rooms that have been used in past studies. Although the movement of the flow in the periphery would be qualitatively similar to the moving room, the radial optic flow in the central field of view differs from the moving room in one respect. Since the images are displayed on a screen at a fixed distance from the subjects, no changes in accommodation and vergence would occur. Alternately, movement of the front wall of a moving room would induce changes in accommodation and vergence. We believe that changes in accommodation and vergence would play a negligible role in generating postural responses for the following reasons. First, pilot studies performed in our laboratory (Jasko et al. 2003) using identical optic flow stimuli have demonstrated that optic flow in the peripheral field of view elicits postural responses to a much greater extent than optic flow in the central field of view. Consequently, vergence and accommodation would not contribute to postural cues generated in the peripheral retina. Secondly, the depth of the simulated radial flow in the current study and the actual radial flow in previous moving room studies is at a distance where it is unlikely that the influence of vergence and accommodation is important. For example, assuming an interpupillary distance of 5 cm and a radial flow depth of 150 cm, the difference in angle of vergence is approximately 2° .
Although the protocols performed by the adults and children were slightly different, we do not believe that the differences would substantially affect the results or conclusions. First, the length of the optic flow stimulation during the 0.25 Hz trial was 60 s for children, and 90 s for adults. The length of the trials for the children was specified so that at least nine cycles of optic flow would occur (e.g., 0.1 Hz trials are 90 s in duration), yet the trial length would be minimized because of children’s propensity for losing interest and wanting to move around. We believe that nine cycles of stimulation provides adequate time for entraining to the stimulation, if it will occur. A remote possibility is that during the additional 30 s of optic flow given to the adults, the sway of the adults adapted during the last 30 s, resulting in lower levels of sway (Loughlin and Redfern 2001). However, we did not find any systematic evidence of adaptation in our sample. Another difference between the protocols performed by the children and adults relates to when the baseline trials were performed. In children, 30 s of quiet standing was added to each trial before the optic flow started. For the adults, a separate 30 s baseline trial was performed. Three pieces of evidence suggest that the addition of 30 s of baseline did not influence the resulting amount of sway during the optic flow period. (1) In most cases, we could distinguish a clear transition in postural responses once the optic flow started, as shown in Fig. 2. (2) The ratio of frequency-specific sway obtained during the optic flow stimulation to sway obtained during baseline was 1.43 in children, and 1.59 in adults, suggesting that the time at which the baseline was performed did not effect how much sway was elicited during the optic flow. (3) There was no correlation between the magnitude of sway obtained during the baseline and optic flow periods.
From a developmental perspective, a sample of children with the age span of 7–12 years could be considered to be heterogeneous, and postural responses may not be similar across this range. Unfortunately, we are unable to comment about the change in sway as a function of age from 7–12 years, because of the low number of subjects at each age. Nonetheless, knowing that children in this age range have different postural responses than adults provides rationale for targeting this age group in future studies.
References
Barela JA, Godoi D, Junior PBF, Polastri PF (2000) Visual information and body sway coupling in infants during sitting acquisition. Infant Behav Dev 23:285–297
Barela JA, Jeka JJ, Clark JE (2003) Postural control in children. Coupling to dynamic somatosensory information. Exp Brain Res 150:434–442
Bertenthal BI, Bai DL (1989) Infants’ sensitivity to optical flow for controlling posture. Dev Psychol 25:936–945
Bertenthal BI, Rose JL, Bai DL (1997) Perception-action coupling in the development of visual control of posture. J Exp Psychol Hum Percept Perform 23:1631–1643
Bertenthal BI, Boker SM, Xu M (2000) Analysis of the perception-action cycle for visually induced postural sway in 9-month-old sitting infants. Infant Behav Dev 23:299–315
Butterworth G, Hicks L (1977) Visual proprioception and postural stability in infancy. A developmental study. Perception 6:255–262
Cherng RJ, Chen JJ, Su FC (2001) Vestibular system in performance of standing balance of children and young adults under altered sensory conditions. Percept Mot Skills 92:1167–1179
Delorme A, Frigon JY, Lagace C (1989) Infants’ reactions to visual movement of the environment. Perception 18:667–673
Dietz V, Gollhofer A, Kleiber M, Trippel M (1992) Regulation of bipedal stance: dependency on “load” receptors. Exp Brain Res 89:229–231
Dijkstra TM, Schoner G, Giese MA, Gielen CC (1994) Frequency dependence of the action-perception cycle for postural control in a moving visual environment: relative phase dynamics. Biol Cybern 71:489–501
Forssberg H, Nashner LM (1982) Ontogenetic development of postural control in man: adaptation to altered support and visual conditions during stance. J Neurosci 2:545–552
Foster EC, Sveistrup H, Woollacott MH (1996) Transitions in visual proprioception: A cross-sectional developmental study of the effect of visual flow on postural control. J Mot Behav 28:101–112
Foudriat BA, Di Fabio RP, Anderson JH (1993) Sensory organization of balance responses in children 3–6 years of age: a normative study with diagnostic implications. Int J Pediatr Otorhinolaryngol 27:255–271
Giese MA, Dijkstra TM, Schoner G, Gielen CC (1996) Identification of the nonlinear state-space dynamics of the action-perception cycle for visually induced postural sway. Biol Cybern 74:427–437
Higgins CI, Campos JJ, Kermoian R (1996) Effect of self-produced locomotion on infant postural compensation to optic flow. Dev Psychol 32:836–841
Isableu B, Ohlmann T, Cremieux J, Amblard B (1998) How dynamic visual field dependence-independence interacts with the visual contribution to postural control. Hum Mov Sci 17:367–391
Jasko JG, Loughlin PJ, Redfern MS, Sparto PJ (2003) The role of central and peripheral vision in the control of upright posture during anterior–posterior optic flow. Proceedings of the 27th Annual Meeting of the American Society of Biomechanics
Jeka JJ, Schoner G, Dijkstra T, Ribeiro P, Lackner JR (1997) Coupling of fingertip somatosensory information to head and body sway. Exp Brain Res 113:475–483
Jouen F, Lepecq J-C, Gapenne O, Bertenthal BI (2000) Optic flow sensitivity in neonates. Infant Behav Dev 23:271–284
Kamm K, Thelen E, Jensen JL (1990) A dynamical systems approach to motor development. Phys Ther 70:763–775
Kay BA, Warren WH Jr (2001) Coupling of posture and gait: mode locking and parametric excitation. Biol Cybern 85:89–106
Kirshenbaum N, Riach CL, Starkes JL (2001) Non-linear development of postural control and strategy use in young children: a longitudinal study. Exp Brain Res 140:420–431
van der Kooij H, Jacobs R, Koopman B, van der Helm F (2001) An adaptive model of sensory integration in a dynamic environment applied to human stance control. Biol Cybern 84:103–115
Lee DN, Aronson E (1974) Visual proprioceptive control of standing in human infants. Percept Psychophys 15:529–532
Lee DN, Lishman JR (1977) Vision—the most efficient source of proprioceptive information for balance control. Agressologie 18:83–94
Lestienne F, Soechting J, Berthoz A (1977) Postural readjustments induced by linear motion of visual scenes. Exp Brain Res 28:363–384
Loughlin PJ, Redfern MS (2001) Spectral characteristics of visually induced postural sway in healthy elderly and healthy young subjects. IEEE Trans Neural Syst Rehab Eng 9:24–30
Magnusson M, Enbom H, Johansson R, Pyykko I (1990a) Significance of pressor input from the human feet in anterior-posterior postural control. The effect of hypothermia on vibration-induced body-sway. Acta Otolaryngol (Stockh) 110:182–188
Magnusson M, Enbom H, Johansson R, Wiklund J (1990b) Significance of pressor input from the human feet in lateral postural control. The effect of hypothermia on galvanically induced body-sway. Acta Otolaryngol (Stockh) 110:321–327
Mardia KV, Jupp PE (2000) Directional Statistics. John Wiley and Sons, Chichester, England
Nashner LM (1971) A model describing vestibular detection of body sway motion. Acta Otolaryngol (Stockh) 72: 429–436
Nougier V, Bard C, Fleury M, Teasdale N (1998) Contribution of central and peripheral vision to the regulation of stance: developmental aspects. J Exp Child Psychol 68:202–215
Oie KS, Kiemel T, Jeka JJ (2002) Multisensory fusion: simultaneous re-weighting of vision and touch for the control of human posture. Cognit Brain Res 14:164–176
Percival DB (1994) Spectral Analysis of Univariate and Bivariate Time Series. In: Stanford JL, Vardeman SB (eds) Statistical methods for physical science. Academic Press, New York, pp 313–348
Peterka RJ (2002) Sensorimotor integration in human postural control. J Neurophysiol 88:1097–1118
Peterka RJ, Benolken MS (1995) Role of somatosensory and vestibular cues in attenuating visually induced human postural sway. Exp Brain Res 105:101–110
Peterka RJ, Black FO (1990a) Age-related changes in human posture control: motor coordination tests. J Vestib Res 1:87–96
Peterka RJ, Black FO (1990b) Age-related changes in human posture control: sensory organization tests. J Vestib Res 1:73–85
Portfors-Yeomans CV, Riach CL (1995) Frequency characteristics of postural control of children with and without visual impairment. Dev Med Child Neurol 37:456–463
Riach CL, Hayes KC (1987) Maturation of postural sway in young children. Dev Med Child Neurol 29:650–658
Schmuckler MA (1997) Children’s postural sway in response to low- and high-frequency visual information for oscillation. J Exp Psychol Hum Percept Perform 23:528–545
Schoner G (1991) Dynamic theory of action-perception patterns: the “moving room” paradigm. Biol Cybern 64:455–462
Shumway-Cook A, Woollacott MH (1985) The growth of stability: postural control from a developmental perspective. J Mot Behav 17:131–147
Sparto PJ, Schor RH (2004) Directional Statistics. In: Stergiou N (ed) Innovative analyses of human movement. Human Kinetics, Champaign
Sparto PJ, Jasko JG, Loughlin PJ (2004) Detecting postural responses to sinusoidal sensory inputs: a statistical approach. IEEE Trans Neural Syst Rehab Eng 12:360–366
Stoffregen TA (1985) Flow structure versus retinal location in the optical control of stance. J Exp Psychol Hum Percept Perform 11:554–565
Stoffregen TA, Schmuckler MA, Gibson EJ (1987) Use of central and peripheral optical flow in stance and locomotion in young walkers. Perception 16:113–119
Thelen E, Kelso JAS, Fogel A (1987) Self-organizing systems and infant motor development. Dev Rev 7:39–65
Winter DA, Patla AE, Prince F, Ishac M, Gielo-Perczak K (1998) SEPSness control of balance in quiet standing. J Neurophysiol 80:1211–1221
Acknowledgments
This research was supported in part by the National Institutes of Health under Grants K25-AG01049, P30-DC05205, R01-DC02490, and by the Eye and Ear Foundation. In addition, we would like to thank Dr. Larry Hodges, Chad Wingrave, Sabarish Babu, Leigh Mahoney, and Jeffrey Jacobson for providing technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sparto, P.J., Redfern, M.S., Jasko, J.G. et al. The influence of dynamic visual cues for postural control in children aged 7–12 years. Exp Brain Res 168, 505–516 (2006). https://doi.org/10.1007/s00221-005-0109-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-005-0109-8