Abstract
Olive oil quality has received much attention during the last 2 decades. However, in some countries e.g. Morocco data reported so far are still deficient and incomplete since only a few researchers have so far addressed the impact of pedoclimatic factors on its quality. For this purpose, thirty-eight Moroccan extra virgin olive oils from the “Picholine Marocaine” variety were collected over two successive crop years (2018 and 2019) and nineteen soil samples were sampled during the first agricultural campaign considering the appropriate sampling equipment recommended. Twenty-three polar phenolic compounds and five Vitamin E isomers (α, β, γ, δ tocopherol and α-tocotrienol) were detected, using HPLC–PDA-ESI–MS and NP-HPLC-FLD, respectively. The most abundant phenolic class was represented by secoiridoids and derivates with an average amount of 1112.3 mg kg−1, whereas among Vitamin E homologs, α-tocopherol was the most abundant, accounting for 91% of total vitamin E. The correlation test and the Principal Components Analysis revealed the significant influence of the pedoclimatic factors on some classes of phenolic compounds (phenolic acids, flavonoids, and secoiridoids); nevertheless, no correlation was detected between phenolic alcohols and other variables under study. Furthermore, the dendrogram cluster allowed to classify the studied areas into five zones, indicating the discrimination of olive oil quality in terms of geographical origin. Besides, phenolic compounds and tocopherols showed significant dependence on the crop year of production. Further, the harvest time turned out to be another factor that can influence the antioxidant fraction quantitatively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agriculture is one of the chief sectors of growth and allows to fight poverty and enhance food security. In this contest, the olive tree (Olea europaea L.) has considerable socio-economic importance in the Mediterranean countries [1]; it is known as a perennial, long-lived, and evergreen tree which is probably among the oldest domesticated trees in the basin [2], and its growth in the region is positively related to the sustainability of soil resource management practices [3]. Morocco is an agricultural country where edaphic and climatic conditions are diverse. The agricultural sector plays a role as a real driver of economic increase [4] and it is well known for the abundance cultivation of the olive tree in almost the entire national territory, except for the Atlantic coastal strip. With up to 96% of the “Picholine Marocaine” as the main variety produced, olives sector contributes up to 5% to the national agricultural Gross Domestic Product (GDP) [5]. Throughout the Country's history, its growth began in the first millennium BC. Since then it underwent a significant expansion, with an olive crop area of 84.000 ha in 2011[6, 7], withal one million ha in 2016 thanks to the efforts under-taken in the framework of the Green Morocco Plan (GMP) [8]. At present, considering its importance in the GMP, it represents the highest cultivated fruit in the country representing 65% of the tree-growing area [7], and interestingly, almost 75% of the harvest fruits go for olive oil production [6].
Extra-virgin olive oil (EVOO) is increasingly recognized as the most prestigious edible oil because of its considerable benefits in the human diet, prevention, and disease treatment [9]. Due to the rising demand for this food product, olive tree cultivation is enlarging worldwide [1]. Statistically, the Mediterranean countries are the dominant producer and consumer with about 97% and 91% of world olive oil production and consumption, respectively [10]. Additionally, Morocco is among the largest producing countries where the production tripled in the last 60 years, reaching 33,79,000 tons in the 2017/18 crop year [11].
The composition of EVOO consists of about 98%-99% of glycerols [12, 13] and ≈2% of minor compounds [14], including more than 230 chemical compounds, such as aliphatic and triterpenic alcohols, sterols, hydrocarbons, etc. Moreover, carotenoids, tocopherols, and hydrophilic phenols [15,16,17,18,19] represent the fraction of bioactive compounds, key components of EVOO. They have received much attention over the last 2 decades due to their responsibility for free radical-scavenging activities, the antioxidant properties [20], and the anti-cancer activity [21].
Being EVOO quality an essential factor in competitiveness [22], all over the world research works showed an increased interest in its composition and the impact of some factors on this quality [23] e.g. the type of crushers for olive oil extraction [24], storage conditions [25], maturity stage of the fruit [26], geographic origin [27] such as altitude [28]. Besides, other authors substantiated the effect of climate (rainfall and temperature) [29, 30], and soil [31]. Nevertheless, to the best of our knowledge, most Moroccan studies were only carried out in a small number of areas. While some research works were carried out on the impact of the geographical origin, so far too little attention was paid to the soil of olive trees. Accordingly, this research project aimed to obtain some findings useful to address these research gaps.
This study had two primary aims: first, to widen the current knowledge about the EVOO bioactive fraction in the well-known EVOO production areas by identifying and quantifying phenolic compounds and tocopherols (Vitamin E); second, to investigate the impact of pedoclimatic factors and the crop year on these phytochemical compounds and emphasize possible distinction among the geographical origins. For this purpose, the current work was carried out throughout nineteen central producing sites of EVOO for two crop seasons, 2018/19 and 2019/20. Taking into account that the distribution of olive trees in Morocco highlights four or five large growing areas where the edaphic and climatic factors vary, the sampling sites choice was based on in-depth research that considered several parameters such as the regional distribution and the abundance of olive trees, the climate, and the EVOO production.
Materials and methods
Samples collection
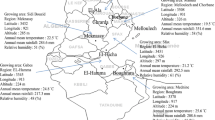
The present work was carried out on thirty-eight Moroccan EVOO samples obtained by milling olives from Picholine Marocaine cultivar. Sampling was carried out during two consecutive productive seasons 2018/19 and 2019/20, in nineteen Moroccan mills sited in the regions of EVOO production, as reported in Fig. 1. During the 2018/2019 productive season, nineteen samples were collected in nineteen mills of North-Center-West Moroccan area: two samples from Tangier-Tetouan-Al Hoceima (Sp1, Sp2), one from Rabat-Sale-Kenitra (Sp3), seven from Fes-Meknes (Sp4-Sp10), four from Beni Mellal-Khenifra (Sp11–Sp14) and five from Marrakech-Safi region (Sp15-Sp19). Sampling collection in the second study year (2019/2020) was performed in the same Moroccan mills where samples were collected in the first year (2018/2019). The EVOO mills of sampling were equipped with similar technology and procedure. To protect the oil from oxygen and light, the samples were stored in dark-colored glass bottles that were closed hermetically and kept away from light. Soil sampling was conducted in November 2018 while considering the appropriate sampling equipment (an Edelman's Auger), the earth depth (0–60 cm), and the samples conditioning. For more details about the study areas, Table S1 (in supplementary materials) provides the geolocation (latitude, longitude and altitude) of the sampling sites.
Chemicals and reagents
Tyrosol (p-HPEA), purity ≥ 95%, hydroxytyrosol (3,4-DHPEA), purity ≥ 90%, oleuropein aglycone (3,4-DHPEA-EA), purity ≥ 98, gallic acid, purity ≥ 97.5%, caffeic acid, purity ≥ 98%, luteolin, purity ≥ 97%, apigenin, purity ≥ 99%, ethyl gallate, purity ≥ 96% reference materials, and the LC–MS grade: acetonitrile, n-hexane, methanol, formic acid, and water were obtained from Merck Life Science (Merck KGaA, Darmstadt, Germany). Tocopherols (α, β, γ, δ) and tocotrienol (α) standards were purchased from Extrasynthase (Genay Cedex, France).
Phenolic compounds analysis
Phenols extraction
Extraction was carried out according to Ricciutelli and co-workers [32]. Briefly, 1 mL of each EVOO was diluted in 1 mL of n-hexane. The sample was extracted with 1 mL of methanol/water (3:2, v/v), shaken for 5 min and extracted for 2 min in an ultrasonic bath (temperature, 37 °C; Hz, 60 W). Then was centrifuged for 10 min at 3000 rpm. The aqueous phase (1 mL) was recovered and washed with 1 mL of n-hexane. Before HPLC injection, 20 µL of internal standard (I.S.) ethyl gallate (1000 ppm) were added to each extract. Each sample was analyzed in triplicate.
Phenols determination by HPLC–PDA/MS
HPLC analysis was performed using a Shimadzu (Kyoto, Japan) instrument, composed of binary solvent pumps LC-20AD, a SPD-M20A photodiode array detector (PDA) and a LCMS-2020 mass spectrometer detector (MS). MS detector was equipped with an electrospray ionization (ESI) source operating in negative ionization (NI) mode and single quadrupole MS. Data acquisition was performed by Shimadzu LabSolution software (Ver. 5.65, Shimadzu, Kyoto, Japan).
Separations were performed on an Ascentis Express C18 (150 × 4.6 mm, 2.7 μm) analytical column (Merck Life Science, Merck KGaA, Darmstadt, Germany). The mobile phase for HPLC–PDA/ESI–MS analyses was water with 0.1% formic acid (A) and acetonitrile with 0.1% formic acid (B) working in the gradient mode 0 min, 10%; 4 min, 35% B; 12 min, 47% B; 12.5 min, 60%; 16 min, 75%; 21 min, 100% B with a flow rate 1.0 mL min−1. All analyses were performed at room temperature of the column and the injection volume was 5 μL. PDA was monitored at a wavelength of 280 nm. The ESI ion source conditions were as follows: DL (desolvation line) temperature, 280 °C; nebulizing gas (nitrogen) flow, 1.5 L min−1; drying gas (nitrogen) flow rate, 5 L min−1; heat block temperature, 300 °C. The mass scan range was set in the range of m/z 100–800; event time was 0.5 s. SIM (single ion monitoring) mode was used for phenol quantification: gallic acid (170 m/z), 3,4-DHPEA (154 m/z), p-HPEA (138 m/z), 3,4-DHPEA-EA (378 m/z), ligstroside aglycone (p-HPEA-EA, 362 m/z), oleocanthal (p-HPEA-EDA, 304 m/z), oleacein (3,4-DHPEA-EDA, 320 m/z), apigenin (270 m/z), luteolin (286 m/z).
Tocopherols and tocotrienols determination by NP-HPLC-FLD
EVOO samples were analyzed without any pre-treatment. In order to quantify α, β, γ-tocopherol and α-tocotrienol in a single analysis, each sample was diluted with n-hexane (1:10, 1:15 or 1:30, v/v) before NP-HPLC analysis. Each EVOO was analyzed in triplicate.
HPLC analyses were carried out using a Shimadzu Nexera-X2 system (Shimadzu, Milan, Italy), including a CBM-20A controller, two LC-30AD dual-plunger parallel-flow pumps, a DGU-20ASR on-line degasser, an autosampler SIL-30AC, a CTO-20AC column oven and a RF-20AXS fluorescence detector with cell capacity of 12 μL, xenon lamp and wavelength range 200–750 nm. Data acquisition was performed by the LCMSsolution Ver. 5.85 software (Shimadzu, Milan, Italy).
Analyses were carried out on an Ascentis Si, 250 × 4.6 mm I.D. with a particle size of 5 µm (Merck KGaA, Darmstadt, Germany) column. The injection volume was 5 µL. Analyses were carried out in isocratic mode: n-hexane and isopropanol (99:1, v/v). Flow-rate was 1.7 mL min−1. Column oven was set at 25 °C. Data were acquired using a Fluorimetric detector at 290 nm for the excitation wavelength of and 330 for emission.
RP- and NP-HPLC methods validation
To quantify the vitamin E and phenols content in the EVOOs samples calibration curves were constructed by using every single available standard, according to the method previously developed and validated by Dugo et al. [15]. Briefly, for tocopherols and tocotrienols quantification five different concentrations of each component, in the range between 0.1 and 200 mg L−1, were analyzed five consecutive times by NP-HPLC. On the other hand, for hydrophilic phenols quantification, five different concentrations of each component, in the range between 0.1 and 1000 mg L−1, were analysed five consecutive times by RP-HPLC. Before injection, 20 μL of internal standard (I.S.) ethyl gallate (1000 ppm) was added to 1 mL of each standard solution.
Soil physicochemical characterization
Upon receipt, the soil samples were dried in the open air to limit microbial activity. They were then ground to separate the soil from the gravel and pebbles prior to be turned into a homogeneous powder. Afterwards they were manually sieved using 0.2 and 2 mm mesh screens, respectively and the suspension density according to the Stokes law [33] was evaluated. The active acidity (pHwater) was measured using a pH-meter according to a ratio of soil: water (1:2.5), and the conductivity by employing a conductivity meter. Total limestone (CaCO3) was executed using Bernard's Calcimeter Method, total organic carbon and organic matter by the Walkley and Black method, and total nitrogen using the Kjeldahl method. Moreover, the available potassium has been extracted using standard ammonium acetate then quantified by Flame Photometer. Each sample was analyzed in duplicate.
Statistical analysis
The R software version 4.1.0 was used for the Principal Components Analysis (PCA), the Hierarchical Clustering on Principal Components (HCPC) (after data standardization) and the correlation test (Supplementary material) by Pearson's correlation coefficient (Table S2). The two-ways ANOVA was conducted using XLSTAT 2018, and the analysis of the differences between the categories via Tukey test (HSD) with a confidence interval of 95%. ArcGIS® software version 10.6.1 was used to visualize the location of sampling sites in Fig. 1.
Results and discussion
Phenolic compounds
Phenolic compounds are part of polar fractions usually obtained by methanol–water extraction. In the present study, 23 hydrophilic phenols classified into four major classes were detected: phenolic acids, phenolic alcohols, secoiridoids and derivates, and flavonoids.
As can be seen from Table 1 that the total phenol content ranges from 110,1 mg kg−1 (Sp6) to 4105.7 mg kg−1 (Sp2) and from 52.8 mg kg−1 (Sp10) to 1420.8 mg kg−1 (Sp5) for 2018/2019 and 2019/2020 crop season respectively, indicating a certain gap of antioxidants in EVOO from the nineteen sampling sites in Morocco. Interestingly, the amount of phenolic compounds in almost all the tested EVOO falls within the average (250 mg kg−1) recommended by European Commission Regulation [34] and it is slightly consistent with what is mentioned in the book of Boskou [35] where wide ranges (50–1000 mg kg−1) for the levels of total polar phenols in EVOO were reported.
Diamantakos et al. [36] analyzed the phenolic content by qNMR in 5764 olive oil samples from Greece and more than 30 varieties for 11 years. They found a maximum concentration of 4003 mg kg−1 of total phenols in oils produced in September 2017 from Kalamon variety. They also observed a significant correlation between the phenols and the harvest period. In another research work, some authors conducted a 3-year study to investigate 44 varieties of olive oils. According to the results, total phenolic compounds varied from 260 to 4497 mg kg−1 during the 2017–2018 crop season [37]. In addition, a study conducted on 25 and 80 cultivars for 2014/15 and 2015/16 crop seasons, respectively, showed high amounts in various phenolic compounds like p-HPEA-EDA (up to 2931.1 mg kg−1 for the first crop season), 3,4-DHPEA-EA (up to 3501.3 mg kg−1 in 2015/16 season), p-HPEA-EA and 3,4-DHPEA-EDA [38]. Strikingly, as far as we know, these are the first and the only highest values reported in the literature.
Regarding the classes of phenolic compounds, with an average of 1112.3 (for 2018) and of 705.85 mg kg−1 (for 2019), secoiridoids and derivates represented the primary class, followed by phenolic alcohols with a total average of 86.69 and 20.45 mg kg−1 for 2018 and 2019, respectively, and phenolic acids in the range from 6.00 (Sp3) to 48.10 (Sp1) mg kg−1 for 2018, and from 3.54 (Sp18) to 34.33 (Sp3) for 2019. The smallest class was one of the flavonoids with an average of 10.25 and 9.54 mg kg−1 for 2018 and 2019, respectively. While studying the phenolic profile of “Picholine Marocaine” for three consecutive crop seasons in Meknes, Bajoub and his co-authors [39] found phenolic alcohols and secoiridoids in the highest amount, with flavonoids in higher content than phenolic acids. Our results agree with the ones reported by Bouymajane et al. [40], who found the same abundance order of phenolic classes in Moroccan EVOOs. Furthermore, according to Boskou [35], the aglycons derived from secoiridoids were the most abundant phenolic compounds in the EVOOs investigated.
Among minor constituents of EVOO, the dialdehydic form of elenolic acid linked to 3,4-DHPEA (3,4-DHPEA-EDA), decarboxymethyl 3,4-DHPEA-EA, and 3,4-DHPEA are principally responsible for the exceptional resistance of EVOO to oxidation. Nonetheless, p-HPEA, lignans, and p-HPEA-EA are weaker antioxidants [35]. In accordance with Tuck and Hayball [20], glycoside oleuropein (a heteroside of elenolic acid), 3,4-DHPEA and p-HPEA present the highest amount (around 90%) of EVOO phenolic compounds. Comparatively, in the present work, 3,4-DHPEA-EA appeared in good amount with a total average of 615.83 and 367.01 mg kg−1, followed by elenolic acid 166.66 and 184.49 mg kg−1 and p-HPEA-EA with a total average of 136.92 and 89.90 mg kg−1 for olive oil in 2018 and 2019, respectively. Such data are higher with respect to the ones recently reported for five Algerian cultivars [41].
In accordance with some Moroccan findings [39, 40], our investigation on the two crop years revealed that p-HPEA amount was higher than 3,4-DHPEA within to phenolic alcohols class. Such an aspect is linked to the age of the studied e.g. Di Stefano and Melilli [42] proved that during olive oil aging, secoiridoids are transformed into hydrophilic substances.
With regards to flavonoids, luteolin was the highest compound (7.06 and 9.32 mg kg−1 for 2018 and 2019, respectively), followed by luteolin glucoside (4.32 and 1.12 mg kg−1, respectively for the two crop years). Likewise, Boskou [35] reported some research work that found around 0.2–7 mg kg−1 of luteolin in Greek VOO, while others reported a value of 10 mg kg−1 in selected Spanish VOO.
Tocopherols (Vitamin E)
Tocopherols are fat-soluble compounds consisting of a single benzene ring with a hydrocarbon tail attached [43]. They are recognized for their dual beneficial action: the advantage of being the vitamin E occurring in human blood and tissues, as well as the possession of vigorous anti-oxygen activity [35]. From the eight E-vitamers (tocopherols (α, β, γ, δ) and tocotrienols (α, β, γ, δ)), α-tocopherol is found in the free form [13] and alone accounts for 90% of all tocopherols. In the EVOO samples investigated in this work, five vitamin E isomers (α, β, γ, δ tocopherol and α-tocotrienol) were detected. Total vitamin E ranged between 38.4 (Sp10)-213.0 mg kg-1 (Sp14) for 2018 and between 48.4 (Sp2)-147.3 (Sp7) for 2019, with an average of 139.8 ± 36.46 and 92.60 ± 30.00 mg kg−1, respectively. α-tocopherol was the most abundant isoform (91.5% and 87% of total vitamin E, respectively for 2018 and 2019) with an average of 122.7 ± 36.84 and 80.39 ± 27.68 mg kg−1, a range of 32.9 ± 0.4 (Sp10)-201.1 ± 0.08 (Sp14) and of 33.9 ± 0.4 (Sp10)-129.2 ± 0.4 (Sp7). It was followed by γ-tocopherol (7 and 6.5%) with a total average of 6.6 ± 1.46 and 6.06 ± 2.48 mg kg−1, for 2018 and 2019, respectively. However, the abundance order of β-tocopherol and α-tocotrienol is dissimilar between the 2 years; for 2018 season: β-tocopherol was more abundant than α-tocotrienol (1%), and an inverted trend can be appreciated for 2019 olive oils. δ-tocopherol was found in trace, with an average of 0.66 ± 0.15 and of 0.47 ± 0.34 mg kg−1 for 2018 and 2019, respectively.
In general the results attained match those observed in earlier studies and, specifically, the average amount of α-tocopherol fits with that fixed by USDA [44]. In addition, wide ranges of α-tocopherol have been reported in Italian, Spanish, and Greek oils, and the typical values are from 100 to 250 mg kg−1 oil [35]. Such results agree with the ones found by Różańska et al. [45] who investigated bioactive compounds in Italian EVOO and revealed a total vitamin E ranging between 70.2 and 232.2 mg kg−1, with an average of 169.0 ± 37.7 mg kg−1, as well, α-tocopherol was the major tocopherol isomer. Moreover, the finding of δ-tocopherol seems to be consistent with that of the “Picholine Marocaine” shown in ref. [46], in which the δ-tocopherol was reported as 0.5 mg kg−1. Also, Bedbabis et al. [47] found amounts of δ-tocopherol of 0.42 and 0.35 mg kg−1 in oils from the olives irrigated with well water and treated wastewater, respectively. Further, in the case of Greek VOO, Psomiadou et al. [48] reported a mean value for δ-tocopherol of 4 mg kg−1.
Pedoclimatic conditions
Table 2 provides the climatic and edaphic conditions in the study sites. As can be seen Sp10 was collected from a heavy rainfall site (639 mm); in contrast, Sp18 had the lowest value of precipitation (145 mm). The yearly temperature average was between 14.04 and 18.22 °C and the wind speed was within 1.7–4.34 m/s.
The particle size is a pivotal variable in the agriculture field. The percentage of sand in our samples ranged between 1.64% (Sp2) and 57.19% (Sp11), which is within the values (≤ 75%) established by the IOC [49], whereas silt and clay fractions were above the standard values. Exhaustively, 53% of the samples were Silt Loam, 21% are Silty Clay Loam, 16% Clay Loam, and about 5% Sandy Loam, and Silty Clay textures. Such balanced proportions seem to be favorable for olive growing.
The most prominent finding to emerge is that soil physicochemical parameters, particularly pH (except for Sp1), electrical conductivity (EC) and limestone were within the optimal values for olive growing areas [49]: pH varied in a range between 5.43 (Sp1) and 8.84 (Sp16) and EC values did not exceed 2dS/m. Furthermore, the organic matter ranged from 0.57 to 3.29% with 84% of samples > 1% (the optimal). Potassium amounts were within the range of 40.68–393.32 ppm, and the C/N ratio between 5.35 and 32.38 indicating the wide disparity between the sampling sites.
The influence of geographical origin
Geographical origin regroups three main parameters; location (latitude, longitude, and altitude), soil composition, and climate that encompasses temperature, rainfall, humidity, and wind speed. By nature, the environment differs from one site geographically to another, though weather conditions are changing speedily due to global warming. Soil is a surface rich in organic and mineral constituents, where plants and microorganisms nourish and find their needs in essential elements. As this latter is prominent in feeding plants, its texture is vital for the tree roots and the necessary water infiltration. Therefore, soil composition variability could influence olive tree, fruit, and thus EVOO quality. Considering the variability of all these parameters, we attempted to investigate their impact on VOO bioactive profile through the correlation test and the PCA.
In fact, to study the impact of pedo-climatic factors, EVOOs were collected during the same period (November 2018) and from the same cultivar “Picholine Marocaine", but different geographical origins characterized by the distinct location and climate. The latters have a direct relationship with the fruit ripeness thus the maturation factor was considered as similar to that of the geographic origin’s climate. This is in agreement with Bajoub and his co-authors [50, 51] who dismissed the ripening index while examining the geographic origin discrimination in EVOOs from “Picholine Maroccaine” in a large Northern Moroccan area.
Principal component analysis (PCA)
Based on the results of the correlation test (Table S2), the PCA was plotted. Figure 2 presents the distribution of variables and observations (19 × 25 matrix). Five principal components were extracted because their cumulative variance was 54.4% PC1 × PC2 and PC1 × PC3 were plotted since they were sufficient for the interpretability of the major correlative links between the studied variables. The two first eigenvectors carry 43.8% of dataset information, dimensions 1 and 2 had, respectively, 26.2 and 17.6% of affinity between VOO phytochemical compounds, soil, climate, and geographical origin parameters. Together, Dim 1 (26.2%) and Dim 3 (10.6%) carried 36.8% of information. The relatively low variations in the data described by PC1 and PC2 could be caused by the low variability of the parameters.
Variables score plots. Top (PC1 x PC2) and bottom (PC1 x PC3) of VOO phenolic fraction, pedo-climate, and geographical origin parameters of the study zone. G.Ac: gallic acid, HY-Decarboxymethyl-E.Ac: hydroxydecarboxymethyl elenolic acid, Lut: luteolin, Api: apigenin, HY-Decarboxymethyl-Ol.Ag: hydroxy decarboxymethyl 3,4-DHPEA-EA, Dehydro. Ol.Ag: Dehydro 3,4-DHPEA-EA, DEDA: decarboxymethyl elenolic acid dialdehyde, Methyl-Ol.Ag: Methyl 3,4-DHPEA-EA, A-tocopherol: α-tocopherol, B-tocopherol: β-tocopherol, G-tocopherol: ϒ-tocopherol, D-tocopherol: δ-tocopherol. Cond: conductivity, CaCO3: limestone, K: available potassium, WS.2 M: wind speed at 2 m, RH.2 M: Relative humidity at 2 m
As shown in Fig. 2A, the group of variables chosen for the PCA analysis was well scattered along with the two first components, and the most striking result to emerge is that latitude, rainfall, and longitude contribute negatively to Dim 1 revealing a negative effect on α-tocotrienol and flavonoids (luteolin, apigenin, and luteolin glucoside). In contrast, these latter compounds were influenced positively by conductivity. Investigating the effect of location on VOO of two main Tunisian olive cultivars, “Chemlali” and “Chétoui” in three different sites, Guerfel et al. [29] concluded that EVOOs obtained from the two varieties showed different characteristics in the three locations studied. Similarly, Romero et al. came to an end that latitude enlarges the effect of the geographical origin [52]. Some studies carried out on other matrices confirmed the association between salinity (conductivity) and phenolic compounds, mostly flavonoids, e.g. Neocleous et al. [53] observed that in some instances the use of saline waters enhances health-beneficial phenolic compounds in baby leaves of lettuce. Besides, Talhaoui et al. [54] reported that salinity stress associated with high sunlight reveal an enhancement of the biosynthesis of other phenolic compounds, particularly flavonoids.
On the other side, the second component provided other correlative links: relative humidity, wind speed, and C/N ratio showed a positive correction to flavoniods and alpha-tocotrienol. Consequently, they correlated positively to methyl 3,4-DHPEA-EA and impact in a negative way hydroxy decarboxymethyl 3,4-DHPEA-EA, α, β, and γ-tocopherol. Via PC1 × PC3 variables plot (B), negative relationships were observed: the percentage of soil limestone (CaCO3) influenced δ-tocopherol amount. Likewise, soil parameters (pHwater, and available potassium) and two bioactive compounds (gallic acid and hydro decarboxymethyl elenolic acid) were linked negatively. Crucially, soil pH is the most substantial property, considering its effects on all other soil variables [55]. Investigating the influence of pH on plant phenolic compounds stability, Friedman and Jürgens [56] demonstrated that gallic acid was not stable to high pH, and this explains the high amount of gallic acid correctly in VOO from Sp1 characterized by slightly acidic soil (5.43 ± 0.13).
Regarding climatic parameters, the present findings seem to be consistent with other research papers, which found that the cumulative rainfall appears to significantly influence phenolic and o-diphenol content [57]. Moreover, between the tree cultivation zones studied in Guerfel’s paper [29], different phenolic profiles were observed, and the authors supposed the provenance of this result from the climate and particularly the rainfall. In contrast to earlier findings, Proietti and Regni [58] reported that the influence of summer and autumn temperatures on the EVOO phenols was higher in the ones produced in cold environments, while Romero et al. [57] reported the effect of the minimum temperatures during the harvest period in the case of α-tocopherol. However, no correlation was found between bioactive compounds and temperature in our results.
Additionally to PCA, the correlation matrix (Table S2) highlighted the positive influence (r = 0.51, p < 0.05) and (r = 0.53, p < 0.05) of silt percentage on α and β-tocopherol, respectively. However, it had a negative impact (r = −0.58, p < 0.01) on decarboxymethyl elenolic acid dialdehyde (DEDA). The findings observed mirror those of the precedent studies that examined the effect of soil on EVOO quality: Rouas et al. [28] suggested that the limestone percentage of soil was the cause of the quantitative differences in all analyzed EVOO parameters, Rached et al. [31] claimed that different soil types affected total phenols, and, finally, Ranalli et al. [59] evaluated the skeleton percentage of soil and its positive correlation with EVOO phenols content. Moreover, the test exhibited a significant positive correlation (r = 0.61, p < 0.01) between altitude and dehydro 3,4-DHPEA-EA. Somehow, our results support previous findings in the literature considering how phenols and stability correlate positively with the zone elevation of the olive tree [27]. Nonetheless, working on “Mastoides” olive, Mousa et al. [60] showed a negative correlation between phenol compounds and altitude.
To summarize, the correlation test and PCA revealed several exciting relationships: all the vitamin E isomers, two phenolic acids (gallic acid and hydroxy decarboxymethyl elenolic acid), flavonoids (luteolin, apigenin, luteolin glucoside), and four secoiridoids and derivate (dehydro 3,4-DHPEA-EA, methyl 3,4-DHPEA-EA, hydroxy decarboxymethyl 3,4-DHPEA-EA, decarboxymethyl elenolic acid dialdehyde (DEDA) were linked to location (latitude, longitude, and altitude), climate (except for temperature), and some soil parameters: texture (the percentage of silt), pHwater, conductivity, available soil potassium, limestone (CaCO3), and C/N ratio. Nevertheless, no correlation was detected between phenolic alcohols and other variables under study.
Hierarchical cluster analysis of principal components (HCPC)
A cluster dendrogram was conducted to classify the sampling sites which share common characteristics regarding EVOO bioactive compounds and the geographical conditions. The dendrogram (Fig. 3) suggested a 5-group solution. The first cluster included one sampling site (Sp10), distinguished by the highest values of C/N ratio, methyl 3,4-DHPEA-EA, and limestone (CaCO3), also the lowest amounts of α, β and γ-tocopherol. The variables hydroxy decarboxymethyl elenolic acid, dehydro 3,4-DHPEA-EA, gallic acid, and available potassium were most significantly associated with cluster 2 (Sp1, Sp9, and Sp11). Comparing the averages of these variables to the overall average in all clusters, it can be concluded that cluster 2 was characterized by high amounts of bioactive compounds mentioned previously and low rates of available potassium. Additionally, a high similarity was observed between Sp9 and Sp11.
Dendrogram of the hierarchical cluster analysis of principal components (HCPC) using Ward’s criterion on the first and the second principal components. The grey dashed rectangles points out the cut in the tree, resulting in five clusters: (1, Red), (2, Yellow), (3, Green), (4, Blue), and (5, Purple)
Cluster 3 regrouped seven sampling sites (from Sp2 to Sp8) mainly differentiated by heavy rainfall and low elevations. Further, it was divided into two significant sub-clusters. For instance, Sp2 and Sp8 contained a high percentage of CaCO3 and showed zero value in decarboxymethyl elenolic acid dialdehyde. Moreover, Sp3, Sp5, and Sp7 provided high similarity in sand percentage. Cluster 4 contained two significant sub-clusters and six sampling sites (from Sp13 to Sp17), Sp13 and Sp14 revealed the highest amount of hydroxy decarboxymethyl 3,4-DHPEA-EA among all the studied sites. Besides, Sp12 and Sp16 had the lowest percentage of CaCO3 among all the zones studied and the lowest β-tocopherol among the sampling sites of the same cluster. Generally, this cluster is distinguished by high amounts of β -tocopherol, hydroxy decarboxymethyl 3,4-DHPEA-EA, and α-tocotrienol. On the contrary, CaCO3, rainfall and relative humidity are in low value. Comparing the average of the fifth cluster variables to the overall averages, a high rate of wind speed, apigenin, luteolin glucoside, methyl 3,4-DHPEA-EA, conductivity, and a low quantity of γ-tocopherol and rainfall was observed.
On the whole, most of the classified groups showed very close geographical neighbourhoods; except only the zones of clusters one and two, all the other fifteen sampling sites were clustered geographically very well. Briefly, the significant discrimination observed between the sampling areas in terms of phytochemical compounds composition and location conditions indicate the strong impact of the production zone on the Moroccan EVOO quality. Interestingly, our findings could enable the geographical traceability of EVOO quality in the country.
Crop season impact
As mentioned in several studies, the crop year could have a significant influence on the quality of the EVOO produced. In our turn, we sought to assess the impact of the production year on the bioactive fraction in Moroccan EVOO of “Picholine Marocaine”. The histograms in Fig. 4 show comparative analysis, and the repeated-measures ANOVAs in Fig. 5 provide the summary statistics.
Phenolic compounds and tocopherol content expressed in mg kg−1 in two successive crop years 2018/2019 from 19 Moroccan areas. Values represent average ± standard deviation (n = 3). Similar letters indicate no significant (p ≥ 0.05) difference between treatments, whereas all paramters have shown a significance with (p ≤ 0.0001). Two-way ANOVA was performed to evaluate crop year, site, and their interaction effects. sampling [Chefchaouen (Sp1), Ouazzane (Sp2), Sidi Kacem (Sp3), Meknes (Sp44, Sp5), Taounate (Sp6), Taza (Sp7), Fes (Sp8), Sefrou (Sp9), Boulmane (Sp10), Khenifra (Sp11), Beni Mellal (Sp12, Sp13), Azilal (Sp14), Kella di Sraghena (Sp15, Sp16), Marrakech (Sp17), Chichaoua (Sp18) and Essaouira (Sp19)]
As shown in the graph above (Fig. 4), the two crop seasons revealed an evident diversity concerning the total average of the phenol groups and Vitamin E. Except for flavonoids, all the other classes showed significant variability. The post hoc analysis confirmed these observations; the effects of crop seasons, sampling sites and their interaction on vitamin E and phenolic compounds were significant (p < 0.0001).
Besides, these tremendous distinctions can be seen clearly in Fig. 5. For instance, polyphenol amounts in Ouazzane (Sp2) are highly different between 2018 and 2019 crops years. Equally, oils from Azilal (Sp14) showed significant differences with regard to tocopherols. EVOOs from different sites shared quantitative resemblance in the same crop year. In terms of phenolic compounds values from: Fes and Azilal, Essaouira and Khenifra, and Chefchaouen, Marrakceh, and Sefrou were similar in 2018 crop year, whereas, values from: Chefchaouen, Taza and Taounate were similar for 2019 season. In terms of tocopherols, Beni Mellal, Sefrou, Essaouira and Fes contained a similar quantity in 2018, whereas El Kelaa des Sraghna, Khenifra, and Sefrou were similar for the 2019 crop season.
However, considering the different harvest time during the two successive years conducted in November and December, respectively, the dissimilarity observed can be explained in part by the different harvest periods of the olive fruits.
Consistent with the present results, previous studies demonstrated a significant dependence between bioactive compounds and production season; evaluating the variations in the quality of EVOO for two crop seasons and from three different varieties (Arbequina, Arbosana, and Koroneiki) grown in Morocco, Mansouri et al. [61] came to an end that phenols and oxidative stability were linked to the crop season of production, Romero et al. [57] found a considerable variability with a significant effect of the year (p < 0.001), as well as, Bajoub et al. [39]. On the other side, some authors observed minor incidence of the crop season. Nonetheless, they suggested that rather than the year weather, the fruits harvest period in each season impacted the antioxidant conditions decreasing as a consequence of the olive fruits ripeness [62]. Notably, tocopherol amounts were reported to decrease in ripe fruits [63]. Similarly, according to Dag et al. [64], the late harvest was associated with a rapid decline in phenolic content.
Conclusion
This study set out to determine the various impact of edaphic, climatic conditions and the crop year on the antioxidant fraction of olive oil produced in nineteen Moroccan production areas over two successive years, 2018/19 and 2019/20.
The Moroccan olive oil samples disclosed 23 hydrophilic and five lipophilic phenolic compounds. Expectedly, our findings support the relevance of the geographical origin impact, executed in previous studies [2, 27]. Significantly, the correlation test and the PCA revealed new relationships between some bioactive compounds and the geographical zone parameters, especially climatic conditions and pedologic variables that unfold remarkable influence. Furthermore, the second notable finding was the quantitative impact of the crop year on the classes of phenolic compounds and tocopherols: the two-way ANOVA results showed significant variations in phytochemical compound amounts over the 2 years of study. In addition to that, the harvest time significantly influenced the EVOO phenolic fraction.
All in all, the results attained in this work confirm preceding findings providing additional evidence on the tremendous impact of edaphic conditions on Moroccan EVOO quality. The findings achieved are encouraging and may be extended to a larger sample size.
References
Besnard G, Breton C, Baradat P, Khadari B, Bervillé A (2001) Cultivar Identification in Olive Based on RAPD Markers. J Am Soc Hortic Sci 126:668–675. https://doi.org/10.21273/JASHS.126.6.668
Bajoub A, Hurtado-Fernández E, Fernández-Gutiérrez A, Carrasco-Pancorbo A, Ouazzani N (2015) Quality and chemical profiles of monovarietal north Moroccan olive oils from “Picholine Marocaine” cultivar: registration database development and geographical discrimination. Food Chem 179:127–136. https://doi.org/10.1016/j.foodchem.2015.01.101
Gargouri K, Sarbeji M, Barone E (2006) Assessment of soil fertility variation in an olive orchard and its influence on olive tree nutrition. In: Second international seminar on biotechnology and quality of olive tree products around the mediterranean basin, pp 5–10
Akesbi N (2006) Evolution et perspectives de l’agriculture marocaine. Rapport 50:85–198
MAPMDREF (2014) Filière Oléicole, Département de l’agriculture. http://www.agriculture.gov.ma/pages/acces-fillieres/filiere-oleicole. Accessed 24 Sept 2020
IOC (2012) General discription of olive growing in Morocco 1–10
IOC (2019) The olive grove in Morocco. J Int Olive Counc 1–89
MAPMDREF (2019) Agriculture en chiffres 2018. Accessed 24 Sept 2020
Lozano-Sánchez J, Segura-Carretero A, Menendez JA, Oliveras-Ferraros C, Cerretani L, Fernández-Gutiérrez A (2010) Prediction of extra virgin olive oil varieties through their phenolic profile. potential cytotoxic activity against human breast cancer cells. J Agric Food Chem 58:9942–9955. https://doi.org/10.1021/jf101502q
Bonazzi M (1997) Les politiques Euro-Mediterraneennes et l’huile d’olive: Concurrence ou partage du travail. MEDIT 3:27–32
IOC (2019) IOC, imprinting the future. 1–27
Servili M, Montedoro G (2002) Contribution of phenolic compounds to virgin olive oil quality. Eur J Lipid Sci Technol 104:602–613. https://doi.org/10.1002/1438-9312(200210)104:9/10%3c602::AID-EJLT602%3e3.0.CO;2-X
Boskou D, Blekas G, Tsimidou M (2006) Olive oil composition. Olive oil, 2nd edn. Elsevier, Amsterdam, pp 41–72
Bengana BM, Bakhouche A, Lozano-Sánchez J, Youcef A, Youyou A, Segura Carretero A, Fernández-Gutiérrez A (2013) Influence of olive ripeness on chemical properties and phenolic composition of Chemlal extra-virgin olive oil. Food Res Int 54:1868–1875. https://doi.org/10.1016/j.foodres.2013.08.037
Dugo L, Russo M, Cacciola F, Mandolfino F, Salafia F, Vilmercati A, Fanali C, Casale M, De Gara L, Dugo P, Mondello L, Rigano F (2020) Determination of the phenol and tocopherol content in Italian high-quality extra-virgin olive oils by using LC-MS and multivariate data analysis. Food Anal Methods 13:1027–1041. https://doi.org/10.1007/s12161-020-01721-7
Fanali C, Della Posta S, Vilmercati A, Dugo L, Russo M, Petitti T, Mondello L, De Gara L (2018) Extraction, analysis, and antioxidant activity evaluation of phenolic compounds in different Italian extra-virgin olive oils. Molecules 23:3249. https://doi.org/10.3390/molecules23123249
Klikarová J, Rotondo CF, Česlová L, Dugo P, Mondello L, Rigano F (2019) The phenolic fraction of Italian extra virgin olive oils: elucidation through combined liquid chromatography and NMR approaches. Food Anal Methods 12:1759–1770. https://doi.org/10.1007/s00217-020-03484-1
Fanali C, Della Posta S, Dugo L, Russo M, Gentili A, Mondello L, De Gara L (2020) Application of deep eutectic solvents for the extraction of phenolic compounds from extra-virgin olive oil. Electrophoresis 41:1752–1759. https://doi.org/10.1002/elps.201900423
Klikarová J, Česlová L, Kalendová P, Dugo P, Mondello L, Cacciola F (2020) Evaluation of Italian extra virgin olive oils based on the phenolic compounds composition using multivariate statistical methods. Eur Food Res Technol 246:1241–1249. https://doi.org/10.1007/s00217-020-03484-1
Tuck KL, Hayball PJ (2002) Major phenolic compounds in olive oil: metabolism and health effects. J Nutr Biochem 13:636–644. https://doi.org/10.1016/S0955-2863(02)00229-2
Emma MR, Augello G, Di Stefano V, Azzolina A, Giannitrapani L, Montalto G, Cervello M, Cusimano A (2021) Potential uses of olive oil secoiridoids for the prevention and treatment of cancer: a narrative review of preclinical studies. Int J Mol Sci 22:1234. https://doi.org/10.3390/ijms22031234
Ait Hmida A (2016) Stratégie de valorisation de l’huile d’olive par l’origine et la qualité: évaluation du projet Tyout-Chiadma, première AOP au Maroc
Russo M, Bonaccorsi IV, Cacciola F, Dugo L, De Gara L, Dugo P, Mondello L (2020) Distribution of bioactives in entire mill chain from the drupe to the oil and wastes. Nat Prod Res. https://doi.org/10.1080/14786419.2020.1752208
Boskou D, Blekas G, Tsimidou M (2005) Phenolic compounds in olive oil and olives. Curr Top Nutraceutical Res 3:125–136
Ben-Hassine K, Amani T, Ferchichi S, Mlaouah A, Benincasa C, Romano E, Flamini G, Lazzez A, Grati-kamoun N, Perri E, Malouche D, Hammami M (2013) Physicochemical and sensory characteristics of virgin olive oils in relation to cultivar, extraction system and storage conditions. Food Res Int 54:1915–1925. https://doi.org/10.1016/j.foodres.2013.09.007
Yamani ME, Sakar EH, Boussakouran A, Rharrabti Y (2020) Influence of ripening index and water regime on the yield and quality of “Moroccan Picholine” virgin olive oil. OCL 27:19. https://doi.org/10.1051/ocl/2020015
Issaoui M, Flamini G, Brahmi F, Dabbou S, Ben Hassine K, Taamali A, Chehab H, Ellouz M, Zarrouk M, Hammami M (2010) Effect of the growing area conditions on differentiation between Chemlali and Chétoui olive oils. Food Chem 119:220–225. https://doi.org/10.1016/j.foodchem.2009.06.012
Rouas S, Rahmani M, El Antari A, Baamal L, Idrissi Janati D, Souizi A, Maat N (2016) Effect of geographical conditions (altitude and pedology) and age of olive plantations on the typicality of olive oil in Moulay Driss Zarhoun. Mediterr J Biosci 1:128–137
Guerfel M, Ouni Y, Taamalli A, Boujnah D, Stafanoudaki E, Zarrouk M (2009) Effect of location on virgin olive oils of the two main Tunisian olive cultivars. Eur J Lipid Sci Technol 111:926–932. https://doi.org/10.1002/ejlt.200800108
Tsimidou MZ (2013) Analytical methodologies: phenolic compounds related to olive oil taste issues. In: Aparicio R, Harwood J (eds) Handbook of olive oil: analysis and properties. Springer, Boston, pp 311–333
Ben Rached M, Galaverna G, Cirlini M, Boujnek D, Zarrouk M, Guerfel M (2017) Pedologic factors affecting virgin olive oil quality of “Chemlali” olive trees (Olea europaea L.). J Oleo Sci 66:907–915. https://doi.org/10.5650/jos.ess17066
Ricciutelli M, Marconi S, Boarelli MC, Caprioli G, Sagratini G, Ballini R, Fiorini D (2017) Olive oil polyphenols: a quantitative method by high-performance liquid-chromatography-diode-array detection for their determination and the assessment of the related health claim. J Chromatogr A 1481:53–63. https://doi.org/10.1016/j.chroma.2016.12.020
Laurant A (1974) Abaque simplifié permettant de calculer les pourcentages de fractions fines dans les analyses granulométriques effectuées au densimètre. Ann Société Géologique Belg
Commission Regulation (EU) No 432/2012 (2012) Establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and healthText with EEA relevance. Off J Eur Union 40:281–320
Boskou D (2008) Olive oil: minor constituents and health, 1st edn. CRC Press, Boca Raton
Diamantakos P, Ioannidis K, Papanikolaou C, Tsolakou A, Rigakou A, Melliou E, Magiatis P (2021) A new definition of the term “high-phenolic olive oil” based on large scale statistical data of Greek olive oils analyzed by qNMR. Molecules 26:1115. https://doi.org/10.3390/molecules26041115
Miho H, Moral J, Barranco D, Ledesma-Escobar CA, Priego-Capote F, Diez CM (2021) Influence of genetic and interannual factors on the phenolic profiles of virgin olive oils. Food Chem 342:128357. https://doi.org/10.1016/j.foodchem.2020.128357
Miho H, Díez CM, Mena-Bravo A, Sanchez de Medina V, Moral J, Melliou E, Magiatis P, Rallo L, Barranco D, Priego-Capote F (2018) Cultivar influence on variability in olive oil phenolic profiles determined through an extensive germplasm survey. Food Chem 266:192–199. https://doi.org/10.1016/j.foodchem.2018.06.002
Bajoub A, Hurtado-Fernández E, Ajal EA, Ouazzani N, Fernandez-Gutierrez A, Carrasco-Pancorbo A (2015) Comprehensive 3-year study of the phenolic profile of Moroccan monovarietal virgin olive oils from the Meknès Region. J Agric Food Chem 63:4376–4385. https://doi.org/10.1021/jf506097u
Bouymajane A, Oulad El Majdoub Y, Cacciola F, Salafia F, Trozzi A, Rhazi Filali F, Dugo P, Mondello L (2020) Characterization of phenolic compounds, vitamin E and fatty acids from monovarietal virgin olive oils of “Picholine marocaine” cultivar. Molecules 25:5428. https://doi.org/10.3390/molecules25225428
Boussahel S, Di Stefano V, Muscarà C, Cristani M, Melilli MG (2020) Phenolic compounds characterization and antioxidant properties of monocultivar olive oils from Northeast Algeria. Agriculture 10:494. https://doi.org/10.3390/agriculture10110494
Di Stefano VD, Melilli MG (2020) Effect of storage on quality parameters and phenolic content of Italian extra-virgin olive oils. Nat Prod Res 34:78–86. https://doi.org/10.1080/14786419.2019.1587434
Tocopherols. In: McCord Res. https://mccordresearch.com.au/library/tocopherols/. Accessed 20 Jan 2021
FoodData Central. https://fdc.nal.usda.gov/. Accessed 16 Dec 2020
Różańska A, Russo M, Cacciola F, Salafia F, Polkowska Z, Dugo P, Mondello L (2020) Concentration of potentially bioactive compounds in Italian extra virgin olive oils from various sources by using LC-MS and multivariate data analysis. Foods 9:1120. https://doi.org/10.3390/foods9081120
Wiesman Z (2009) Chapter 11—olive-oil quality biotechnologies. In: Wiesman Z (ed) Desert olive oil cultivation. Academic Press, San Diego, pp 257–302
Bedbabis S, Trigui D, Ben Ahmed C, Clovodeo ML, Camposeo S, Vivaldi GA, Rouina BB (2015) Long-terms effects of irrigation with treated municipal wastewater on soil, yield and olive oil quality. Agric Water Manag 160:14–21. https://doi.org/10.1016/j.agwat.2015.06.023
Psomiadou E, Tsimidou M, Boskou D (2000) α-Tocopherol content of greek virgin olive oils. J Agric Food Chem 48:1770–1775. https://doi.org/10.1021/jf990993o
IOC (2007) Production techniques in olive growing. International Olive Council, Madrid, Spain
Bajoub A (2016) Virgin olive oil: Potential of different omics approaches to authenticate its geographical and botanical origin. Universidad de Granada
Bajoub A, Carrasco-Pancorbo A, Ajal EA, Ouazzani N, Fernandez-Gutierrez A (2015) Potential of LC–MS phenolic profiling combined with multivariate analysis as an approach for the determination of the geographical origin of north Moroccan virgin olive oils. Food Chem 166:292–300. https://doi.org/10.1016/j.foodchem.2014.05.153
Romero N, Saavedra J, Tapia F, Sepulveda B, Aparicio R (2016) Influence of agroclimatic parameters on phenolic and volatile compounds of Chilean virgin olive oils and characterization based on geographical origin, cultivar and ripening stage. J Sci Food Agric 96:583–592. https://doi.org/10.1002/jsfa.7127
Neocleous D, Koukounaras A, Siomos AS, Vasilakakis M (2014) Assessing the salinity effects on mineral composition and nutritional quality of green and red “baby” lettuce: responses of baby lettuce to salinity. J Food Qual 37:1–8. https://doi.org/10.1111/jfq.12066
Talhaoui N, Taamalli A, Gómez-Caravaca AM, Fernandez-Gutierrez A, Segura-Carretero A (2015) Phenolic compounds in olive leaves: Analyticaldetermination, biotic and abiotic influence, and health benefits. Food Res Int 77:92–108. https://doi.org/10.1016/j.foodres.2015.09.011
Nisha C, Barnali M, Shabana P, Meena D (2017) Analysis of soil samples for its physico-chemical parameters from Kadi City. Raman Sci Technol Found 3:36–40
Friedman M, Jürgens HS (2000) Effect of pH on the stability of plant phenolic compounds. J Agric Food Chem 48:2101–2110. https://doi.org/10.1021/jf990489j
Romero MP, Tovar MJ, Ramo T, Motilva MJ (2003) Effect of crop season on the composition of virgin olive oil with protected designation of origin “Les garrigues.” J Am Oil Chem Soc 80:423–430. https://doi.org/10.1007/s11746-003-0715-z
Proietti P, Regni L (2016) Handbook for a sustainable management of the olive groves, vol 9. www.olive4climate.eu
Ranalli A, De Mattia G, Patumi M, Proietti P (1999) Quality of virgin olive oil as influenced by origin area. Grasas Aceites 50:249–259
Mousa YM, Gerasopoulos D, Metzidakis I, Kiritsakis A (1996) Effect of altitude on fruit and oil quality characteristics of ‘Mastoides’ olives. J Sci Food Agric 71:345–350. https://doi.org/10.1002/(SICI)1097-0010(199607)71:3%3c345::AID-JSFA590%3e3.0.CO;2-T
Moumen AB, Belhaj K, Mansouri F, Fauconnier M-L, Richard G, Sindic M, Elamrani A, Serghini Caid H (2018) Effect of crop season on the quality and composition of extra virgin olive oils from Greek and Spanish varieties grown in the Oriental region of Morocco. Emir J Food Agric. https://doi.org/10.9755/ejfa.2018.v30.i7.1738
Baccouri B, Zarrouk W, Krichene D, Nouairi I, Ben Youssef N, Daoud D, Zarrouk M (2007) Influence of fruit ripening and crop yield on chemical properties of virgin olive oils from seven selected Oleasters (Olea europea L.). J Agron 6:388–396. https://doi.org/10.3923/ja.2007.388.396
Preedy VR, Watson RR (2010) Olives and olive oil in health and disease prevention. Academic Press, Cambridge
Dag A, Kerem Z, Yogev N, Zipori I, Lavee S, Ben-David E (2011) Influence of time of harvest and maturity index on olive oil yield and quality. Sci Hortic 127:358–366. https://doi.org/10.1016/j.scienta.2010.11.008
Acknowledgements
The authors thank Merck Life Science and Shimadzu Corporations for their continuous support. This work was supported by the National Center for Scientific and Technical Research of Morocco (CNRST) through the excellence grant under n° 19UAE2018. Miss. T. Lechhab. is grateful to ERASMUS+ KA107 for its financial support of her Ph.D. during her stay at Messina University. The authors are grateful to Mr Lechhab Jilali for his collaboration.
Author information
Authors and Affiliations
Contributions
TL: conceptualization; investigation; formal analysis; validation; writing-original draft; writing-review and editing. FS: supervision; writing-original draft. WL: writing-review and editing; YOEM: data curation, formal analysis, investigation, software, validation. MR: data curation; methodology; writing-original draft preparation. MRTC: investigation; validation. ET: investigation. PD: resources; writing-review and editing; supervision. LM: resources; project administration; FC: conceptualization; methodology; resources; writing-review and editing; supervision.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that there is no conflict of interest.
Compliance with ethics requirements
This study does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lechhab, T., Salmoun, F., Lechhab, W. et al. Determination of bioactive compounds in extra virgin olive oils from 19 Moroccan areas using liquid chromatography coupled to mass spectrometry: a study over two successive years. Eur Food Res Technol 247, 2993–3012 (2021). https://doi.org/10.1007/s00217-021-03842-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-021-03842-7