Abstract
In this study, the effect of emulsifier and preparation technique on physicochemical properties and antioxidant/antimicrobial activity of plant oil (lemongrass and oregano, 1:1)/citrus extracts incorporated in oil-in-water (O/W) microemulsion in comparison to coarse emulsion, was investigated. The optimization of O/W microemulsion was performed by two surfactants with different hydrophilic–lipophilic balance values and two techniques of preparation such as sonication and microfluidization. The stability of microemulsions during storage time regarding to the droplet size, ζ-potential, viscosity, total polyphenolics content, total flavonoids content, antioxidant, antiradical and antimicrobial activity were assessed. Micro-emulsification increased the bioactivity of plant oil/citrus extracts by increasing the surface area of droplets induced by size decrement. Microfluidized and sonicated emulsions showed the same relatively stable profile during storage time. Micro-emulsification improves the functionality of plant oil/citrus extracts, such as antioxidant and antiradical activity. An increment about 16–32 times in antimicrobial activity of plant oil/citrus extracts was also attained. Furthermore, the morphology of microemulsions observed by transmission electron microscopy, confirmed a mono-dispersed nano-sized emulsion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, one of the top research areas in food safety and quality is the extraction and characterization of new natural ingredients with biological activity that can be further incorporated into functional foods, contributing to consumer’s well-being [1]. Therefore, the increasing demand by consumers for foods and beverages containing natural preservatives has generated intensive research on innovative preservation methods [2]. Natural compounds derived from plants such as essential oils (EOs), phenolic compounds and related compounds are recognized as strong antimicrobials/antioxidants. In recent years, citrus phenolic compounds have attracted great attention due to their strong antimicrobial properties, high yields, aromas and flavors and particularly their flavonoid components [3]. Citrus flavonoids have nutritional properties and also a large spectrum of biological activity such as antioxidant, antimicrobial against a wide range of Gram-negative/positive bacteria. They also exhibited antiviral activity against rhino- and poliomyelitis viruses [4].
Similarly to most bioactive compounds, natural antimicrobials are chemically reactive species which may interact with food matrix ingredients causing to change their antimicrobials activity. To inhibit growth of microorganisms, antimicrobial compounds must directly interact with the target microorganisms and partition into either the microbial membranes or the microbial intracellular space. It should be also mentioned that physical and chemical processes can alter the structure and functionality of antimicrobials, as they can reduce the effectiveness of the antimicrobials in the vicinity of food pathogens or spoilage organisms [5, 6]. Moreover, the limited water solubility of some antimicrobial compounds reduces their effectiveness and the homogeneity of their distribution in food matrices which is required to insure the total inhibition of microbial growth [7].
An innovative approach to overcome these problems is the encapsulation of bioactive compounds in nano-sized-Biosystems. The Biosystems can increase their physical stability and form a protective layer around the core, isolating it from its surrounding environment until its release is triggered, avoiding undesirable interactions of the bioactive ingredient with other food components or chemical reactions that could lead to its degradation, possible undesirable consequences on taste and odour, as well as negative health [8,9,10].
One of these innovative Biosystems includes microemulsion, a transparent colloidal, optically isotropic and thermodynamically stable system with wide applications in the chemical, food, cosmetic and pharmaceutical industries [11]. Several characteristics make microemulsions advantageous system for natural antimicrobials protection in food systems. This colloidal carrier has a high loading capacity, a good thermodynamic stability and can overcome the diffusional barrier of bacterial membranes to hydrophobic/hydrophilic molecules due to their high solubilizing capacity, nano-sized structures also favors the molecules bioavailability and increases their permeation [12].
The aim of this study is the optimisation of microemulsion that contains natural biomolecules such as citrus phenolic compounds in combination with plant oil extracts using two techniques; sonication and microfluidization and different hydrophilic–lipophilic balance (HLBs). The physicochemical and biological stability of microemulsions during 1 month of storage was assessed in comparison with coarse emulsion by considering the droplet size, ζ-potential, viscosity, total polyphenolics content (TPC), total flavonoids content (TFC), antioxidant and antimicrobial activity.
Materials and methods
Materials
Citrus extract (BIOSECUR F440D®) was kindly provided by Biosecur Lab Inc. (Mont St-Hilaire, QC, Canada). The organic plant oil extracts (lemongrass and oregano, 1:1) used were purchased from Bio-Lonreco Inc. (Dorval, QC, Canada). ULTRALEC® P Deoiled Soya Lecithin was provided by ADM Ltd. (Lloydminster, AB, Canada). Sucrose monopalmitate (Habo Monoester P90) was kindly provided by COMPASS Food Pte Ltd. (Tuas, Singapore). All other reagents of analytical grade were obtained from Sigma-Aldrich (Oakville, ON, Canada).
Microemulsion formulations
The microemulsion formulations were optimized according to the method that was developed in our laboratory [10]. The O/W contains plant oil extracts and BIOSECUR F440D® with ratio of 1:3 (W/W) was optimized with different emulsifier:oil phase ratio (1 and 1.5) and various HLB values in presence of lecithin (HLB ~ 7) and lecithin–sucrose monopalmitate (HLB = 12 with 45:55 ratio, HLB = 14 with 36:64 ratio) as emulsifiers. The colloidal suspension containing plant oil extracts with emulsifiers dispersed in water, were subjected to sonication or microfluidization to obtain the homogeneous emulsion solution.
Sonication method
The oil-in-water (O/W) microemulsion, contains plant oil extracts, BIOSECUR F440D® with ratio of 1:3 (W/W), 1% (W/W) lecithin and sucrose monopalmitate with different ratio (45:55%, 36:64%) as emulsifiers, was prepared by sonication. The emulsifiers were solubilized in bi-distilled water under agitation (1 h at 20 °C). The BIOSECUR F440D® and plant oil extracts were also added to emulsifier solution under stirring. Thereafter, the pre-emulsion was homogenized with a high sheer laboratory homogenizer (Ultra-Turrax T25, Germany) at 10,000 rpm for 40 s. The colloidal suspension was subjected to sonication (20 °C) at 40 kHz, and 70% of full power for 5, 10, 15 and 20 min (1 s ON and 1 s OFF) to obtain a homogeneous microemulsion (Sonicator Vibra cell 75115, Fisher Scientific Co.).
Microfluidization method
The O/W mixture with the same compositions as ones prepared for the sonication experiments were used to form microemulsion by microfluidization. The pre-emulsions was prepared with Ultra-Turrax homogenizer as mentioned above. After that, the colloidal suspension was subjected to microfluidization, using an electric-hydraulic M-110P Microfluidizer® equipped with a Diamond Interaction Chamber for emulsions downstream (Microfluidics International Corp., Newton, MA, USA). Optimization was performed at pressures of 15,000, 20,000 and 25,000 psi in 1–2 cycles (at 25 °C). Coarse emulsions with the same composition as microemulsions were prepared with a magnetic stirrer (30 min) to compare with microemulsion.
Physicochemical characterization
Size and ζ-potential
The mean diameter, size distribution and ζ-potential of microemulsions were determined upon empirical dilution of the samples using dynamic light scattering (DLS) technique employing a Zetasizer Nano ZS (Malvern Instruments Ltd., Germany). As, viscosity directly affects the Brownian motion of nano-particles and thus the calculated emulsion size result, therefore, sample should be diluted to an appropriate concentration. The software used is DTS Nano, version 6.12 supplied by the manufacturer (Malvern Instruments Inc., MA, USA). To avoid multiple scattering effects, microemulsions were diluted (1:50), and then the sample was put into a standard capillary electrophoresis cell equipped with gold electrodes. All measurements were carried out at 20 °C by considering a medium viscosity of 1.33 and medium refractive index of 1.333. Results were presented as an average diameter of particle (z average) with the polydispersity index (PDI) and ζ-potential of the microemulsion [13].
Viscosity
The viscosity of samples (16 mL) was determined using a Brookfield LVDV-II+ viscometer (Brookfield Eng. Lab., MA, USA) equipped with a UL (Ultra-Low) adapter using the “closed tube” method to control the temperature. The ULA spindle was used in measurements, as well as the Wingather V2.1 program for data collection and calculations. The absolute viscosity (cP) of the samples was determined at room temperature according to the type of rotational axis and the shear rate.
Turbidity (τ)
Turbidity analysis of the microemulsions was carried out by measuring the absorbance of undiluted samples at 600 nm using a UV–visible spectrophotometer Scinco S-3100 (Betatek Inc., ON, Canada).
Total polyphenolic contents (TPC)
Total phenolic content in each emulsion was determined by spectrophotometry according to the Folin–Ciocalteu method. The sample was mixed with H2O and Folin–Ciocalteu’s reagent and vortexed for 30 s. After 6 min reaction, 2 mL of sodium carbonate monohydrate 20% (w/v) were added and the total mixture was heated at 40 °C (in a water bath) for 20 min. The absorbance of the final solution (blue color) was measured at 760 nm, using a UV–visible spectrophotometer Scinco S-3100 (Betatek Inc.) with a blank (water and reagents) placed in the reference cell. A standard curve with gallic acid was established and TPC results were expressed as microgram gallic acid equivalents (GAE) per milliliter of sample [14].
Total flavonoids content (TFC)
Total flavonoid content was estimated using a colorimetric method described previously [15]. Briefly, 0.25 mL of the sample or (+)-catechin standard solution was mixed with 1.25 mL of distilled water in a test tube followed by the addition of 75 µL of a 5% NaNO2 solution. After 6 min, 150 µL of a 10% AlCl3·6H2O solution was added and allowed to stand for another 5 min before adding 0.5 mL of 1 M NaOH. The mixture was brought to 2.5 mL with distilled water and mixed well. The absorbance was measured immediately against the blank at 510 nm using a spectrophotometer Scinco S-3100 (Betatek Inc.) in comparison with the standards prepared similarly with known (+)-catechin concentrations. TFC results were expressed as microgram of catechin equivalents per milliliter of sample.
Antioxidant activity assays
The antioxidant activity of the microemulsion was assessed by two common techniques. For all antioxidant assays, standard chemicals were used for comparison.
Phosphomolybdate assay for total antioxidant capacity (TAC)
The total antioxidant capacity (TAC) assay of samples was carried out by the phosphomolybdenum method described by Ahmed et al. [16] with slight modification. The sample was dissolved in 1 mL methanol. Ascorbic acid was used as a standard. A stock solution of ascorbic acid (5000 mg/L) was prepared in distilled water, from which dilutions were made ranging from 25 to 500 mg/L. In a test tube, 300 µL of the sample was mixed with 3 mL phosphomolybdate reagent (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The test tube was covered with aluminum foil and incubated at 95 °C for 90 min. The mixture was then allowed to reach room temperature and its absorbance was recorded at 765 nm. Blank was run using the same procedure but containing an equal volume of methanol instead of the samples. The TAC (%) was estimated using Eq. (1):
DPPD assay to determine the free radical scavenging capacity (FRSC)
The antioxidant capacity of samples evaluated according to a modified procedure of the DPPD (N,N-diethyl-p-phenylenediamine) colorimetric method as described by Salmieri and Lacroix [17]. This method serves to measure the effect of the presence of antiradical agents against reactive oxygen species (ROS) such as superoxide anions (·O2−), hydroxyl radicals (·OH), singlet oxygen (1O2) and their by-products (H2O2, HOCl) generated by the electrolysis of a saline solution. A volume of 200 µL of sample was added to a cell containing 3 mL of 0.15 M NaCl and submitted to electrolysis for 1 min (10 mA DC, 400 V) using a power supply (Bio-Rad, model 1000/500, Mississauga, ON, Canada). After electrolysis, an aliquot of 200 µL was added to 2 mL of DPPD solution (25 mg/mL). The generated oxidative species react instantly with DPPD, producing a red coloration that can be measured at 515 nm using a spectrophotometer Scinco S-3100 (Betatek Inc.). The FRSC (antiradical capacity) is then equivalent to the capacity of samples to inhibit the accumulation of oxidative species (able to oxidize DPPD) and, consequently, to reduce the red coloration. The reaction advancement was quantified using a non-electrolyzed NaCl solution (no oxidative species, ascribed to 100% scavenging, i.e., positive control) and an electrolyzed NaCl solution (in the absence of antioxidants, ascribed to 0% scavenging, i.e., negative control). The scavenging percentage was calculated according to Eq. (2):
Trolox® (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), a water-soluble vitamin E analogue, was employed as a standard, and its calibration curve was obtained using concentrations ranging from 100 to 700 µg/L in 30% (v/v) ethanol. FRSC results were expressed as microgram of Trolox equivalents per milliliter of sample.
Transmission electron microscopy (TEM)
Visualization of morphology and structure of microemulsions was carried out by TEM with a negative staining method. To perform TEM observations, a drop of diluted-emulsion was placed on Formvar–carbon-coated copper grid (200 mesh and 3 mm diameter HF 36) for 5 min. The excess of the liquid was removed using the filter papers. After drying the grid at room temperature, a drop of phosphotungstic acid (3%) was placed on the copper grid containing the emulsion and left for 3 min at room temperature. Thereafter again, the excess of the liquid was removed using the filter papers and after drying the grid at room temperature, the micrographs were collected using a transmission electron microscope (HITACHI, H-7100) operating at 75 kV. Micrographs were recorded using an AMT Image capture Engine or Olympus TEM CCD camera.
Minimal inhibitory concentration (MIC) determination
Antimicrobial activity of citrus phenolic compounds/plant oil extracts incorporated in microemulsion was evaluated by MIC determination.
Preparation of cultures
Stock cultures of E. coli O157:H7 EDL933, Listeria monocytogenes 2812, Aspergillus niger (ATCC 1015) and Penicillium chrysogenum (ATCC 10106) were, respectively, stored at − 80 °C in Tryptic Soy Broth (TSB) medium for bacteria (Oxoid LTD., Basingstoke, England) and Potato Dextrose Broth (PDB) for fungi containing glycerol (10% V/V). On the day of the experiment, the bacterial and fungal concentration of each culture was adjusted to 106 CFU/mL or 106 conidia/mL, respectively for MIC determination.
Microbial analysis
The MIC value of each antimicrobial agent was determined in sterilized flat-bottomed 96-well microplate according to the serial microdilution method as described by Turgis et al. [18]. Briefly, serial dilutions of the microemulsion were made in Mueller Hinton Broth (MHB, DIFCO) for bacteria and in Potato Dextrose Broth (PDB, DIFCO) for fungi and dispensed into 96-well microplates. Each sample was diluted by MHB medium pre-added in 96-well microplate, to obtain serial concentrations. Each well was then inoculated with pathogenic strain at a concentration of 106 CFU/mL or 106 conidia/mL. The microplate was incubated aerobically for 24 h at 37 or 28 °C, for bacteria and fungi, respectively. Then, the absorbance was measured at 595 nm in an Ultra Microplate Reader (Biotek instruments, Winooski, VT, USA). The MIC was defined as the lowest concentration of microemulsion showing a complete growth inhibition of the tested bacterial strains (related to a measured absorbance that is equal to the absorbance of the blank).
Statistical analysis
The experiments were conducted in triplicate (n = 3). An analysis of variance (ANOVA) and Duncan’s multiple-range tests were employed to statistically analyze all results. The analysis was performed using STATISTICA 8.0 software (STATSOFT Inc., Thulsa, USA). Differences between means were considered significant when the confidence interval was lower than 5% (p ≤ 0.05).
Results and discussions
Sonication method
Sonication generates the emulsions by cavitation phenomena. As bubbles grow and migrate, they collapse in fluid and leads to an extremely high pressure. High pressure creates strong waves to disturb the fluid near the sonicating tip, break up immiscible liquid phase and causes to mix the emulsion’s two-phases. An increase in input power produces more cavities and stronger waves. This cavitation mechanism apparently differs from that by microfluidizer, in which the shear force plays a major role [19].
Sonication time
To optimize the sonication method, lecithin-based emulsions were prepared at different times of sonication (5, 10, 15 and 20 min). It was observed by increasing the time of sonication, the droplet size decreased from 170 to 90 nm (Fig. 1). The droplet size also decreased with increasing sonication intensities at 40, 60 and 70% of full power. Obviously, high-power sonication induced stronger mechanical vibrations and produced more powerful shock waves, thereby resulting in smaller emulsion droplets than those produced in low-power sonication.
The obtained results highlighted that after 20 min of sonication, the particles sizes were only weakly affected by the sonication times, as 20 min of sonication appeared to be sufficient to obtain the desired nano-sized emulsion. Some studies have shown that beyond a certain critical size, even in additional energy applied to break the droplets, the droplet size remains constant [20]. Based on preliminary obtained results, 20 min of sonication was considered to obtain the desired size.
HLB optimization
Indeed, the HLB index also provides a useful starting point in the emulsifier selection to provide an emulsion of good stability. Regards to this subject and to prepare a more stable microemulsion, lecithin was combined with sucrose monopalmitate (HLB = 18) to prepare different HLB values as following; SME 12 (sonicated microemulsion with HLB 12) and SME 14 (sonicated microemulsion with HLB 14).
Microemulsions with selected HLBs prepared in different sonication times (Fig. 2). It was observed, HLB caused by emulsifiers’ combination had significant effect on droplet size of emulsion like as sonication time. As by increasing the HLB value more than 10, preferable HLB for O/W emulsions, the emulsion droplet size significantly decreased, e.g., for 5 min of sonication the size decreased from 170 nm (HLB = 7) to 97 nm (HLB = 14) (p ≤ 0.05). Surfactants as surface-active agents reduced the interfacial tension necessary enough to break up the droplets to smaller size but not too much to simply disrupt the droplets. These findings are in accordance with results of Kaci et al. (2016) and Arab Tehrany et al. [21, 22] who described the effect of lecithin on emulsion properties.
a Effect of sonication time on particle size of emulsion with different HLB values. b Presents the effect of emulsifier: oil ratio on droplet size reduction of sonicated emulsion. c Presents the effect of HLB (7, 12 and 14) on color of sonicated emulsions (20 min). Different lowercase letters represent significant differences in droplet size of emulsion (p ≤ 0.05)
At effective HLB values, surfactant presents appropriate surface activity at the droplet surface to provide the protection against coalescence and coagulation [23]. No significant difference in droplet size of two emulsions with HLB 12 and 14 prepared at 10 min (90–95 nm), 15 min (77–80 nm) and 20 min (68–75 nm) of sonication, was observed (p > 0.05). Furthermore, by increasing the sonication time, the appearance of emulsion became clear from yellow color which indicates the formation of the microemulsion with smaller size (Fig. 2a).
Emulsifier:oil ratio
Concentration of emulsifier depends on different factors such as minimum preferred droplet size, emulsion application, emulsifier chemical structure, oil phase quantity and quality and also long-term stability of a specified emulsion system [23]. Here it was observed, by increasing the emulsifier concentration, the droplet size decreased because of interfacial tension’s decrement induced by emulsifier (Fig. 2b). However, emulsions with high surfactant concentrations (emulsifier:oil phase of 1.5:1) and small droplet size (< 45 nm) may have a potential risk to be toxic for human cells and also may not be commercially interesting for food companies because of legal and cost concerns. Additionally, based on some empirical rules, the surfactant forms micelles in an aqueous solution when its concentration exceeds some critical level, known as the critical micelle concentration (CMC). Thus, when surfactant is added to a solution above the CMC, the number of micelles tends to increase as same as the size or shape of the individual micelles [23]. As emulsifier:oil phase ratio of 1:1 presented also a stable microemulsion with acceptable particle size, it was considered in this study.
Microemulsion characterization
Microemulsions with preferred HLB values (12 and 14) and emulsifier:oil ratio of 1, prepared by sonication (20 min), characterized by considering the size, ζ-potential, PDI, pH and viscosity (Table 1). Emulsion with HLB 14 presented smaller droplet size but no significant differences (p > 0.05) were observed in the size, ζ potential, PDI, pH and viscosity of microemulsion prepared with two different HLBs (Table 1). Microemulsions with particle size about 58–60 nm, low PDI and viscosity of 1.21 cP were prepared by 20 min of sonication (Fig. 3).
ζ-Potential is an important parameter to determine the stability of various colloidal dispersions. The values of the zeta potential significantly show the extent of electrostatic repulsion between particles in dispersion. For molecules and particles in nano-scale, a high zeta potential (absolute value higher than 30) presents higher stability. When the potential is less, the dispersion tends eventually to aggregate. Sonicated microemulsions with positive ζ-potential ≈ 51–55 mV presented a remarkable factor of stability.
Microfluidization method
Microfluidizer is high shear fluid processors, which converts pressure energy into shear and forces, resulting in desired droplet size that happens in an interaction chamber. The degree of emulsification was determined by the design of the microchannel inside the interaction chamber, the introduced pressure, and the emulsification time (number of cycles that the suspension passed through the chamber). Microfluidizer consistently generate significantly higher shear than other methods and more uniform particle size reduction [24].
Microemulsions optimization with HLB 12 and 14 at different pressures (15,000, 20,000 and 25,000 psi) and number of cycles (1 and 2) have done by microfluidization method. It was observed, by increasing the intensity of disruptive energy during the emulsification or by recirculating the emulsion (increasing the time of emulsification), the size of emulsion and viscosity decreased (170–70 nm) and the color became clear (Fig. 4). The obtained results are in good agreement with the results of Salvia-Trujillo et al. [25]. They observed the average droplet size, viscosity and whiteness index of microemulsions decreased by increasing the processing pressure and the number of passes [25].
a The effect of pressure and number of microfluidization on microemulsion size (HLB 14). b Presents the emulsions prepared with lecithin/sucrose monopalmitate with two HLB (12 and 14) in three pressures (15,000, 20,000 and 25,000 psi), (A, B and C presents HLB 12), (A′, B′ and C′ presents HLB 14) after two cycles. Different lowercase letters represent significant differences in droplet size of emulsion (p ≤ 0.05)
Based on preliminary results, increment of emulsification pressure more than 25,000 psi, had no significant effect (p > 0.05) on droplets size. Furthermore, according to the obtained results, emulsion prepared by microfluidization at 20,000 psi, after two passages through the microfluidizer presented the same droplet size as 20 min of sonication.
To compare the effect of HLB on droplet size of emulsion prepared by microfluidization (in 20,000 psi—2 cycles), emulsions with HLB 12 and 14 were prepared (Table 1). A negligible difference between the droplet sizes of emulsion with higher HLB value (14) was observed. Although, no significant differences (p > 0.05) in size, ζ potential, PDI, pH and viscosity of microfluidized microemulsions prepared with two different HLBs were noted.
Antimicrobial analysis
The effect of emulsification on antimicrobial activity of plant oil/citrus extracts loaded in microemulsion was compared with coarse emulsion (Table 2). Microemulsions prepared with two techniques presented the same antimicrobial activity against pathogenic bacteria and spoilage fungi, L. monocytogenes, E. coli, A. niger and P. chrysogenum, respectively. No significant difference was observed between microemulsions prepared with two techniques (p > 0.05). However, a significant difference between microemulsions and coarse emulsion was observed (p ≤ 0.05). Emulsification improved noticeably the antimicrobial properties of microemulsions (MIC decreased) in compared to coarse emulsions. Micro-emulsification increased the antimicrobial activity of emulsions about 16–32 times higher than coarse emulsion against foodborne pathogen bacteria like as L. monocytogenes, E. coli, and spoilage fungi such as A. niger and P. chrysogenum, by decreasing the droplets size.
As emulsion droplet size decreases, its bioavailability increases, which facilitates the penetration and translocation of emulsion in bacterial membrane and increases the antimicrobial activity. These findings are in good agreement with the results of Salvia-Trujillo et al. [26, 27]. Salvia-Trujillo et al. [26] observed that microfluidized nanoemulsions induced a significant reduction in population of E. coli in compared to coarse emulsion and ultrasonicated nanoemulsions [24]. Furthermore, they found that edible nanoemulsion-based coatings with lemongrass EO droplets in the nano-range exhibited a faster and greater inactivation of E. coli during storage time in compared to conventional emulsions [27]. Salvia-Trujillo et al. [27] also observed that microfluidized nanoemulsions contain lemongrass or clove essential oil showed an enhanced antimicrobial activity against E. coli in compared to coarse emulsions [28]. Furthermore, Otoni et al. [29] concluded that droplet size reduction to a few hundreds of nanometers improved the antimicrobial properties of the nanoemulsified films against spoilage fungi in bakery products.
It should be also mentioned that the antimicrobial activity of microemulsion not only depends on its droplet size but also on their compositions. The antifungal activities of plant oil/citrus extracts loaded in microemulsion are related to their major phenolic constituents. These phenolic compounds interact with cell membrane and alter its permeability, leading to leakage of cell contents, which are essential to microorganism survival [30].
The presence of citrus extract in microemulsion is an effective factor on its antimicrobial activity. A possible synergic effect between citrus and plant oil extracts led to this noticeable antibacterial and antifungal activity. Citrus phenolic compounds have a large spectrum antibacterial activity against a wide range of Gram-negative/positive bacteria [31]. Randazzo et al. [32] also observed that citrus extract induced a largest spectrum of inhibition against 76 strains of L. monocytogenes. Citrus extract mainly acts on the membrane and causes conformational and/or compositional damages in some components of the cell membrane [32]. Many studies have also shown that phenolic compounds present in citrus extract can affect enzyme activity, cause protein denaturation, modify cell permeability, interfere with the bacterial membrane function, induce permeability in the cell membrane causing release of the cell constituents, and decrease the ATP concentration and intracellular pH. Their activity can also relate to the inactivation of membrane-bound proteins [32, 33].
Emulsion stability
Size
The stability of emulsions prepared by tow techniques were assessed (Fig. 5). As it was observed, coarse emulsions presented the droplet size about 345–390 nm at day 0 and then after, a significant increment was observed during the first month of storage (440–480 nm) (p ≤ 0.05). As after 1 month of storage, the precipitation was observed. A significant difference (p ≤ 0.05) was observed between coarse emulsion and microemulsions prepared with sonication and microfluidization. In the first days of storage, a slight increase in droplet size of all microemulsions was observed which can be explained by the required time to attain the thermodynamically stable system after micro-emulsification. Microemulsions with HLB 12 prepared by two techniques (MME 12, SME 12) presented negligibly more stable profiles during 2 months of storage (4 °C). No significant difference (p > 0.05) was observed between the size assessments of microfluidized-emulsion and sonicated-emulsion with HLB 12.
Particle size distribution of sonicated/microfluidized samples in terms of storage time. MME 12 (microfluidized microemulsion with HLB 12), MME 14 (microfluidized microemulsion with HLB 14), SME 12 (sonicated microemulsion with HLB 12), SME 14 (sonicated microemulsion with HLB 14) in comparison with coarse emulsion (coarse emulsion with HLB 12, coarse emulsion with HLB 14)
Microemulsions with HLB 14 prepared by two techniques (MME14, SME14) presented negligibly higher droplet size profiles during the storage time (p > 0.05). This size difference was also observed more noticeably in coarse emulsion with HLB 14 in compared to ones with HLB 12 (p ≤ 0.05). This increment in emulsion size happened during the first week of storage and then after a stable profile was obtained. The results confirmed that micro-emulsification with two techniques increased the emulsion stability by decreasing the droplet. These results are in good agreement with the results of Salvia-Trujillo et al. [25] and Juttulapa et al. [33].
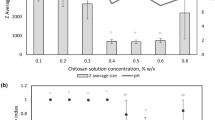
Zeta potential
Zeta potential (ζ) is an important tool for understanding the state of the nanoparticle surface and predicting the long-term stability of emulsion. No significant increment was observed in zeta potential of microemulsions prepared with two methods and HLB values (p > 0.05). Coarse emulsion showed ζ-potential about 50 mV in the first days but a continuous decrement to 21.2 mV was observed during the first month of storage that confirmed large droplets with non-homogenous charge noticeably tend to coagulate more than smaller size.
Viscosity
Viscosity and concentration of dispersed phase are also effective factors on nano-sized emulsions stability. The properties like as viscosity, density, polarity, interfacial tension and concentration of oil phase determine its bulk physicochemical properties. These properties may have an appreciable influence on the formation, stability, and quality attributes of a food emulsion [34]. As by increasing the viscosity of oil phase, droplet size increased and higher disruption energy needs to create the smaller size. Furthermore, the minimum size of droplets that can be produced by some methods depends on the ratio of the viscosity of the dispersed phase to that of the continuous phase. However, some studies also show that the droplet size is independent of viscosity of liquid samples in systems that breakup is occurring in turbulent flow [23].
As it is observed in Fig. 6a, no significant increment (p > 0.05) in viscosity of microemulsions was observed during the storage time. Coarse emulsion with larger droplet size had viscosity higher than microemulsions and viscosity increment was observed during the first month of storage. Coagulation can lead to viscosity increment and emulsion instability.
a Viscosity and b turbidity evolution of microemulsions during 2 months of storage. MME 12 (microfluidized microemulsion with HLB 12), MME 14 (microfluidized microemulsion with HLB 14), SME 12 (sonicated microemulsion with HLB 12), SME 14 (sonicated microemulsion with HLB 14) in comparison with coarse emulsion (coarse emulsion with HLB 12, coarse emulsion with HLB 14)
Turbidity (τ)
The turbidity measurement of emulsions was performed and a significant difference between coarse emulsions and microemulsions was observed (p ≤ 0.05) although no significant difference between microemulsions was detected (p > 0.05). A slight increment in turbidity of emulsions was observed but microemulsions showed stable values during the storage time. A significant difference (p ≤ 0.05) between coarse emulsions with two HLB values can be related to the major presence of lecithin in emulsion with HLB 12 in compared to HLB 14 (Fig. 6b).
Emulsification led to decrease in the turbidity of emulsion by droplet size decrement. This highlights the importance of controlling both the mean particle size and the width of the particle-size distribution (PDI) in samples that appear optically transparent. These findings are in good agreement with the results of Gharibzahedi et al. [35] and Xu et al. [11].
Total polyphenolics content (TPC)
The Total phenolic content (TPC) of microemulsions expressed as µg GAE/mL of microemulsion (Fig. 7a). In the first day of analysis, all microemulsions presented the same total polyphenol contents (257–264 µg GAE/mL) in microemulsion. However, during the storage at 4 °C, a significant decrement was observed in sonicated microemulsion with HLB 14 during 2 weeks of storage compared to other microemulsions. A gradual reduction in TPC of all samples was observed during 1 month of storage and then after they reached to a stable point, as MME 14 and MME 12 with respective TPC of 264 and 260 µg GAE/mL reached to 185 and 200 µg GAE/mL, respectively after 35 days of storage. The same decrement was observed for sonicated microemulsions, e.g., SME 14 and SME 12 with respective TPC of 264 and 261 µg GAE/mL reached to 186 and 172 µg GAE/mL, respectively after 35 days of storage. A slight difference in total phenolic content of MME 12 and MME 14 was observed. Sonicated microemulsion with HLB 12 presented relatively more stable behavior in compared to one with HLB 14. As phenolic compounds are chemically active compounds, they can be polymerized thereby losing their activity and solubility. Oxidation can be considered as an effective parameter to decrease the total polyphenolic contents of emulsions. In other side, coarse emulsions presented lower TPC (80–112 µg GAE/mL) than all microemulsions and also its content decreased during the storage time (35–44 µg GAE/mL) (p ≤ 0.05).
Total phenolic content (TPC) (a) and total flavonoids content (TFC) (b) of microemulsions prepared with microfluidizer and sonication expressed as microgram Gallic acid equivalent (GAE) and microgram Catechin equivalent per milliliter of sample, respectively, in comparison with coarse emulsion. For each sample, different letters represent significant differences in TPC and TFC of samples (p ≤ 0.05)
Total flavonoids content (TFC)
According to the obtained results, microemulsions with HLB 12 prepared with two techniques presented the lower TFC compared to those with HLB 14. The conspicuous presence of lecithin with high oxidation potential in microemulsions with HLB 12 compared to those with HLB 14, can be the possible reducing factor of TFC. Some evidences have shown that natural lecithin can be chemically or enzymatically oxidized and hydrolyzed to break off one of the hydrocarbon tails causing to destabilize the O/W emulsions [36]. Almost all samples showed the same profile of TFC during 1 month of storage which represents relative stability of microemulsions. In other side, coarse emulsions presented lower TFC than other microemulsions and also its content decreased gradually during the storage time (p ≤ 0.05). It was observed that the technique of emulsification had lesser effect on TFC and TPC in compared to HLB caused by emulsion composition (Fig. 7b).
Antioxidant activity
At the first day of analysis, all microemulsions presented the same total antioxidant capacity (TAC) about 70–75% and then after a continuous reduction was observed during the storage time (40–45%) (Fig. 8a). No significant differences were observed between the microemulsions (p > 0.05). The profile of TAC reduction is quietly consistent with total phenolic content (TPC) reduction. The good correlations between TPC and TAC confirmed that the phenol compounds were mainly responsible for the antioxidant activity of the extracts. The antioxidant activity of phenolic compounds depends largely on their chemical structure of components. The obtained results are in good agreement with the results of Zhu [37].
Total antioxidant capacity (TAC) (a) and free radical scavenging capacity (FRSC) (b) of microemulsions prepared with microfluidizer and sonication expressed as percentage and microgram of Trolox equivalents per milliliter of samples, respectively, compared to coarse emulsion. For each sample, different letters represent significant differences in TAC and FRSC of samples (p ≤ 0.05)
Coarse emulsions obviously showed significantly lower TAC (44–45%) than microemulsions with the same HLB values (p ≤ 0.05). A significantly rapid decline in TAC of coarse emulsions was observed during the storage time (7–9%) (p ≤ 0.05). These results confirmed that micro-emulsification protected the bioactive molecules against degradation during the storage time, as it was observed the rate of TAC decrement in the coarse emulsion was significantly higher than microemulsions (p ≤ 0.05).
Antiradical activity
The free radical scavenging capacity (FRSC) of microemulsions expressed as microgram Trolox equivalent per milliliter of sample (Fig. 8b). No significant difference (p > 0.05) in FRSC of microemulsions with HLB 14 (0.44–0.046 µg Trolox eq/mL) in compared to those with HLB 12 (0.40–0.42 µg Trolox eq/mL) was observed. In the following days, almost all samples showed a slight reduction during 1 month of storage (0.32–0.35 µg Trolox eq/mL), in similar behaviors as observed for total flavonoid contents (TFC). This correlation between FRSC and TFC, may be confirmed that flavonoids can be considered as the major responsible free radical scavenging in citrus/plant oil extracts incorporated in microemulsions. These findings are in good consistent with the results of Zhu [37]. Furthermore, no significant effect induced by emulsification technique on TAC and FRSC of microemulsions was observed (p > 0.05).
Coarse emulsion showed a rapid continuous reduction during storage time (from 0.40 µg Trolox eq/mL at day 1 to 0.09 µg Trolox eq/mL at day 35). This phenomenon confirmed again the protection effect of micro-emulsification on bioactive molecules loaded in emulsion. Some studies explained that the large surface area of the droplets as a result of their size reduction to the nanometer range and also formation of free radicals due to cavitation during the emulsification process can lead to TFC and FRSC reduction of microemulsions [38].
Conclusion
Based on obtained results, droplet size of emulsion depends not only on physical parameters of technique such as sonication time, microfluidizer pressure, number of passages but also on emulsion parameters such as emulsifier:oil ratio, emulsifier composition and its surface-active properties. The results also revealed that technique of emulsification had lesser effect on TFC and TPC in compared to emulsion parameters such as HLB values.
It was observed that microfluidization and sonication are the feasible technologies to reduce the droplet size of plant oil/citrus extracts loaded emulsion up to nano-metric scale and improved their functionality like as antioxidant, antiradical and antibacterial activity compared to coarse emulsion. Micro-emulsification increased the bioavailability of active components in emulsion by droplet size decrement and homogenously distribution of active molecules in emulsion.
This study strongly evidenced that microemulsion are able to amplify the performance of plant oil/citrus extracts by improving their solubility and bioavailability, maintaining the antimicrobial effect as fully functional over a period of months, improving their controlled release as well as preventing undesirable interactions with food matrix. Indeed, microemulsion is able to increase the effectiveness of the natural antimicrobial agents as well as decrease the necessary dosage used in food systems that qualified its application in food companies. From a point of view in the commercialization of natural food preservatives, these results are very promising.
References
Severino R, Ferrari G, Vu KD, Donsì F, Salmieri S, Lacroix M (2015) Antimicrobial effects of modified chitosan based coating containing nanoemulsion of essential oils, modified atmosphere packaging and gamma irradiation against Escherichia coli O157:H7 and Salmonella typhimurium on green beans. Food Control 50:215–222
Takala PN, Salmieri S, Boumail A, Khan RA, Vu KD, Chauve G, Lacroix M (2013) Antimicrobial effect and physicochemical properties of bioactive trilayer polycaprolactone/methylcellulose-based films on the growth of foodborne pathogens and total microbiota in fresh broccoli. J Food Eng 116(3):648–655
Tripoli E, La Guardia M, Giammanco S, Majo Di D, Giammanco M (2007) Citrus flavonoids: molecular structure, biological activity and nutritional properties: a review. Food Chem 104(2):466–479
Kaul TN, Middleton E Jr, Ogra PL (1985) Antiviral effect of flavonoids on human viruses. J Med Virol 15(1):71–79
Côté J, Caillet S, Doyon G, Dussault D, Sylvain JF, Lacroix M (2011) Antimicrobial effect of cranberry juice and extracts. Food Control 22(8):1413–1418
Weiss J, Gaysinsky S, Davidson M, McClements J (2009) In: Barbosa-Cánovas S, Mortimer A, Lineback D, Spiess W, Buckle K, Colonna P (eds) Nanostructured encapsulation systems: food antimicrobials. Global issues in food science and technology, 1st edn. Academic Press, New York
Shah B, Ikeda S, Michael Davidson P, Zhong Q (2012) Nanodispersing thymol in whey protein isolate-maltodextrin conjugate capsules produced using the emulsion–evaporation technique. J Food Eng 113(1):79–86
Donsì F, Annunziata M, Sessa M, Ferrari G (2011) Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT Food Sci Technol 44(9):1908–1914
Flanagan J, Singh H (2006) Microemulsions: a potential delivery system for bioactives in food. Crit Rev Food Sci Nutr 46(3):221–237
Khan A, Vu KD, Chauve G, Bouchar J, Riedl B, Lacroix M (2014) Optimization of microfluidization for the homogeneous distribution of cellulose nanocrystals (CNCs) in biopolymeric matrix. Cellulose 5:3457–3468
Xu Z, Jin J, Zheng M, Zheng Y, Xu X, Liu Y, Wang X (2016) Co-surfactant free microemulsions: preparation, characterization and stability evaluation for food application. Food Chem 204:194–200
Djekic L, Primorac M, Filipic S, Agbaba D (2012) Investigation of surfactant/cosurfactant synergism impact on ibuprofen solubilization capacity and drug release characteristics of nonionic microemulsions. Int J Pharm 433(1):25–33
Maherani B, Arab-Tehrany E, Kheirolomoom A, Cleymand F, Linder M (2012) Influence of lipid composition on physicochemical properties of nanoliposomes encapsulating natural dipeptide antioxidant l-carnosine. Food Chem 134(2):632–640
Caillet S, Salmiéri S, Lacroix M (2006) Evaluation of free radical-scavenging properties of commercial grape phenol extracts by a fast colorimetric method. Food Chem 95(1):1–8
Jan S, Khan MR, Rashid U, Bokhari J (2013) Assessment of antioxidant potential, total phenolics and flavonoids of different solvent fractions of monotheca buxifolia fruit. Osong Public Health Res Perspect 4(5):246–254
Ahmed D, Khan M, Saeed R (2015) Comparative analysis of phenolics, flavonoids, and antioxidant and antibacterial potential of methanolic, hexanic and aqueous extracts from Adiantum caudatum leaves. Antioxidants 4(2):394–409
Salmieri S, Lacroix M (2006) Physicochemical properties of alginate/polycaprolactone-based films containing essential oils. J Agric Food Chem 54:10205–10214
Turgis M, Vu KD, Dupont C, Lacroix M (2012) Combined antimicrobial effect of essential oils and bacteriocins against foodborne pathogens and food spoilage bacteria. Food Res Int 48(2):696–702
Arndt REA (2016) Cavitation. In: Johnson WR (ed) Handbook of fluid dynamics, 2nd edn. CRC Press, New York
Ramisetty KA, Pandit AB, Gogate PR (2015) Ultrasound assisted preparation of emulsion of coconut oil in water: Understanding the effect of operating parameters and comparison of reactor designs. Chem Eng Process 88:70–77
Kaci M, Arab-Tehrany E, Dostert G, Desjardins I, Velot E, Desobry S (2016) Efficiency of emulsifier-free emulsions and emulsions containing rapeseed lecithin as delivery systems for vectorization and release of coenzyme Q10: physico-chemical properties and in vitro evaluation. Colloids Surf B Biointerfaces 147:142–150
Arab Tehrany E, Kahn CJF, Baravian C, Maherani B, Belhaj N, Wang X, Linder M (2012) Elaboration and characterization of nanoliposome made of soya; rapeseed and salmon lecithins: application to cell culture. Colloids Surf B Biointerfaces 95:75–81
McClements J (2004) Food emulsion principle, practices, and techniques, 2nd edn. CRC Press, Florida
Sadeghpour Galooyak S, Dabir B, Zolfaghari M (2015) An innovative numerical approach for simulation of emulsion formation in a Microfluidizer. Colloids Surf A Physicochem Eng Asp 487:169–179
Salvia-Trujillo L, Rojas-Graü MA, Soliva-Fortuny R, Martín-Belloso O (2013) Effect of processing parameters on physicochemical characteristics of microfluidized lemongrass essential oil-alginate nanoemulsions. Food Hydrocoll 30(1):401–407
Salvia-Trujillo L, Rojas-Graü MA, Soliva-Fortuny R, Martín-Belloso O (2014) Impact of microfluidization or ultrasound processing on the antimicrobial activity against Escherichia coli of lemongrass oil-loaded nanoemulsions. Food Control 37(1):292–297
Salvia-Trujillo L, Rojas-Graü MA, Soliva-Fortuny R, Martín-Belloso O (2015) Use of antimicrobial nanoemulsions as edible coatings: Impact on safety and quality attributes of fresh-cut fuji apples. Postharvest Biol Technol 105:8–16
Salvia-Trujillo L, Rojas-Graü A, Soliva-Fortuny R, Martín-Belloso O (2015) Physicochemical characterization and antimicrobial activity of food-grade emulsions and nanoemulsions incorporating essential oils. Food Hydrocoll 43:547–556
Otoni CG, Pontes SFO, Medeiros EAA, Soares NDFF. (2014) Edible films from methylcellulose and nanoemulsions of clove bud (Syzygium aromaticum) and oregano (Origanum vulgare) essential oils as shelf life extenders for sliced bread. J Agric Food Chem 62(22):5214–5219
Lucera A, Costa C, Conte A, Del Nobile MA (2012) Food applications of natural antimicrobial compounds. Front Microbiol 8(3):1–13
Cormier J, Scott R, Janes M (2013) Antibacterial activity of Biosecur® citrus extract surface cleaner against Vibrio vulnificus. Open Microbiol J 8(7):130–134
Randazzo W, Jiménez-Belenguer A, Settanni L, Perdones A, Moschetti M, Palazzolo E, Moschetti G (2016) Antilisterial effect of citrus essential oils and their performance in edible film formulations. Food Control 59:750–758
Juttulapa M, Piriyaprasarth S, Takeuchi H, Sriamornsak P (2017) Effect of high-pressure homogenization on stability of emulsions containing zein and pectin. Asian J Pharm 12(1):21–27
Coupland JN, McClements DJ (1997) Physical properties of liquid edible oils. J Am Oil Chem Soc 74(12):1559–1564
Gharibzahedi SMT, Mousavi SM, Hamedi M, Ghasemlou M (2012) Response surface modeling for optimization of formulation variables and physical stability assessment of walnut oil-in-water beverage emulsions. Food Hydrocoll 26(1):293–301
Gunstone FD, Padley F (1997) Lipid technologies and applications. Marcel Dekker, New York
Zhu Y (2014) Relationship of total phenolic contents, DPPH activities and anti-lipid-oxidation capabilities of different bioactive beverages and phenolic. LSU Master’s Theses, Louisiana State University, School of Nutrition and Food Sciences
Berton-Carabin CC, Ropers MH, Genot C (2014) Lipid oxidation in oil-in-water emulsions: involvement of the interfacial layer. Compr Rev Food Sci Food Saf 13(5):945–977
Acknowledgements
This work was financially supported by the Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec (MAPAQ; Project # IA-115316) and Biosecur Lab Inc. Also, the members of RESALA laboratory appreciate the Biosecur Lab for providing Biosecur products.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Behnoush Maherani declares no conflict of interest. Mohamed Ali Khlifi declares no conflict of interest. Stephane Salmieri declares no conflict of interest. Monique Lacroix declares no conflict of interest. She has received the research grant from Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec (MAPAQ; Project # IA-115316) and Biosecur Lab Inc.
Compliance with ethics requirements
This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Maherani, B., Khlifi, M.A., Salmieri, S. et al. Design of biosystems to provide healthy and safe food. Part A: effect of emulsifier and preparation technique on physicochemical, antioxidant and antimicrobial properties. Eur Food Res Technol 244, 1963–1975 (2018). https://doi.org/10.1007/s00217-018-3108-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-018-3108-2