Abstract
Physicochemical, functional, and nutritional properties of flours from six navy bean varieties cultivated in two different locations in Manitoba, Canada were evaluated. The effect of genotype was found insignificant for protein content, ash content, zeta potential, and surface hydrophobicity among physicochemical properties (p > 0.05). For protein content, total starch content, and zeta potential of navy bean flours, environmental factors were found significant (p < 0.05). Genotype, environment, and genotype × environmental interactions were determined as significant for water holding capacity as well as for trough and final viscosities of flours. There was no significant effect on the pasting temperature of flours (p > 0.05). In vitro protein digestibility results varied by genotype. Although in vitro protein digestibility corrected amino acid score values were significantly affected by genotype, environment, and their interactions similar to slowly digestible starch. The current study established a better understanding of the varietal effects of genotype and environment on a wide range of properties of navy bean flours.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Navy bean is a variety of common beans (Phaseolus vulgaris L.) belonging to the Fabaceae (Leguminosae) family. Dry beans provide essential nutrition globally for a relatively low cost due to their richness in protein, carbohydrates, dietary fiber, and micronutrients. Navy beans are small-sized, white-colored, oval-shaped beans with tremendous health benefits, including putative abilities to inhibit colon carcinogenesis [1], lower the risk of obesity, total cholesterol, and cardiovascular risk factors [2]. In developing countries, legumes and cereals constitute a significant portion of the daily diet of lower income families [3]. In addition, market trends and customer-driven food formulations have led to more new food products containing pulse fractions (flours, protein concentrates, protein isolates, and starch). Legume flours have been used as binders [4], extenders [5], and enrichers of cereal products, such as bread, biscuits, pasta, and extruded snacks [6] in food formulations.
Pulses typically contain 18–28% protein, 1–1.5% lipids (except chickpea, 4–5%), and 60–65% carbohydrates [7]. However, the type and functionality of pulse proteins play a special role in processed food products [6]. Globulins classified as 7S (vicilin) and 11S (legumin) are the major storage proteins in pulses. The differences in the legumin:vicilin (Lg/Vn) ratio may affect the functional properties of pulse proteins, including interfacial behaviours [8, 9]. Moreover, genetic factors and extrinsic factors, such as agronomical practices, climatic factors, and environmental factors alter protein composition and content in pulses [10]. In the previous studies, genotype and environment effects on the functionality of faba bean protein concentrates [11], the starch fraction of field pea and faba bean [12] and faba bean protein isolates [13] were examined. In addition, Balasubramanian et al. [14] studied the genotype and environment effect on canning quality of selected navy, black and pinto beans and reported significant cultivar effects on most canning quality traits. However, limited studies have been published regarding the impact of genotype and environment on the composition, functionality and protein quality of navy beans cultivated in Canada.
Raw material standardization is essential in food formulations for the industry. Therefore, information on the possible effect of varietal and environmental factors on the functional and nutritional characteristics of edible bean flours is beneficial for food product designs. It was hypothesized that genetic factors would be significantly important in the compositional and functional properties of pulse flours. Therefore, in this study, six navy bean varieties grown at two different locations with different temperature and precipitation in Manitoba, Canada in 2019 were investigated to determine the effect of variety and environment and their interactions on the composition, physicochemical properties, functionality, and nutritional properties of edible bean flours, in relation to their potential usage in food formulations.
Materials and methods
Materials
Six popular varieties of navy bean (Portage, Nautica, T9905, Bolt, Indi, Envoy) were grown in three replications in a randomized complete block design at two locations (Morden, 49°11′15.6″ N–98°04′34.5″ W; and Portage la Prairie, 49°57′31.6″ N–98°16′27.4″ W) in Manitoba, Canada, in 2019. Each cultivar was planted in four rows with 5 m in row length and 75 cm in row spacing, and with 125 seeds planted per row. The fields were maintained according to commercial production standard procedures. The growing season weather conditions at the two sites for 2019 (June 1–Sept 15) were as follows: 18.4 and 17.9 °C mean temperature, 246.8 and 195.9 mm total precipitation, and 2260 and 2176 total crop heat unit, for the Morden and Portage la Prairie sites, respectively. The Morden site was slightly warmer and had more precipitation than Portage la Prairie. All four rows were harvested at natural maturity for quality analysis. Broken and damaged seeds and foreign materials were handpicked from the samples before testing commenced.
The total starch assay was purchased from Megazyme International Ltd. (Co. Wicklow, Ireland). All other chemicals were at least reagent grade and purchased from Sigma-Aldrich (Oakville, ON, Canada). Millipore Milli-QTM water purification system was used for deionized water.
Proximate composition
Moisture, ash, and crude fat analyses of navy bean flours were carried out according to the AOAC method 925.10, 923.03, and 920.85, respectively [15]. Nitrogen content was analyzed according to the Dumas combustion method by a Nitrogen/Protein Analyzer (CN628, LECO Corp., St. Joseph, MI, USA), then converted to crude protein content (%N × 6.25) regarding AACC Method 46-30.01 [16]. The total starch content of flours was determined according to the AACC method 76-13.01 using Megazyme Total Starch Kit [16]. The data from all the analyses were reported on a dry basis (db) of the flours.
Total phenolic content
The total phenolics of navy bean flours were determined using Folin–Ciocalteu reagent according to the method of Velioglu et al. [17]. 1 g of flour was weighted into a 50 ml centrifuge tube and 5 mL of 1% HCl in methanol was added. The sample tubes were placed on a rotating shaker for 2 h at room temperature. Then, samples were centrifuged at 1050×g for 10 min at room temperature. Extraction repeated 2 more times and supernatants were collected. 0.75 ml of Folin Ciocalteu Reagent (diluted tenfold with distilled water) was added to 100 µl of extract in a glass tube. Tubes allowed to stand 5 min at room temperature. After that 0.74 mL of sodium bicarbonate (60 g/L) was added to sample tubes and incubated 90 min at room temperature. The absorbance was measured at 725 nm via UV/VIS spectrophotometer (Genesys 10, Thermo Scientific, Fair Lawn, NJ, USA). The results are expressed as gallic acid equivalents.
Surface charge (zeta potential)
The surface charge of samples was determined by measuring the electrophoretic mobility (UE) using a Zetasizer Nano (Malvern Instruments, Westborough, MA, USA) according to Chang et al. [18]. Flour dispersions were prepared with distilled water at a concentration of 0.05% (w/w) based on flour weight. The dispersion was adjusted to pH 7.0 with 0.1 M HCl or NaOH and stirred overnight at 4 °C to ensure the dissolution of particles. The pH of dispersions was adjusted to pH 7.0 prior to analysis.
Surface hydrophobicity
Surface hydrophobicity was determined according to the method of Kato, Nakai [19]. Briefly, all flours (0.025% w/w, based on protein weight) were dissolved in 10 mM sodium phosphate buffer (pH 7.0) and stirred overnight at 4 °C. After that, 0.005–0.025% dilutions were prepared in 10 mM sodium phosphate buffer (pH 7.0). For each concentration, 1.6 mL of sample were mixed with 20 µL of 8 mM 8-Anilino-1-naphthalenesulfonic acid (ANS) solution [dissolved in 10 mM sodium phosphate buffer (pH 7.0)], then samples were vortexed for 10 s and stored in the dark for 5 min. Fluorescence intensity (FI) was measured using a FluoroMax-4 spectrofluorometer (Horiba Jobin Yvon Inc., Edison, NJ, USA) with excitation and emission wavelengths of 390 and 470 nm, respectively. FI values of ANS blank and protein blanks were subtracted from the FI of the protein solutions containing ANS. The initial slope (S0) of FI against protein concentration was calculated by linear regression analysis and used as an index of the protein surface hydrophobicity.
Legumin/vicilin ratio
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions was performed for all flour samples to quantify the relative proportions of legumin and vicilin (Lg/Vn ratio). Fractions were separated according to the method of Laemmli [20]. Protein samples, 1% (w/w), were solubilized in water, and 30 µL of solution was then added to 30 µL of 2 × SDS-PAGE sample buffer (20 mM Tris–HCl buffer at pH 7.6, 10% SDS solution, 2% \(\beta\)-mercaptoethanol, 50% (v/v) glycerol and 0.01% bromophenol blue) and vortexed for 20 s. Samples were then heated for 10 min in an 85ºC water bath, followed by centrifugation at 12,000×g for 5 min. For electrophoresis, 30 µL of the protein–Laemmli sample (0.5% protein v/v) was loaded into a prepared 15% separating gel, and 4% stacking gel and samples were run using an MGV-202 Vertical Mini-Gel System (CBS Scientific, San Diego, CA, USA) for ~ 1.5 h at 120 V and 40 mPa using a Power Source 300 V Electrophoresis Power Supply (VWR, Mississauga, ON, Canada). BLUelf Prestained Protein Ladder (FroggaBio Scientific Solutions, Toronto, Ontario, Canada) ranging from 5 to 245 kDa were used as molecular weight markers. Subsequently, the gel was fixed for 2 h in gel fixing solution containing 50% methanol, 10% glacial acetic acid and 40% water, followed by staining with 0.1% Coomassie blue stain for 2 h, and then de-stained for 4 h with 3:6:1 methanol:water glacial acetic acid (v:v:v) solution. The protein bands were then imaged, where images are used to estimate the molecular weight determination against a set of standards. Protein bands were quantified using implied densitometry using ImageJ® software (National Institutes of Health Bethesda, Maryland, USA). All samples were run in triplicate. The protein bands were measured via volume, where volume was determined by the sum of pixel intensity for all pixels in each section.
Fourier transform mid-infrared (FT-MIR) spectroscopy
The navy bean flour samples were prepared for FT-MIR in the form of pressed potassium bromide (KBr) pellets, with sample material representing 1.1–1.3% of total weight of the KBr pellets. 4.7–5.1 mg of sample were cryogrinding and homogenization with 399.3–401.4 mg of KBr with the use of a Spex SamplePrep Geno/Grinder 2010. Three replicates for each sample mixed KBr with a weight of 97.4–99.0 mg were measured for each sample. 13 mm KBr pellets were pressed utilizing an automated hydraulic press (AutoCrushIR PIKE Technologies Inc., Madison, WI, USA). The FT-MIR data were collected using an Agilent Technologies (Cary 670 series, Agilent Technologies Inc., CA, USA) microscope equipped with a bulk analysis accessory and thermoelectrically cooled Deuterated Lanthanum α-Alanine-doped TriGlycine Sulphate (DLaTGS) detector at the mid-IR beamline at the Canadian Light Source, Saskatoon, Canada. The sample chamber was purged with dry nitrogen to minimize the interference from water vapor and carbon dioxide absorption bands. The MIR data in the spectral range from 4000 to 600 cm−1 wavenumbers at a spectral resolution of 2 cm−1 were recorded with an average of 32 scans per sample. Background spectrum (average of 32 scans) of the instrument was recorded after every 18 samples using a pure KBr pellet.
The data analysis of the FT-MIR data were carried out using Quasar (version 0.5.6) (10. 5281/zenodo.4287478) software. The second derivative spectra were calculated using a Savitzky–Golay algorithm with 9-point smoothing to determine peak position for peak fitting. The amide I region (1600–1700 cm−1) was deconvoluted through peak fitting utilizing the LMFIT python package (version 1.0.1).
Functional properties
Protein solubility
Protein solubility of navy bean flours was determined according to the modified method of Morr et al. [21]. Briefly, 1% (w/w) of flour (based on protein weight) was dispersed in distilled water. The pH of the suspension was adjusted to 7.0 with 0.1 M HCl or NaOH, followed by stirring overnight at 4 °C. The suspension was then centrifuged at 4430×g for 10 min at room temperature. 5.0 g of the supernatant was used for nitrogen and protein (N% × 6.25) determination according to AOAC method 960.52 [15]. Protein solubility was calculated using the following equation (Eq. 1)
Water hydration and oil holding capacities
Water hydration capacity (WHC) and oil holding capacity (OHC) of all flours were determined according to AACC method 56–30.01 [16] and Nidhina and Muthukumar [22] with slight modifications, respectively. In brief, 0.5 g of flour was suspended in 5 g of distilled water (or 5 g canola oil for OHC) in a 10 mL centrifuge tube. The tube was vortexed for 10 s every 5 min for 30 min. The sample was centrifuged at 1000 × g for 15 min followed by decating the supernatant. The weight of sediment was recorded. WHC and OHC values were calculated in g/g using Eq. 2.
Foaming properties
Foaming capacity (FC) and foaming stability (FS) were determined by using the method of Bai et al. [23]. Briefly, 1% (w/w) flour suspensions were prepared and adjusted to pH 7.0 using 0.1M HCl or NaOH prior to stirring overnight. The solution pH was checked and adjusted to 7.0 before the homogenization. 15 mL of the solution was transferred to 400 mL beaker to homogenize using Omni Macro Homogenizer (Omni International, Marietta, GA, USA) equipped with a 20 mm sawtooth probe at speed 4 for 5 min. Then, the sample was transferred to 50 mL graduated cylinder, and the foam volume was recorded at 0 and 30 min. FC and FS were calculated based on Eqs. 3 and 4, respectively.
Emulsification properties
Emulsion activity (EA) and stability (ES) of bean flours were analyzed according to the modified method of Yasumatsu et al. [24]. One gram of flour was suspended in 14.3 mL of Milli Q water and 14.3 mL of canola oil. The suspension was homogenized in 10,000 rpm for 1 min using Omni Macro Homogenizer (Omni International, Marietta, GA, USA) with a 20 mm sawtooth probe. 10 ml of the emulsion was placed in 15 mL centrifuge tubes and centrifuged at 1300×g for 5 min. EA was determined by dividing the height of emulsion layer after the centrifugation by the total height before the centrifugation. ES was determined similarly to EA with a difference that the emulsion was heated at 80 °C for 30 min in a water bath.
Pasting properties
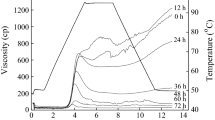
Pasting properties of all flours were measured using a Rapid Visco-Analyzer (RVA Super 3, Newport Scientific, Sydney, Australia) according to the method of Ai et al. [25]. The flours were suspended in distilled water as a total weight of 28.0 g (8% dry solids content) and analyzed using Standard Method 2 in the Thermocline Software. The suspension was equilibrated for 1 min at 50 °C, heated at a rate of 6 °C/min to 95 °C, kept at 95 °C for 5 min, and cooled at a rate of 6 °C/min to 50 °C.
Protein quality
Amino acid composition and amino acid scores
The amino acid contents of the samples except for methionine, cysteine, and tryptophan were determined by acid hydrolysis using the AOAC Official Method 982.30 [15]. The performic acid oxidized hydrolysis procedure according to AOAC Official Method 985.28 was used to determine methionine and cysteine. Tryptophan was determined using the alkaline hydrolysis method, as described by ISO protocol 13904:2016 [26]. Amino acid score was calculated by comparing the amino acid pattern of the sample (mg/g protein) against the FAO 1991 amino acid pattern reference for children from age 2 to age 5 (threonine (34), valine (35), methionine + cysteine (25), isoleucine (28), leucine (66), phenylalanine + tyrosine (63), histidine (19), lysine (58), tryptophan (11) [27]. The limiting amino acid (LAA) was determined by the lowest ratio.
In vitro protein digestibility (IVPD)-corrected amino acid score (IV-PDCAAS)
IVPD of samples was determined using the method described by Hsu et al. [28] with slight modifications. The pH of the flour suspensions (6.25 mg protein/mL) was adjusted to 8.0 with 0.1 N NaOH or HCl and kept in a water bath at 37 °C for 1 h. A multienzyme solution was prepared with 1.6 mg/mL of trypsin (porcine pancreas ≥ 13,000–20,000 BAEE units/mg protein), 3.1 mg/mL chymotrypsin (bovine pancreas ≥ 40 units/mg protein), and 1.3 mg/mL protease (Streptomyces griseus ≥ 15 units/mg) in 10 mL water and the solution was adjusted to pH 8.0 and kept in water bath at 37 °C. Flour suspension (10 ml) was mixed with multienzyme solution (1 ml) in an agitated water bath at 37 °C. The pH drop (ΔpH) for each sample was recorded every 30 s for 10 min. IVPD was calculated using Eq. 5:
where ΔpH10 min refers to the change in pH from initial 8.0 to the end of 10 min.
IV-PDCAAS was determined by multiplying IVPD by the amino acid scores of LAA [29].
In vitro starch digestibility
In vitro starch digestibility in pulse flours was determined according to the method described by Englyst et al. [30]. The analysis was performed under controlled enzymatic hydrolysis followed by colorimetric measurement of the glucose released. Rapidly digestible starch (RDS) and slowly digestible starch (SDS) were measured following incubation with porcine pancreatic alpha-amylase and amyloglucosidase at 37 °C in a water bath. RDS is the glucose released after 20 min and SDS is the glucose released after a further 100 min incubation. RS was measured indirectly by calculating the starch that is not hydrolyzed after 120 min incubation.
Data analysis
An one-way analysis of variance (ANOVA) along with a post-hoc Tukey test was performed to identify the differences of samples. In addition, a two-way analysis of variance (ANOVA) was performed using SPSS software (version 21.0, SPSS, Chicago, IL, USA) to test the effects of genotype and the environment together with their interactions on the compositional, functional characteristics of the navy bean flours. The different parameters were correlated with each other using a Pearson’s two-tailed significance correlation.
Results and discussion
Composition and physicochemical properties
Compositional analyses of the navy bean flours are presented in Table 1. The protein content of navy bean flours ranged from 19.5% (Indi, Portage la Prairie) to 28.5% (Portage, Morden) which is comparable to reported values (15–35%) [31]. Both environment and genotype × environment interaction had a significant effect on the protein content of flours (p < 0.01) (Table 2). The highest protein contents were found in the flours cultivated in Morden, MB, which is slightly warmer and had more rainfall than Portage la Prairie. It has been reported that high temperatures and water deficits might increase protein content [32]. However, studies showed that cultivars might respond to environmental conditions differently [32, 33]. Moreover, ash, crude fat, total starch, and total phenolic contents of navy bean flours were determined to be in a range of 4.2–4.8%, 1.2–2.0%, 30.3–39.7%, and 0.8–1.1 mg GAE/1 g flour, respectively. Genotype and environment had a significant impact on total starch content. Similarly, proximate composition of field peas were significantly affected by genotype and environmental conditions [34]. However, there was no significant effect of genotype, environment, or their interactions on ash content of samples. In addition, genotype has been shown to yield significant impacts on protein and total starch content of field peas and faba beans [12]. In addition, the total phenolic content of different varieties of navy beans differed significantly (p < 0.05). It was reported that both genotype and environment have an essential role in the total phenolic contents of common beans [35]. However, genotype might have stronger effects than the location on the total phenolic contents of common beans [36].
Zeta potential and surface hydrophobicity are critical parameters related to the functionality of the flours, such as emulsification, solubility etc., as they affect the interactions between molecules. Zeta potential values were not found to be significantly different across genotypes (Tables 1, 2). However, they were significantly affected by environmental conditions. In addition, zeta potential values were lower than -30 mV, which creates strong repulsive forces to have a stable hydrocolloid system. Zeta potential of proteins is affected by both electrochemical properties of the particle surface and medium conditions [37]. Zeta potential values are significantly correlated with the compositional properties of flours in terms of protein (r = 0.61, p < 0.001) and total starch contents (r = − 0.58, p < 0.001). Similarly, correlations between protein content and zeta potential were reported in rice flours depends on the charges of amino and carboxyl groups in different pHs [37]. Surface hydrophobicity was significantly affected due to genotype × environment interactions and ranged between 6 and 15 arbitrary unit (a.u.). Surface hydrophobicity results positively correlated with the protein content of flours (r = 0.36, p < 0.05), which is likely due to the amount of aromatic amino acid residues, but negatively correlated with total starch content (r = − 0.39, p < 0.05). Lg/Vn ratios of bean flours depend not only intrinsic but also external factors; however, some varieties can be less or more sensitive to environmental factors [10]. The Lg/Vn ratios of bean flours were significantly affected by genotype (p < 0.01) and ranged between 0.12 and 0.52.
FTIR analyses
The Gaussian spectral deconvolutions of the amide I band were performed on navy bean flour samples. Five Gaussian bands have been determined for navy bean samples, centered at 1615, 1638, 1659, 1678, and 1691 cm−1. In legumes and cereals, the Gaussian bands centered in the range between 1610–1615, 1630–1638, 1650–1660, 1660–1680, and 1690–1695 were assigned to amino acid side chains (A1), β-sheet, α-helix, β-turn (T), and amino acid side chains (A2), respectively [38]. Relative spectral weights of the secondary structure components of navy bean flours were found to be 14–15%, 35–37%, 21–23%, 13–14%, and 12–15% for A1, β-sheet, α-helix, β-turn, and A2, respectively (Table 3). Only genotype × environment interactions were significantly affected the small variations of secondary structures except α-helix. In addition, the growing environment significantly affected the spectral weight of the α-helix in navy bean samples. 1610–1615 cm−1 (A1) and 1690–1695 cm−1 (A2) ranges have indicated intermolecular protein complexes and amino acid chains [39]. Even though protein complexes as aggregates might not be expected in raw flours, A1 and A2 in raw common beans were determined 10% and 26%, respectively [40]. The highest relative spectral weights (35–37%) of navy bean flours were assigned to β-sheet secondary structure. Similar to our findings, β-sheet structure of soybean, lentil, and barley seeds were reported more than 30% even 37–44% for chickpea [38]. Furthermore, 1650–1660 cm−1 were assigned to α-helix structure which contributed up to 23% of the secondary structures of navy beans. Although the contribution of α-helix structure for soybeans were reported only 12%, it was found around 18–20% for most legumes [38].
Functional properties
The functional properties of navy bean flours are presented in Table 4. The OHC of navy bean flours was found to be 0.9–1.0 g/g, and was only significantly different due to genotype (p < 0.05). OHC negatively correlated with the Lg/Vn ratio (r = − 0.53, p < 0.001). Lipid type and the matrix structure mainly affect OHC; however, surface hydrophobicity and amino acid distribution of proteins also have an important impact on OHC [41]. However, there was no significant correlation between surface hydrophobicity and OHC results of navy bean samples (p > 0.05).
The WHC of navy bean samples was significantly affected by genotype, environment, and genotype × environment interactions, and ranged between 1.4 and 1.7 g/g. The highest WHC was found in var. Bolt and in the Morden location. WHC was positively correlated with protein content (r = 0.50, p < 0.05), surface hydrophobicity (r = 0.37, p < 0.05), and zeta potential (r = 0.39, p < 0.05) whereas it was negatively correlated with starch content (r = − 0.47, p < 0.05), Lg/Vn ratio (r = − 0.53, p < 0.001) and IVPD (r = − 0.50, p < 0.05). Protein content had a significant impact on the WHC of samples. Since protein concentration as well as surface polarity/hydrophobicity, protein conformation, and amino acid conformation [42, 43], compositional balance (protein–starch) may cause a negative correlation between WHC and starch content. In addition, a negative correlation between WHC and Lg/Vn indicated that an increase in vicilin led to an increased WHC of samples. The latter may be explained by the higher glutamic acid and aspartic acid levels within the vicilin fraction that are known to increase the binding of water [44].
Foaming properties of pulse flours can be desired or unwanted in relation to end product characteristics. FC for various pulse fours has been reported to fall between 166 and 203% [45]. In the current study, FC of navy bean flours was determined to fall between 160 and 276% and FS as 80–86%. A significant genotype × environment interaction was only found for the FC of samples, whereas no significant effects were determined for FS. Positive correlation between FC and lipid content (r = 0.34, p < 0.05) and total starch content (r = 0.42, p < 0.05) were observed. Although proteins played more important role to prepare stable foams through formation interfacial protein membranes at the air–water interface, interactions of starch with proteins might enhance the oil–water interface [46]. In addition, it was reported that molecular architecture of starch polymers (amylose/amylopectin ratio) had an important effect on foaming ability [47].
EA of navy bean flours ranged between 25 and 30% and was not affected by genotype or environment, but there was a significant genotype × environment interaction. EA results of var. Nautica, var. Envoy and var. Portage were not varied with location and were found 28%, 25–26% and 30%, respectively. In the same manner, EA of legume flours was reported as 20–26%, which was significantly higher than values previously reported for cereal flours (5–17%) [45]. Moreover, significant environment and genotype × environment interactions were observed for the ES of navy bean flours which was found in a range of 60–91%. Specifically, in Morden, MB, var. Envoy had the highest ES (91%). In addition, ES was positively correlated with zeta potential values (r = 0.62, p < 0,001) and the surface hydrophobicity (r = 0.37, p < 0.05) of navy beans.
For protein solubility, the only significant effect was genotype × environment interaction. Protein solubility results ranged between 88 and 97% for navy bean flours. Similarly, protein solubility values of yellow pea and faba bean flours were reported as 78% and 92%, respectively [48].
Pasting properties of pulse flours are important parameters to design cereal–pulse blend products as well as new products based on pulse flours. Pasting characteristic of starch is the main parameter for the RVA curves. In addition, protein–starch interactions affect pasting properties which can indicate the flour quality. Pasting properties of navy bean flours were presented in Table 5. The peak viscosity ranged from 439 cP (Envoy & Portage, Morden) to determine 715 cP (Bolt, Portage la Prairie), breakdown viscosity ranged from 0 to 102 cP (Portage, Portage la Prairie), and final viscosity ranged from 693 cP (Envoy, Morden) to 1216 cP (Bolt, Portage la Prairie). Pasting viscosities are mainly affected by starch content; however, other compounds such as protein, fibers, gums and their interactions with starch may influence the viscosity [49]. Compositional differences among navy bean flours may lead to a difference in viscosities during pasting. Furthermore, total starch content was positively correlated with peak viscosity (r = 0.38, p < 0.05), final viscosity (r = 0.46, p < 0.05), and setback viscosity (r = 0.55, p < 0.05) values of navy bean flours. Protein content of flours was negatively correlated with their peak viscosity (r = − 0.50, p < 0.05), final viscosity (r = − 0.36, p < 0.05), and setback viscosity (r = − 0.41, p < 0.05) values. The lowest peak setback, final, and trough viscosities were found in flours from the Morden, MB location. There was no difference between different genotypes; however, environment and genotype × environment interactions were significant for peak viscosity and setback viscosity of navy beans. Besides, trough and final viscosities of flours were significantly affected by genotype, environment and genotype × environment interactions. In addition, neither genotype nor environment significantly affected pasting temperatures of samples ranged between 89 and 95 °C. An absence of a peak caused lack of breakdown viscosities in some samples, which was also seen in yellow pea, green pea, and faba bean flours [45]. Besides, the peak time was found around 13 min without a significant difference between samples (p > 0.05).
Nutritional properties
Protein quality data in terms of the limiting amino acid, amino acid score, in vitro protein digestibility (IVPD), and in vitro protein digestibility corrected amino acid score (IV-PDCAAS) for navy bean flours are presented in Table 6. The limiting amino acid for navy bean flours were determined to be the sulfur amino acids, methionine and cysteine, with scores ranging between 0.79 and 1.03. Similarly, it was previously reported that the limiting amino acids in beans was either tryptophan or the sulfur amino acids, but sulfur amino acids were limiting in navy beans [50]. In vitro protein digestibility (IVPD) values of navy bean flours were found to be 70–73%, and with significant effects due to genotype and environment–genotype interactions. Processing of navy beans, including baking, cooking, and extrusion yields in vitro protein digestibility values of 77–79% [50]. IVPD was shown to be positively correlated with protein solubility (r = 0.42, p < 0.05) and the Lg/Vn ratio (r = 0.52, p < 0.05), but negatively correlated with lipid content (r = − 0.69, p < 0.001), total phenolic content (r = − 0.56, p < 0.001), and water holding capacity (r = − 0.50, p < 0.05) of flours. Protein–phenolic interaction phenomena can be used to explain the negative correlation between protein digestibility and phenolic content of navy bean flours. There are reversible (hydrogen bonding, hydrophobic binding, and van der Waals forces) and irreversible interactions (covalent bonding) between phenolics and proteins that may cause changes in solubility, digestibility, and thermal stability of proteins [51].
IV-PDCAAS ranged from 60 to 74% by the significant effects of genotype, environment, and their interactions (p < 0.05). The lowest IV-PDCAAS value was determined in Bolt, but the highest was found in Indi. In addition, the highest IV-PDCAAS value was found in samples derived from Portage la Prairie. The IV-PDCAAS values of baked, cooked, and extruded navy beans were reported 61%, 55%, and 55%, respectively [50]. Since raw flours are not consumed directly, the IV-PDCAAS results of raw flours may not indicate the actual values. In addition, the IV-PDCAAS of raw navy bean flours was comparable with IV-PDCAAS of other raw pulse flours which were 72–82% for chickpea and soybean; however, they were higher than those observed for green and yellow peas, 52–55% for green and yellow peas [45]. Moderately high IV-PDCAAS indicated high protein quality in navy beans. IV-PDCAAS of navy bean flour was found negatively correlated with protein content (r = − 0.85, p < 0.001) which was previously reported as negatively changing of amino acid profile via an increase in the content of the nonessential amino acids [52].
Rapidly digestible starch (RDS, 0.6–6.5%), slowly digestible starch (SDS, 3.2–6.3%), and resistant starch (RS, 18.2–23.4%) values were measured for navy bean flours (Table 5). RS values were found higher than the RDS ad SDS values, which indicates high enzymatic resistance due to strong protein and fiber matrices in bean flours [48]. RS data was found to be positively correlated with total starch (r = 0.64, p < 0.05) and total phenolic content (r = 0.62, p < 0.05), but negatively correlated with protein (r = − 0.45, p < 0.05) and ash content (r = − 0.37, p < 0.05). RDS was negatively correlated with protein (r = − 0.53, p < 0.05) and positively correlated with ash content (r = 0.47, p < 0.05). Similarly, SDS was found to be positively correlated with ash content (r = 0.56, p < 0.05) but negatively correlated with total phenolic content (r = − 0.56, p < 0.05). Genotype did not affect RDS, but a significant environment (p < 0.01) and environment × genotype interaction (p < 0.05) were detected. Overall, RDS results from Portage la Prairie sites were found significantly higher than the Morden sites. On the other hand, SDS was significantly affected by all factors. RS values were only affected by genotype (p < 0.05). In addition, proportions of amylopectin B1 chains which forms a crystalline structure may increase resistance to enzyme hydrolysis [53]. The data are in accordance with the previous studies on raw bean powders [25, 48].
Conclusion
Genotype had a significantly impact on some compositional properties of raw navy bean flours, including lipid content, total starch content, total phenolic content, and Lg/Vn ratio. Environmental conditions varied protein content, total starch content, and zeta potential of flours. Protein content positively correlated with zeta potential and surface hydrophobicity results. In addition, OHC and WHC of navy bean flours were varied significantly by genotype. Genotype, environmental conditions and their interactions had an impact on pasting properties of flours. In addition, IV-PDCAAS values of raw navy bean flours were found higher than the previously reported processed flours. Raw bean flours are not consumed directly without thermal processing; therefore, the IV-PDCAAS results may be overestimated. Moreover, this study showed starch and protein digestibility can be affected by genotype and environmental conditions.
References
Borresen EC, Brown DG, Harbison G, Taylor L, Fairbanks A, O’Malia J, Bazan M, Rao S, Bailey SM, Wdowik M (2016) A randomized controlled trial to increase navy bean or rice bran consumption in colorectal cancer survivors. Nutr Cancer 68(8):1269–1280
Zhu Z, Jiang W, Thompson HJ (2012) Edible dry bean consumption (Phaseolus vulgaris L.) modulates cardiovascular risk factors and diet-induced obesity in rats and mice. Br J Nutr 108(S1):S66–S73
Du S-k, Jiang H, Yu X, Jane J-l (2014) Physicochemical and functional properties of whole legume flour. LWT Food Sci Technol 55(1):308–313
Modi VK, Mahendrakar NS, Rao DN, Sachindra NM (2004) Quality of buffalo meat burger containing legume flours as binders. Meat Sci 66(1):143–149
Serdaroğlu M, Yıldız-Turp G, Abrodímov K (2005) Quality of low-fat meatballs containing legume flours as extenders. Meat Sci 70(1):99–105
Monnet A-F, Laleg K, Michon C, Micard V (2019) Legume enriched cereal products: a generic approach derived from material science to predict their structuring by the process and their final properties. Trends Food Sci Technol 86:131–143
Tiwari BK, Singh N (2012) Pulse chemistry and technology. Royal Society of Chemistry
Swanson BG (1990) Pea and lentil protein extraction and functionality. J Am Oil Chem Soc 67(5):276–280
Bora PS, Brekke CJ, Powers JR (1994) Heat induced gelation of pea (Pisum sativum) mixed globulins, vicilin and legumin. J Food Sci 59(3):594–596
Mertens C, Dehon L, Bourgeois A, Verhaeghe-Cartrysse C, Blecker C (2012) Agronomical factors influencing the legumin/vicilin ratio in pea (Pisum sativum L.) seeds. J Sci Food Agric 92(8):1591–1596
Martinez M, Stone AK, Yovchev AG, Peter R, Vandenberg A, Nickerson MT (2016) Effect of genotype and environment on the surface characteristics and functionality of air-classified faba bean protein concentrates. Eur Food Res Technol 242(11):1903–1911
Hood-Niefer SD, Warkentin TD, Chibbar RN, Vandenberg A, Tyler RT (2012) Effect of genotype and environment on the concentrations of starch and protein in, and the physicochemical properties of starch from, field pea and fababean. J Sci Food Agric 92(1):141–150
Singhal A, Stone AK, Vandenberg A, Tyler R, Nickerson MT (2016) Effect of genotype on the physicochemical and functional attributes of faba bean (Vicia faba L.) protein isolates. Food Sci Biotechnol 25(6):1513–1522
Balasubramanian P, Slinkard A, Tyler R, Vandenberg A (1999) Genotype and environment effect on canning quality of dry bean grown in Saskatchewan. Can J Plant Sci 79(3):335–342
AOAC (2012) Official methods of analysis of AOAC international, 19th edn. AOAC International, Maryland
AACC (2000) Approved methods of analysis, 10th edn. American Association of Cereal Chemists, St. Paul
Velioglu Y, Mazza G, Gao L, Oomah B (1998) Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem 46(10):4113–4117
Chang C, Tu S, Ghosh S, Nickerson MT (2015) Effect of pH on the inter-relationships between the physicochemical, interfacial and emulsifying properties for pea, soy, lentil and canola protein isolates. Food Res Int 77:360–367
Kato A, Nakai S (1980) Hydrophobicity determined by a fluorescence probe method and its correlation with surface properties of proteins. Biochim Biophys Acta BBA Protein Struct 624(1):13–20
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Morr CV, German B, Kinsella JE, Regenstein JM, Buren JV, Kilara A, Lewis BA, Mangino ME (1985) A collaborative study to develop a standardized food protein solubility procedure. J Food Sci 50(6):1715–1718
Nidhina N, Muthukumar SP (2015) Antinutritional factors and functionality of protein-rich fractions of industrial guar meal as affected by heat processing. Food Chem 173:920–926
Bai T, Stone AK, Nickerson MT (2018) Effect of tempering moisture and infrared heating temperature on the functionality of Desi chickpea and hull-less barley flours. Cereal Chem 95(4):508–517
Yasumatsu K, Sawada K, Moritaka S, Misaki M, Toda J, Wada T, Ishii K (1972) Whipping and emulsifying properties of soybean products. Agric Biol Chem 36(5):719–727
Ai Y, Jin Y, Kelly JD, Ng PKW (2017) Composition, functional properties, starch digestibility, and cookie-baking performance of dry bean powders from 25 Michigan-grown varieties. Cereal Chem 94(3):400–408
ISO (2016) Animal feeding stuffs determination of tryptophan content. International Organization for Standardization
FAO/WHO (1991) Protein quality evaluation-report of the joint FAO/WHO expert consultation; food and nutrition paper 51. Food and Agriculture Organization of the United Nations, The World Health Organization. Rome, Italy
Hsu HW, Vavak DL, Satterlee LD, Miller GA (1977) A multienzyme technique for estimating protein digestibility. J Food Sci 42(5):1269–1273
Bai T, Nosworthy MG, House JD, Nickerson MT (2018) Effect of tempering moisture and infrared heating temperature on the nutritional properties of desi chickpea and hull-less barley flours, and their blends. Food Res Int 108:430–439
Englyst HN, Kingman SM, Cummings JH (1992) Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr 46:S33
Ganesan K, Xu B (2017) Polyphenol-rich dry common beans (Phaseolus vulgaris L.) and their health benefits. Int J Mol Sci 18(11):2331
Al-Karaki GN, Ereifej KI (1999) Relationships between seed yield and chemical composition of field peas grown under semi-arid Mediterranean conditions. J Agron Crop Sci 182(4):279–284
Nikolopoulou D, Grigorakis K, Stasini M, Alexis M, Iliadis K (2006) Effects of cultivation area and year on proximate composition and antinutrients in three different kabuli-type chickpea (Cicer arientinum) varieties. Eur Food Res Technol 223(6):737–741
Nikolopoulou D, Grigorakis K, Stasini M, Alexis MN, Iliadis K (2007) Differences in chemical composition of field pea (Pisum sativum) cultivars: effects of cultivation area and year. Food Chem 103(3):847–852
Yang QQ, Gan RY, Ge YY, Zhang D, Corke H (2018) Polyphenols in common beans (Phaseolus vulgaris L.): chemistry, analysis, and factors affecting composition. Compr Rev Food Sci Food Saf 17(6):1518–1539
Espinosa-Alonso LG, Lygin A, Widholm JM, Valverde ME, Paredes-Lopez O (2006) Polyphenols in wild and weedy Mexican common beans (Phaseolus vulgaris L.). J Agric Food Chem 54(12):4436–4444
Wongsagonsup R, Shobsngob S, Oonkhanond B, Varavinit S (2005) Zeta potential (ζ) analysis for the determination of protein content in rice flour. Starch-Stärke 57(1):25–31
Carbonaro M, Maselli P, Nucara A (2012) Relationship between digestibility and secondary structure of raw and thermally treated legume proteins: a Fourier transform infrared (FT-IR) spectroscopic study. Amino Acids 43(2):911–921
Murayama K, Tomida M (2004) Heat-induced secondary structure and conformation change of bovine serum albumin investigated by Fourier transform infrared spectroscopy. Biochemistry 43(36):11526–11532
Carbonaro M, Maselli P, Dore P, Nucara A (2008) Application of Fourier transform infrared spectroscopy to legume seed flour analysis. Food Chem 108(1):361–368
Chau CF, Cheung PCK (1998) Functional properties of flours prepared from three Chinese indigenous legume seeds. Food Chem 61(4):429–433
Traynham TL, Myers DJ, Carriquiry AL, Johnson LA (2007) Evaluation of water-holding capacity for wheat–soy flour blends. J Am Oil Chem Soc 84(2):151
Yu J, Ahmedna M, Goktepe I (2007) Peanut protein concentrate: production and functional properties as affected by processing. Food Chem 103(1):121–129
Lam ACY (2016) Effect of cultivar and environment on the physicochemical and functional properties of pea protein isolates. Master's thesis, University of Saskatchewan, Saskatoon
Stone AK, Nosworthy MG, Chiremba C, House JD, Nickerson MT (2019) A comparative study of the functionality and protein quality of a variety of legume and cereal flours. Cereal Chem 96(6):1159–1169
Aluko RE, Mofolasayo OA, Watts BM (2009) Emulsifying and foaming properties of commercial yellow pea (Pisum sativum L.) seed flours. J Agric Food Chem 57(20):9793–9800
Sjöqvist M, Gatenholm P (2005) The effect of starch composition on structure of foams prepared by microwave treatment. J Polym Environ 13(1):29–37
Setia R, Dai Z, Nickerson MT, Sopiwnyk E, Malcolmson L, Ai Y (2019) Impacts of short-term germination on the chemical compositions, technological characteristics and nutritional quality of yellow pea and faba bean flours. Food Res Int 122:263–272
Abdel-Aal E-SM, Ragaee S, Rabalski I, Warkentin T, Vandenberg A (2018) Nutrient content and viscosity of Saskatchewan-grown pulses in relation to their cooking quality. Can J Plant Sci 99(1):67–77
Nosworthy MG, Medina G, Franczyk AJ, Neufeld J, Appah P, Utioh A, Frohlich P, House JD (2018) Effect of processing on the in vitro and in vivo protein quality of beans (Phaseolus vulgaris and Vicia Faba). Nutrients 10(6):671
Ozdal T, Capanoglu E, Altay F (2013) A review on protein–phenolic interactions and associated changes. Food Res Int 51(2):954–970
Witten S, Böhm H, Aulrich K (2015) Effect of variety and environment on the contents of crude nutrients, lysine, methionine and cysteine in organically produced field peas (Pisum sativum L.) and field beans (Vicia faba L.). Landbauforschung Appl Agric For Res 65(34):205–216
Chung HJ, Liu Q, Donner E, Hoover R, Warkentin TD, Vandenberg B (2008) Composition, molecular structure, properties, and in vitro digestibility of starches from newly released Canadian pulse cultivars. Cereal Chem 85(4):471–479
Acknowledgements
FT-MIR analysis of the research described in this paper was performed at the Canadian Light Source, a national research facility of the University of Saskatchewan, which is supported by the Canada Foundation for Innovation (CFI), the Natural Sciences and Engineering Research Council (NSERC), the National Research Council (NRC), the Canadian Institutes of Health Research (CIHR), the Government of Saskatchewan, and the University of Saskatchewan.
Funding
Financial support was provided through the AgriScience Program (Canadian Pulse Science Research Cluster) under the Canadian Agricultural Partnership.
Author information
Authors and Affiliations
Contributions
BG participated in planning of the experimental design, performing physicochemical and functional analyses, analyzing the results and writing the first draft of the manuscript. DK performed physicochemical and functional analyses. NW performed starch digestibility analysis. AH provided the navy been seeds from breeding trials. KT, SR, and ML was responsible for FT-MIR analysis. JDH was responsible for the AA quality analysis and was second reader of the manuscript. MTN was the primary supervisor of the project, contributed to the experimental design and was second reader of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors have no conflict of interest to declare.
Compliance with ethics requirement
This article does not contain any studies with human or animal studies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guldiken, B., Konieczny, D., Wang, N. et al. Effect of variety and environment on the physicochemical, functional, and nutritional properties of navy bean flours. Eur Food Res Technol 247, 1745–1756 (2021). https://doi.org/10.1007/s00217-021-03745-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-021-03745-7