Abstract
The volatile compounds of peaches (Prunus persica L.) obtained from five cultivars (Chongyanghong, Y1; Ruiguang 19, Y2; Zaohongxia, Y3; Zaohong 2, Y4; and Wuyuehuo, Y5) were analyzed by gas chromatography–olfactometry (GC–O), gas chromatography–mass spectrometry (GC–MS) and GC–flame photometric detection (FPD). A total of 40 odor-active volatile compounds were observed in the GC–O experiments. Amongst those compounds, hexanal, (Z)-3-hexen-1-ol, (E)-2-hexenal, 3-mercaptohexanol, nonanal, γ-nonalactone, and γ-decalactone contributed greatly to aroma of peach. In addition, thirty-four quantified compounds were demonstrated as important odorants according to odor activity values (OAVs > 1). Amongst these compounds, hexanal (OAV: 28–89), pentanal (OAV: 9–16), (E)-2-heptenal (OAV: 19–60), (E)-2-hexenal (OAV: 26–86), (E)-2-octenal (OAV: 10–42), (E)-2-nonenal (OAV: 8–94), γ-decalactone (OAV: 13–34), δ-decalactone (OAV: 2–19), (R)-(−)-linalool (OAV: 29–76) and phenyl acetaldehyde (OAV: 4–59) were the most powerful compounds in five varieties of peach.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The peach (Prunus persica L.), is rich in proteases, sugars and other organic compounds in addition to other trace elements and 17 amino acids which are required by human body [1]. The unique aroma of peach is derived from hundreds of volatile compounds that develop during the maturity and ripening stages. These volatile compounds mainly consist of alcohols, esters, lactones, aldehydes, ketones and terpenoids [2,3,4]. However, not all of the volatile compounds are responsible for the overall aroma of peach. The olfactory impact of these compounds depends on whether their concentrations are greater than their odor perception threshold values, which has led to the use of an odor activity value (OAV) to identify impact odorants [5,6,7].
Although the majority of aroma volatiles in fruits are esters, aldehydes, and terpenoid hydrocarbons, small quantities of other specific volatile sulfur compounds contribute to the aromas associated with various different foods and often define the characteristic flavor of the food. For example, 1-p-menthene-8-thiol and 4-mercapto-4-methyl-2-pentanone are character impact compounds found in grapefruit [8]. Also, 4-mercapto-4-methyl-2-pentanone, 3-(mercapto)hexyl acetate and 3-mercapto-1-hexanol are important in blackcurrant aroma [9], and methyl ethyl disulfide and diethyl disulfide in the aroma of durian [10]. Sulfur-containing amino acids, such as cysteine, cystine, and methionine, are the major precursors for the formation of the sulfur-containing compounds [11].
Intensive investigations have focused on the evolution of peach and nectarine aromas during the processes of ripening and maturation [4, 12,13,14]. Several studies have also investigated the effect of culture techniques and management on the composition and content of volatiles. Volatiles may be modified by bagging [15], sun light [16], and post-harvest treatments [17]. Other studies have also investigated the aroma compounds from different cultivars [2, 18, 19]. However, an investigation of the key aroma, sulfur compounds and sensory profile in peach has not yet been reported. The aims of the current study were (1) to identify the key aroma compounds in peach samples by GC–O and OAV, (2) to identify volatile sulfurs in peach samples using flame photometric detection (FPD), and (3) to characterize the aroma profile of peach samples by sensory evaluation.
Materials and method
Standard compounds
Acetaldehyde, ethyl acetate, 2-methylbutanal, pentanal, ethyl butanoate, 1-penten-3-one, butyl acetate, hexanal, 3-methylbutyl acetate, β-myrcene, 1-penten-3-ol, limonene, heptanal, 2-pentylfuran, ethyl 2-butenoate, (E)-2-hexenal, cis-ocimene, pentanol, hexyl acetate, terpinolene, octanal, cis-3-hexenyl acetate, (Z)-2-penten-1-ol, (E)-2-heptenal, 6-methyl-5-hepten-2-one, hexanol, (Z)-3-hexen-1-ol, nonanal, (E,E)-2,4-hexadienal, (E)-2-octenal, 1-octen-3-ol, heptanol, (E,E)-2,4-heptadienal, furfural, 2-ethyl-1-hexanol, decanal, benzaldehyde, (E)-2-nonenal, linalyl acetate, octanol, α-cedrene, β-copaene, (E)-2-decenal, nonanol, phenyl acetaldehyde, acetophenone, α-terpineol, α-citronellol, γ-hexalactone, cis-linalool oxide, decanol, geranylacetone, benzyl alcohol, phenylethyl alcohol, β-ionone, γ-nonalactone, γ-decalactone, δ-decalactone, methanethiol, ethanethiol, propanethiol, 2-methylthiophene were purchased from Alfa Aesar Corporation (Tianjin, China). (R)-(−)-Linalool, 3-methylthiophene, thiazole, 2-isopropyl-4-methylthiazole, 4-mercapto-4-methyl-2-pentanone, 3-mercaptohexanol, 8-mercaptomenthone, 3-methyl-2-butene-1-thiol and a homologous series of alkanes (C6-C30) were purchased from Sigma-Aldrich (St. Louis, MO). All of the chemical standards used above were of GC quality.
Materials
The volatile compounds of five peach cultivars (Prunus persica L.) were studied: ‘Chongyanghong’ (Y1, Hebei province), ‘Ruiguang 19’ (Y2, Beijing), ‘Zaohongxia’ (Y3, Liao’ning province), ‘Zaohong 2’ (Y4, He’nan province) and ‘Wuyuehuo’ (Y5, Shangdong province). The samples were supplied by Shanghai Bairun Flavour & Fragrance Co., Ltd. 1 kg of peaches was crushed and manually deseeded to acquire the peach musts. All musts were kept in a refrigerator (4 °C) until analyzed.
Solid-phase microextraction (SPME)–absorption of aroma compounds
One 75-µm carboxen–polydimethyl siloxane (CAR–PDMS) fiber was preconditioned on gas chromatograph for 30 min before it was used. The injector temperature of gas chromatograph was set at 250 °C. Because the volatile compounds in musts were sensitive to high temperature, the extraction temperature was set at 30 °C. The other optimized SPME experimental conditions were investigated, i.e., 30 min of extraction time and a sample volume of 6 g. The fiber was directly introduced into the GC injector for desorption for 4 min.
Calibration of standard curves
According to our previous research [20], model solution was prepared containing 20 mg/g sucrose, 10 mg/g glucose, 10 mg/g fructose, 3 mg/g citric acid, 1 mg/g (-)-quinic acid in Milli-Q deionized water [21, 22]. A standard stock solution containing 4 mg/kg of methanethiol, 2 mg/kg of ethanethiol, 2 mg/kg of propanethiol, 2 mg/kg of 2-methylthiophene, 2 mg/kg 3-methylthiophene, 2 mg/kg of thiazole, 0.2 mg/kg of 2-isopropyl-4-methylthiazole, 0.02 mg/kg of 4-mercapto-4-methyl-2-pentanone, 1 mg/kg of 3-mercaptohexanol, 0.2 mg/kg of 8-mercaptomenthone and 1 mg/kg of 3-methyl-2-butene-1-thiol in Milli-Q deionized water.
The standard solution was diluted with water according to the proportion of 1:5, 1:10, 1:20, 1:30, 1:40 and 1:50, respectively. 0.01 mL of those diluted solutions containing sulfur compounds and 0.01 mL of the internal standard solution with 0.2 mg/kg of dipropyl disulfide were mixed with model solution. Then the volatile compounds in solution were absorbed by fiber, which was employed in the peach must. The calibration curves were employed to calculate the concentrations of volatile compounds in peach musts. Similarly, 0.01 mL of each of the diluted solutions prepared by other non-sulfur compounds with 0.01 g internal standard solutions containing 5 mg/kg of 2-octanol was introduced into the model solution. Then, the calibration curves for non-sulfur compounds were established. The experiment conducted was repeated thrice.
GC–olfactometry analysis
The GC separation consisted of an Agilent 7890A chromatograph equipped with a flame ionization detector (FID) and an ODP-2 Olfactory Detector Port (Gerstel, Mulheim an der Ruhr, Germany). This system allowed us to simultaneously obtain a FID signal for the quantification and the odor characteristics of each compound detected by sniffing port. GC effluent was split 1:1 among the FID and sniffing port. Samples were separated on the HP-Innowax analytical fused silica capillary column (60 m × 0.25 mm × 0.25 µm, Agilent, Santa Clara, CA) and HP-5 analytical fused silica capillary column (60 m × 0.25 mm × 0.25 µm, Agilent, Santa Clara, CA). Conditions for GC–O analysis were as follows: the flow rate of carrier gas (hydrogen) was 2 mL/min; the oven temperature was first increased from 40 °C (6 min), ramped at 3 °C/min to 100 °C, and then ramped at 5 °C/min to 230 °C (20 min); the injector and FID detector temperatures were set at 250 and 280 °C, respectively. Moist air was pumped into the sniffing port at 50 mL/min to quickly remove the odorant eluted from the sniffing port. The aroma intensity (AI) was evaluated according to the previous paper [20].
GC–MS identification of aroma compounds
A 7890 gas chromatograph with a 5975C mass selective detector (MSD) (Agilent Technologies, USA) was employed. Two dissimilar columns, HP-Innowax analytical fused silica capillary column (60 m × 0.25 mm × 0.25 µm, Agilent, Santa Clara, CA) and HP-5 analytical fused silica capillary column (60 m × 0.25 mm × 0.25 µm, Agilent, Santa Clara, CA), were used for analyzing the volatile compounds. The injection port was set in a splitless mode for 3 min at 250 °C. The carrier gas was helium that was set at a constant flow rate of 1 ml/min. The MSD was used for chemical identification. Its electron impact energy was 70 eV. The ion source temperature was set at 230 °C. The quadrupole mass filter was operated at 150 °C. The transfer line temperature was at 250 °C. The chromatograms were recorded by monitoring the total ion currents in 30–450 m/z. The oven temperature was held at 40 °C for 6 min, then ramped to 100 °C at the rate of 3 °C/ min and ramped at the rate of 5 °C/min to 230 °C for the last 20 min. The volatile compounds were determined by comparing retention indices, retention times of standard compounds and Wiley7n.l Database (Hewlett–Packard, Palo Alto, CA). The RIs of unknown compounds were determined via sample injection with a homologous series of alkanes (C6–C30) (Sigma-Aldrich, St. Louis, MO).
Gas chromatography–FPD
The Agilent-7890A GC equipped with a flame photometric detection (FPD) was used in the sulfur mode. Two different phases of columns were employed to separate the volatile compounds. The types of columns were HP-Innowax (60 m × 0.25 mm i.d. × 0.25 µm film thickness, Agilent Technologies, USA) and HP-5 (60 m × 0.25 mm i.d. × 0.25 µm film thickness, Agilent Technologies, USA). The oven temperature was held at 40 °C for 6 min, then ramped to 100 °C at the rate of 3 °C/ min and ramped at the rate of 5 °C/min to 230 °C for the last 20 min. The temperature of FPD detector was set at 250 °C. PMT voltage was set at 500 V. The sulfur compounds were identified with retention times of standard compounds and RIs on both columns. The method of GC–MS analysis was referred for the quantification of sulfur compounds.
Odor activity values (OAV)
The OAV of a compound was calculated by dividing the calculated concentrations with the literature sensory thresholds, which was obtained from the literature.
Sensory analysis
The peaches were evaluated by a well-trained panel of ten members (five males and five females). Before the quantitative descriptive analysis, 10 g peaches was placed in a 100-ml plastic cup covered with Teflon and was subjected to a panelist in laboratory without peculiar smell at 25 °C. Then, the panelists had profoundly discussed aroma compositions of the peaches through three preliminary sessions (each for 2 h), until all of them had agreed with the degree of aromatic flavor. Subsequently, the organoleptic characteristic descriptors were quantified using six sensory descriptors (“alcohol”, “fruity”, “floral”, “green and grassy”, “sweet”, and “harmony”) to evaluate aroma defects and positive features. The complete blocks were estimated for each sample in triplicate for each treatment at random. The mean value of each sample was presented by the triplicate mean score based on ten-point scales.
Statistical analysis
The quantitative descriptive sensory analysis was submitted to analysis of variance (ANOVA). Duncan’s multiple comparison tests and Pearson’s correlation coefficients were calculated using XLSTAT ver.7.5 (Addinsoft, New York, NY, USA).
Results and discussion
GC–O results for peach samples
By application of GC–O, the aroma compounds detected in the peach samples are summarized in Table 1. The aroma compounds were confirmed in comparison with their RIs, odor characteristics and mass spectra obtained from standard compounds. A total of 40 odor-active volatile compounds were observed in the GC–O experiments. There were four unidentified volatile compounds perceived in five of the peach samples.
As presented in Table 1, the Y3 sample had the most aroma-active compounds amongst the other peach samples. Of those compounds, hexanal (AI: 2.8–4.5), (Z)-3-hexen-1-ol (AI: 1.6–3.2), (E)-2-hexenal (AI: 2.1–3.9), 3-mercaptohexanol (AI: 2.3–2.9), nonanal (AI: 1.1–3.6), γ-nonalactone (AI: 2.4–2.9), γ-decalactone (AI: 2.1–3.2), δ-decalactone (AI: 2.2–2.9), β-ionone (AI: 1.7–3.7) and 4-mercapto-4-methyl-2-pentanone (AI: 2.5–3.2) were the most powerful aroma-active compounds contributing to the aroma profile of the peach samples, indicating that these compounds are the major contributors of the characteristic aroma which is common to the cultivars investigated. Similar findings also show that C6 compounds, alcohols, aldehydes and lactones are the major contributors to peach aroma [2]. These C6 compounds (hexanal, (Z)-3-hexen-1-ol, (E)-2-hexenal) are known products of enzyme-catalyzed breakdown of unsaturated fatty acids. Lactones, particularly γ-decalactone and δ-decalactone, are described as “character impact” compounds in peach aroma, which contributed to the “peachy” background to peach [2].
However, GC–O could not clearly provide information on the potent odorants in the sample as it was measured based on aroma intensity or the odor threshold of the compounds in air. Moreover, loss of the volatile compounds during the isolation and concentration steps was not fully taken into account [23]. Accurate quantification is normally performed to characterize the important aroma compounds through the OAV using the odor threshold of compounds [24, 25].
Quantitative analysis of sulfur volatiles in peach samples
As shown in Table 2, eleven sulfur volatile compounds were detected in this investigation. These were identified based on their retention index in two dissimilar columns compared with standard chemicals and a sulfur-specific FPD response indicates that the detected peaks contained sulfur. On the basis of their chemical structure, these compounds mainly included thiol, thiazole and thiophene. Quantitatively methanethiol, ethanethiol, propanethiol, 3-methylthiophene and 2-methylthiophene showed relatively high amounts compared to other sulfur compounds. It is worth noting that 3-mercaptohexanol (3MH), 8-mercaptomenthone, 2-isopropyl-4-methylthiazole and 4-mercapto-4-methyl-2-pentanone (4MMP) were present in trace amounts in these samples. However, the contribution of each volatile compound to the overall fruit aroma was determined from their aroma intensity and odor activity values. 4-Mercapto-4-methyl-2-pentanone and 3-mercaptohexanol could contribute to the characteristics of passion fruit, broom, black current and citrus, passion fruit, grapefruit, respectively [26]. These were found in peach samples for the first time. According to the previous studies conducted in grapes, 4MMP and 3MH were released from precursors of which the cysteinylated [S-3-(hexan-1-ol)-l-cysteine (Cys-3MH) and S-4-(4-methylpentan-2-one)-l-cysteine (Cys-4MMP)] and glutathionylated [S-3-(hexan-1-ol)-glutathione (Glut-3MH) and S-4-(4-methylpentan-2-one)-glutathione (Glut-4MMP)] precursors have been identified. From Table 2, the amounts of 4 MMP and 3 MH varied significantly in each of the samples. The different concentrations of these compounds detected between the samples may be attributed to the variety and geographical variations, such as climatic conditions, terrain, water availability and other environmental factors [27, 28]. It is also worth noting that 4MMP and 3MH may contribute greatly to aroma of peach samples due to their extremely low thresholds of 0.8 and 60 ng/kg, respectively [26]. These data agree with that from a previous study which demonstrated these compounds contribute significantly to the aroma profiles of grape wine [9, 26]. 2-Isopropyl-4-methylthiazole, named peach thiazole in the flavor field, is considered a peach and tropical aroma [29]. Concentrations were almost five times as high in the Y2 sample (0.033 µg/kg) compared to the Y5 sample (0.007 µg/kg). It is well known that the concentration of the aroma compounds may not actually reflect the influence on their contribution to the aroma profile in the samples.
Quantitative analysis of volatile compounds
The concentrations and odor activity values (OAVs) of the volatile compounds obtained by GC–MS are displayed in Tables 3 and 4. The major volatile compounds of peach samples were hexanol (2442.54–17991.25 µg/kg), (E)-2-hexenal (2169.55–7077.94 µg/kg), (Z)-3-hexen-1-ol (588.14–1845.51 µg/kg), benzaldehyde (1187.78–10803.38 µg/kg), hexanal (632.04–2005.42 µg/kg). In contrast, (E)-2-octenal (30.39–127.05 µg/kg), (E)-2-nonenal (3.25–37.47 µg/kg), octanal (1.11–25.93 µg/kg) and phenyl acetaldehyde (16.11–236.42 µg/kg) were present at relatively low amounts in each of the samples.
The contributions of compounds to the aroma of samples depended not only on the amounts of the compound but also the odor detection threshold values of compounds. According to the results obtained by Guth, those with OAVs greater than 1 were considered to contribute to the aroma of the samples [30]. Table 4 shows the contributions of the different compounds to the aroma of five samples (OAVs > 1), which indicated that twenty-six, twenty-six, thirty-four, twenty-seven and twenty-nine quantified compounds could be found in the samples at concentrations higher than their corresponding odor thresholds, respectively. These compounds might, therefore, contribute to the peach aroma. Amongst these compounds, ten are the most powerful compounds in five varieties of peach: hexanal (OAV: 28–89), pentanal (OAV: 9–16), (E)-2-heptenal (OAV: 19–60), (E)-2-hexenal (OAV: 26–86), (E)-2-octenal (OAV: 10–42), (E)-2-nonenal (OAV: 8–94), γ-decalactone (OAV: 13–34), δ-decalactone (OAV: 2–19), (R)-(−)-linalool (OAV: 29–76) and phenyl acetaldehyde (OAV: 4–59). Interestingly, they were mainly aldehyde compounds. These results were consistent with the findings that the odor threshold values of aldehyde compounds are generally lower than the concentrations of these compounds [20].
For the Y5 sample, the OAVs of the hexanal, (E)-2-heptenal, (E)-2-hexenal and (E)-2-octenal from the Shangdong region are significantly higher than the other regional peaches. These compounds can exert a strong influence on peach aroma. Concerning theY4 sample, the OAVs of pentanal and (E)-2-octenal from the Henan region are the highest amongst the five regional peaches. These compounds are responsible for the green, fresh, citrusy, and fatty notes. The results are consistent with previous investigations which show aldehydes with six to ten carbons are perceived as having green, fatty, or tallow aromas [20]. Although most aldehydes can contribute to special and characteristic green, fatty, or tallow aromas at low levels, they also lead to rancid, painty or other unpleasant favors when present at high levels due to their low threshold. For example, hexanal has a low detectable odor threshold of 4.5 µg/kg [31]. At low concentrations, it contributes to the desirable green, fresh and fatty notes of aroma but presents “oxidized” off-flavors when concentrations accumulate above a critical level. The content of most aldehydes should be controlled within a suitable range which was further confirmed by the findings of sensory evaluation [31].
Two important terpenoid compounds, β-ionone and (R)-(−)-linalool, were detected in the study. (R)-(−)-Linalool, with lilac, lavender sensory properties, has a low threshold value of 10 µg/kg. The highest OAV of this volatile was obtained in sample Y5 (76), and lowest one in sample Y1 (29). β-Ionone, which may be considered a floral aroma, exhibited the highest OAV (172) in Y3 and was absent in the Y5 sample. These compounds could significantly contribute to the overall aroma of the peach samples and agree with the analysis of GC–O in the study.
Lactone compounds such as γ-hexalactone, γ-nonalactone, γ-decalactone and δ-decalactone were also identified in this study, which were compounds that contributed to the characteristic fruity and sweet odors of the peach samples. As summarized in Table 3, sample Y1 exhibited higher amounts of these compounds and OAVs than those in other peach samples. Based on the OAV, the most powerful aroma-active lactone compound was γ-hexalactone in sample Y1. This compound was considered as the key odorant in sample Y1. According to previous investigations, lactones have been reported as character impact compounds in peach aroma which contributed to the background of peaches. The study also presented flavors specific to peach aroma that are associated with C6 aldehydes, C6 alcohols and terpenoids [4, 19].
Sensory analysis
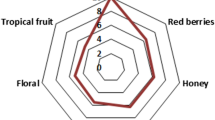
Sensory analysis was performed by evaluating the organoleptic quality of five kinds of peach samples using six descriptors that included “alcohol”, “fruity”, “floral”, “green” and “grassy”, “sweet” and “harmony”. ANOVA was employed to distinguish statistical differences between peach samples through sensory evaluation scores (data not shown). The statistical analysis demonstrated that samples showed dramatic differences in each of the descriptors (p < 0.05) (Fig. 1). These noticeable differences suggested each of the samples had significantly different flavor intensities. The panelists were also a significant influencing factor on all descriptors. This phenomenon was not unusual in characteristic descriptive analysis and indicated that panelists applied different levels of qualitative scoring because of physiological diversities in the perceived intensity or differences in personal preference, such as central or extreme raters [20].
Y1 and Y5 samples were accompanied by “alcohol”, “green” and “grassy” descriptors more frequently than the other samples. The major compounds involved in these descriptors include hexanol, (Z)-3-hexen-1-ol, hexanal, pentanal, (E)-2-heptenal, (E)-2-hexenal, (E)-2-octenal and (E)-2-nonenal, as described by panelists of GC–O. The result was in agreement with previous investigations that showed aldehydes and alcohols are generally associated with “green”, “fresh grass”, “green plants” and “citrusy notes”. Y3 sample was rated with the highest value of the fruity descriptor, whilst Y1 indicated the lowest sensorial score. It is common knowledge that the “fruity” descriptor is the predominant and most fundamental part of the global flavor of peach. Therefore, this descriptor was an important symbol in measuring the quality of peach aroma. According to previous studies, the “fruity” descriptor was mainly associated with ester compounds [20]. In this study, ethyl butanoate, butyl acetate, 3-methylbutyl acetate and hexyl acetate presented relatively high OAVs in the samples. These compounds might contribute to the “fruity” descriptor. “Floral” was also an important aroma descriptor which had its highest aroma score in sample Y3 and the lowest score in sample Y1. It was mainly composed of terpenoids, such as β-ionone and (R)-(−)-linalool. The Y2 sample was accompanied by the “sweet” descriptor more than any of the other samples. This phenomenon indicated that the Y2 sample yielded the highest amount of compounds and was able to influence a “sweet” aroma in its corresponding peach. The major aroma-active compounds in the “sweet” category mainly included lactones, such as γ-hexalactone, γ-nonalactone, γ-decalactone and δ-decalactone, as described by panelists of GC–O. The highest score under the “harmony” descriptor was found in the Y1 sample, whereas the lowest score was found in sample Y5. Notably, by comparing the sensory analysis of the “harmony” and “green and grassy” descriptors, these two descriptors showed the complete opposite when scored by the judges. Undoubtedly, aldehyde compounds played important roles in the overall aroma of peaches. It is also noted that these compounds were positively correlated with the aroma quality of the samples in suitable amounts. Otherwise, these compounds were perceived as offensive and conferred a negative sensory contribution to the aroma of samples [31].
Correlations between sensory descriptors and volatile compounds
An overview of the Pearson correlation analysis conducted between the sensory descriptors and the volatile compounds is shown in Table 5 (shown in the Supporting material). Strong positive correlations were observed in our study between “alcohol” and “green and grassy” (r = 0.945), and between “fruity” and “floral” (r = 0.980). Moderately positive correlations were observed between “floral” and “green and grassy” (r = 0.498), “fruity” and “green and grassy” (r = 0.590). A significantly strong relationship between “alcohol” and “green and grassy” may be explained by the fact that most of the volatile compounds were common in those two descriptors, such as hexanol, (Z)-3-hexen-1-ol, hexanal, pentanal, (E)-2-heptenal, (E)-2-hexenal, (E)-2-octenal and (E)-2-nonenal. The strong negative correlations were reported in this study between “alcohol” and “harmony” (r = − 0.904), “green and grassy” and “harmony” (r = − 0.926), whilst the “sweet” descriptor showed a moderate negative correlation with “alcohol” (r = − 0.497) and with “green and grassy” (r = −0.576).
Regarding the volatile compounds, the groups of high correlation were found. From Table 5, a large number of saturated and unsaturated C5, C6 and C7 aldehydes and alcohols were strongly correlated with each other. For example, strong correlations were also observed between hexanal and (E)-2-hexenal (r = 0.914), (E)-2-heptenal (r = 0.946), hexanol (r = 0.990), and (Z)-3-hexen-1-ol (r = 0.893); between (E)-2-hexenal and (E)-2-heptenal (r = 0.986), (E)-2-octenal (r = 0.872), (E)-2-nonenal (r = 0.816); between (Z)-3-hexen-1-ol and (E)-2-hexenal (r = 0.858), octanal (r = 0.986), (E)-2-heptenal (r = 0.924), and octanol (r = 0.983).
Otherwise, the strong negative correlations also were observed in this study between γ-hexalactone and 2-methylbutanal (r = − 0.964), octanal (r = − 0.936), (Z)-3-hexen-1-ol (r = − 0.876), (E)-2-octenal (r = − 0.878), (E,E)-2,4-heptadienal (r = − 0.940), (R)-(−)-linalool (r = − 0.978), and octanol (r = − 0.903). Interestingly, similar phenomenon was observed between γ-decalactone and 2-methylbutanal (r = − 0.876), pentanal (r = − 0.824), octanal (r = − 0.757), (Z)-3-hexen-1-ol (r = − 0.737), nonanal (r = − 0.615), (E)-2-octenal (r = − 0.602). This result demonstrated that lactone compounds presented a negative correlation with aldehydes and alcohol compounds which was partly the result of the negative relationship between “sweet” and “alcohol” and “green and grassy”.
Conclusions
The volatile compounds of peaches obtained from five cultivars were analyzed by GC–MS, GC–O, GC–PFD and OAV. Of these compounds, hexanal, (Z)-3-hexen-1-ol, (E)-2-hexenal, 3-mercaptohexanol, nonanal, γ-nonalactone, γ-decalactone, δ-decalactone, β-ionone, (R)-(−)-linalool, phenyl acetaldehyde and 4-mercapto-4-methyl-2-pentanone were the most powerful aroma-active compounds contributing to the aroma profile of the peach samples. The data presented in this study lay a foundation for the establishment of a chromatographic library of characteristic aroma compounds from different varieties of peach and can be used to evaluate peach quality. Furthermore, it provides the basis for the identification of varieties and quality control based on characteristic aroma compounds.
References
Kato M, Shibamoto T (2001) Variation of major volatile constituents in various green teas from Southeast Asia. J Agric Food Chem 49:1394–1396
Wang Y, Yang C, Li S, Yang L, Wang Y, Zhao J, Jiang Q (2009)) Volatile characteristics of 50 peaches and nectarines evaluated by HP–SPME with GC–MS. Food Chem 116:356–364
Aubert C, Milhet C (2007) Distribution of the volatile compounds in the different parts of a white-fleshed peach (Prunus persica L. Batsch). Food Chem 102:375–384
Aubert C, Günata Z, Ambid C, Baumes R (2003) Changes in physicochemical characteristics and volatile constituents of yellow- and white-fleshed nectarines during maturation and artificial ripening. J Agric Food Chem 51:3083–3091
Jiang B, Xi Z, Luo M, Zhang Z (2013) Comparison on aroma compounds in Cabernet Sauvignon and Merlot wines from four wine grape-growing regions in China. Food Res Int 51:482–489
Li H, Tao YS, Kang WH, Yin CL (2006) Wine aroma analytical investigation progress on GC (review). J Food Sci Biotechnol 25:99–104
Vilanova M, Martínez C (2006) First study of determination of aromatic compounds of red wine from Vitis vinifera cv. Castañal grown in Galicia (NW Spain). Eur Food Res Technol 224:431–436
Jabalpurwala F, Gurbuz O, Rouseff R (2010) Analysis of grapefruit sulphur volatiles using SPME and pulsed flame photometric detection. Food Chem 120:296–303
Rigou P, Triay A, Razungles A (2014) Influence of volatile thiols in the development of blackcurrant aroma in red wine. Food Chem 142:242–248
Zhu J, Chen F, Wang L, Niu Y, Shu C, Chen H, Xiao Z (2015) Comparison of aroma-active compounds and sensory characteristics of durian (Durio zibethinus L.) wines using strains of Saccharomyces cerevisiae with odor activity values and partial least-squares regression. J Agric Food Chem 63:1939–1947
Lee SM, Kim YS (2011) Determination of volatile sulfur compounds formed by the Maillard Reaction of glutathione with glucose. In: Volatile sulfur compounds in food. ACS symposium, vol 1068, pp 231–241
Zhang B, Shen JY, Wei WW, Xi WP, Xu CJ, Ian F, Chen KS (2010) Expression of genes associated with aroma formation derived from the fatty acid pathway during peach fruit ripening. J Agric Food Chem 58:6157–6165
Lian JG, Lin Q, Liu MY, Wang CZ, Zhang YM, Chen XS (2010) GC-MS analysis of volatile constituents in some late-maturing peach cultivars fruits. J ShanDong Agric Univ 41:503–507
Zhang XM, Jia HJ (2005) Changes in aroma volatile compounds and ethylene production during “Hujingmilu” peach (Prunus persica L.) fruit development. J Plant Physiol Mol Biol 31:41–46
Jia HJ, Araki A, Okamoto G (2005) Influence of fruit bagging on aroma volatiles and skin coloration of ‘Hakuho’ peach (Prunus persica Batsch). Postharvest Bio Tech 35:61–68
Génard M, Bruchou C (1992) Multivariate analysis of within-tree factors accounting for the variation of peach fruit quality. Sci Hortic 52:37–51
Derail C, Hofmann T, Schieberle P (1999) Differences in key odorants of handmade juice of yellow-flesh peaches (Prunus persica L.) induced by the workup procedure. J Agric Food Chem 47:4742–4745
Montevecchi G, Simone GV, Masino F, Bignami C, Antonelli A (2012) Physical and chemical characterization of Pescabivona, a Sicilian white flesh peach cultivar [Prunus persica (L.) Batsch]. Food Res Int 45:123–131
Horvat RJ, Chapman GW, Robertson JA, Meredith FI, Scorza R, Callahan AM, Morgens P (1990) Comparison of the volatile compounds from several commercial peach cultivars. J Agric Food Chem 38:234–237
Zhu J, Chen F, Wang L, Niu Y, Yu D, Shu C, Chen H, Wang H, Xiao Z (2015) Comparison of aroma-active volatiles in oolong tea infusions using GC-olfactometry, GC-FPD, and GC-MS. J Agric Food Chem 63:7499–7510
Qian W, Yan J, Ma M, Cai Z (2015) Determination of sugar and acid components in different maturity stages of yellow peach. Jiangsu Agric Sci 43:287–290
Shen Z, Ma R, Yu M, Cai Z, Song H, Li X (2007) Regularity analysis of main sugar and acid in fruit development of peach. Acta Agric Boreali-Sin 22:130–134
Klesk K, Qian M, Martin RR (2004) Aroma extract dilution analysis of cv. Meeker (Rubus idaeus L.) red raspberries from Oregon and Washington. J Agric Food Chem 52:5155–5161
Buttara M, Intarapichet KO, Cadwallader KR (2014) Characterization of potent odorants in Thai chempedak fruit (Artocarpus integer Merr.), an exotic fruit of Southeast Asia. Food Res Int 66:388–395
Schieberle P, Hofmann T (1997) Evaluation of the character impact odorants in fresh strawberry juice by quantitative measurements and sensory studies on model mixtures. J Agric Food Chem 45:227–232
Coetzee C, Du Toit WJ (2012) A comprehensive review on Sauvignon blanc aroma with a focus on certain positive volatile thiols. Food Res Int 45:287–298
Montero-Prado P, Bentayeb K, Nerin C (2013) Pattern recognition of peach cultivars (Prunus persica L.) from their volatile components. Food Chem 138:724–731
Cheng H, Qin ZH, Guo XF, Hu XS, Wu JH (2013) Geographical origin identification of propolis using GC–MS and electronic nose combined with principal component analysis. Food Res Int 51:813–822
Weenen H, Koolhaas WE, Apriyantono (1996) A sulfur-containing volatiles of durian fruits (Durio zibethinus Murr.). J Agric Food Chem 44:3291–3293
Guth H (1997) Identification of character impact odorants of different white wine varieties. J Agric Food Chem 45:3022–3026
Song S, Zhang X, Xiao Z, Niu Y, Hayat K, Eric K (2012) Contribution of oxidized tallow to aroma characteristics of beeflike process flavour assessed by gas chromatography–mass spectrometry and partial least squares regression. J Chromatogr A 1254:115–124
Acknowledgements
The research was supported by the National Natural Science Foundation of China (No. 2147614090) and National Key Research and Development Program Nanotechnology Specific Project (No. 2016YFA0200304).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhu, J., Xiao, Z. Characterization of the key aroma compounds in peach by gas chromatography–olfactometry, quantitative measurements and sensory analysis. Eur Food Res Technol 245, 129–141 (2019). https://doi.org/10.1007/s00217-018-3145-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-018-3145-x