Abstract
Use of gallic acid (GA) as a standard in the Folin-Ciocalteu assay leads to an underestimation of phenol content in persimmon extracts so does the widely used catechin (C) in vanillin assay for determining condensed tannins in persimmon extracts. To develop suitable standards for addressing the issue of accuracy, we prepared highly purified persimmon tannin (PT) and its three fractions (PT20, PT40 and PT60), as well as three characteristic structural subunits of PT including epicatechin-3-gallate-(4β → 8, 2β → O→7)-epicatechin-3-gallate (A-type ECG dimer), epigallocatechin-3-gallate-(4β → 8, 2β → O→7)-epigallocatechin-3-gallate (A-type EGCG dimer) and epicatechin-(4β → 8)-epicatechin (B-type EC dimer). We compared the color yield of commercially available standards including the dimers GA, EGCG, ECG, C and PT and its three fractions in the Folin-Ciocalteu assay and vanillin assay, respectively. Our results suggested that for estimating phenol content in persimmon extracts, EGCG, ECG, C and GA were poor standards in Folin-Ciocalteu assay. We recommended PT40 as the most suitable standard in determining the phenol content of persimmon extracts. In the vanillin assay, A-type EGCG dimer or A-type ECG dimer were superior than the commonly used C as standards, and ECG was also an alternative when A-type EGCG dimer or A-type ECG dimer were not available.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent studies showed that the persimmon proanthocyanidin (condensed tannin) has been associated with various biological functions, including antioxidant activity [1–3], anti-inflammatory activity, antimicrobial activity [4], hypolipidemic activity [5–8], antidiabetic activity [9] and decreasing the risk of atherosclerosis [10]. Accurate quantification of total phenols or condensed tannins in persimmon-related food or products is essential for assessments of the healthy benefits and resultant product labeling, as well as potential risks.
However, in our previous study, we found that quantification of polyphenols in persimmon-related products using the widely employed colorimetric methods is inaccurate and problematic. For example, Folin-Ciocalteu method is one of the most routinely used assays for determination of total polyphenol concentrations in numerous food products such as fruits, juices and nutritional supplements. But in our previous study, we found that the total phenol content of persimmon extract determined by Folin-Ciocalteu method with gallic acid (GA) as the standard lowered by 50 % than that obtained by the formaldehyde-HCl method. Because this method is not specific to particular groups of phenolic compounds, but serve to quantify the total concentration of phenolic hydroxyl groups in the tested sample [11], making it is prone to be interfered by a wild diversity of compounds. In addition, GA is widely used as the standard for polyphenol analysis in this assay; however, the significant structural difference between polyphenolic compounds found in natural products, such as persimmon and GA, makes GA a poor choice of standard [12].
A similar problem also existed in analyzing condensed tannins concentration of persimmon extracts by the widely used vanillin assay. Color yield of persimmon tannins in vanillin assay is significantly lower than that of proanthocyanidins from other sources such as sorghum and grape seed on a mass basis. The use of catechin (C) as a standard in vanillin assay resulted in the condensed tannin content of persimmon powders being underestimated by up to 50–80 %. The 4-(dimethylamino) cinnamaldehyde (DMAC) assay is a colorimetric method and has been successfully used to quantify proanthocyanidin content in cranberry products [12]. However, we found that the color yield of persimmon tannins in the DMAC method is significantly lower than that in Folin-Ciocalteu method and vanillin assay.
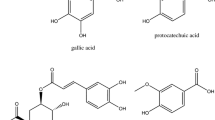
The structural variations in proanthocyanidin have great influence on the color yield in colorimetric assays. For example, quebracho is a 5-deoxy proanthocyanidin that is resistant to oxidative depolymerization [13], so color yields are low in acid butanol assay. The color yield of polymeric proanthocyanidin with vanillin was greatly lower than that of C with vanillin [14]. Similar things were also observed in persimmon tannin. Because the structure of persimmon proanthocyanidin is so unique, complex and heterogeneous [15], it is highly galloylated, is highly polymerized and contains both A-type and B-type interflavan bonds. No commercially available standard is representative of the complex structure of proanthocyanidin found in persimmon. It is not surprising that the use of routine and structural distinguished standards such as GA, C and grape seed proanthocyanidin leads to significant underestimation of persimmon total polyphenolic compounds and condensed tannins in colorimetric assays. In order to develop suitable standards for addressing the issue of accuracy, we prepared high purified persimmon tannin (PT) and its three fractions (PT20, PT40 and PT60), as well as three characteristic structural subunits of PT. The specific objectives of this work were (1) to compare the isolated persimmon proanthocyanidins to its characteristic structural subunits for use as standards in Folin-Ciocalteu and vanillin assays and to find out which structural feature has great influence on the color yield and (2) to develop a suitable standard for accurate determination of total phenol in persimmon products.
Materials and methods
Materials
Vanillin and FC reagent were from Sigma Chemical Co. (St. Louis, MO USA). C, epigallocatechin-3-gallate (EGCG) and epicatechin-3-gallate (ECG) were purchased from Sigma Chemical Co. (St. Louis, MO, USA) with purity of (≥) 95 %. GA and myricetin were purchased from Aladdin Chemistry Co. Ltd (Shanghai, China) with purity of (≥) 97 %. Epicatechin-(4β → 8)-epicatechin (B-type EC dimer) was isolated from Granny Smith apples (Malus domestica) and characterized by HPLC–MS/MS as previously reported [16], and its purity and identity were confirmed by HPLC and mass spectrometry. Epicatechin-3-gallate-(4β → 8, 2β → O→7)-epicatechin-3-gallate (A-type ECG dimer) and epigallocatechin-3-gallate-(4β → 8, 2β → O→7)-epigallocatechin-3-gallate (A-type EGCG dimer) were separated from persimmon as the method previously reported [17] and further prepared by preparative HPLC. Their purity and identity were confirmed by HPLC and mass spectrometry. Persimmon proanthocyanidin was prepared as previously reported [17]. Different persimmon proanthocyanidin fractions (PT20, PT40 and PT60) were isolated from persimmon pulp (Diospyros kaki Gongcheng shuishi) using Toyopearl HW-50F column as previously reported [18]. The prepared persimmon proanthocyanidin and its three fractions (PT20, PT40 and PT60) were characterized by MALDI-TOF and thiolysis-HPLC-ESI-MS as we previously reported [17, 18]. PT20, PT40 and PT60 account for 8.3 ± 0.5, 80 ± 4.3 and 11.7 ± 1.2 % of PT, respectively. Formaldehyde-HCl precipitation method [19] was used to determine the purity of PT and its fractions. All chemicals were of analytical reagent grade, and doubly distilled water was used in all the experiments. AB-8 macroporous resin was purchased from Naikai Chemical Plant (Tianjin, China.) and Toyopearl HW-50F resin was obtained from Tosoh (Tokyo, Japan).
HPLC analysis
Proanthocyanidin oligomers in each fraction were analyzed using a Shimadzu HPLC (Shimadzu, Japan) equipped with an auto-sampler, a binary pump, a column oven and a photodiode array detector. The column used was a 5-μm Hypersil ODS2 (4.6 mm × 200 mm, i.d), with a 4 mm × 4 mm i.d. guard column of the same material (Shim-pack GVP-ODS 2). Separation was accomplished at 30 °C with a gradient of 0.13 % TFA in H2O (A) and 0.1 % TFA in acetonitrile (B) at 0.8 ml/min in a program as follows: 0–10 min, 0–10 % B; 10–15 min, 10–20 % B; 15–20 min, 20–40 % B; 20–25 min, 40–55 % B; 25–35 min, 55 % B and 35–55 min, 55–0 % B and then reequilibrated for 10 min. Samples were filtered through a 0.45-μm cellulose acetate spin filter, and the eluate was monitored at 280 nm.
Folin-Ciocalteu assay
The assay was performed as described previously [20]. Briefly, samples were dissolved in methanol at concentration of 0.5 mg/ml. Each sample (0.5 ml) was mixed with 1 ml of Folin-Ciocalteu reagent ((Sigma Co., USA). After 5 min, 1 ml of 10 % Na2CO3 was added and the mixture was left at 25 °C for 60 min, and then distilled water was added to the reaction mixture to make the final volume of 10 ml. The absorbance was read at 760 nm using a Shimadzu UV-2200 spectrophotometer (Shimadzu, Japan).
Vanillin assay
The assay was performed using 5 mol/l sulfuric acid as a catalyst as described by Waterman and Mole [21]. Briefly, to 1.0 ml of sample (0.5 mg/ml, in methanol), 2.5 ml of 1 % vanillin reagent in methanol and 2.5 ml of 5 mol/l sulfuric acid in methanol were added. After vortex vibrated for 15 s, the samples were kept at 30 °C for 30 min, and then the absorbance values of samples and blank were read at 500 nm using a Shimadzu UV-2200 spectrophotometer (Shimadzu, Japan). Sulfuric acid (5 mol/l) in methanol was used as a blank.
Statistical analysis
Results are presented as the mean ± SEMs of at least three replicates. Statistical tests were performed with the GRAPHPAD INSTAT statistical package version 5.0 (GraphPad, San Diego) and the Excel (Microsoft office, USA).
Results and discussion
HPLC analysis of persimmon tannins and its fractions
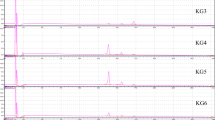
The reversed-phase HPLC chromatogram at 280 nm of persimmon proanthocyanidin and its three fractions are shown in Fig. 1. The isolated persimmon tannins (PT) showed a large hump with several resolved small peaks on it. PT40 and PT60 were characterized by the presence of an intense hump without any resolved small peaks on it, while PT20 contains many resolved peaks and a relative small hump, which indicated that PT, PT40 and PT60 were mainly polymeric polyphenols with diverse isomers and oligomers with different degrees of polymerization, but PT20 contains a large amount of low molecular weight substances. The mean degree of polymerization (mDP) of PT, PT20, PT40 and PT60 were estimated to be 26.0, 2.61, 23.3 and 33.4, respectively, by thiolysis-HPLC analysis as we previously reported [17, 18], providing further support for this result. Except for the absorbance at 278 nm, PT and its three fractions showed no obvious absorbance at other wavelengths, suggesting no other phenolic compounds such as flavonols and anthocyanins exist. The purity of PT, PT20, PT40 and PT60 were estimated to be 96.52, 91.40, 94.40 and 92.21 %, respectively, by formaldehyde-HCl precipitation method [19].
Folin-Ciocalteu assay
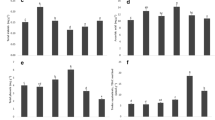
In the Folin-Ciocalteu assay, the chromophore produced is a blue phosphotungstic–phosphomolybdic complex of undefined structure. Although the underlying chemistry of this reaction is not fully understood, it is widely used for measuring total phenol contents in food and plant extracts [22]. Standard curves obtained from GA, C, EGCG, ECG and PT and its three fractions were shown in Fig. 2. Compared to the slope values, intercept coefficients were very small, implying that their impact on estimating phenol content was negligible. The slopes of the GA and three monomeric catechins (C, ECG and EGCG) standard curves were very similar, suggesting that they have similar color yield in this assay. However, the slope of standard curves from PT, PT40 and PT60 were very analogous and about 2.5 times lower than that of GA and the three monomeric catechins, indicating that PT content would be underestimated by 2.5-fold if these standards were used for the Folin-Ciocalteu assay. The slope of standard curve from PT20 was 3 times lower than that of PT, PT40 and PT60, suggesting that the color yield of low molecular weight fraction of PT differs from that of the high molecular polymeric fractions, and the difference may be due to the distinguished difference in the structure of the constituents. Although EC, EGC, EGCG and ECG occurred as the extender units of polymeric persimmon proanthocyanidin with EGCG and ECG being the dominant [17, 18], our data indicated that EGCG, ECG, C and GA were poor standards in Folin-Ciocalteu assay for quantification of total phenol contents of persimmon products. Color development depends on the structure of the flavanol in colorimetric assays. The color yield of procyanidin B3 was found to be less than 50 % of that with C or EC in DMAC assay [23]. Because persimmon proanthocyanidin exists as polymers with myriad combinations of building blocks, the large polymeric persimmon proanthocyanidin may not be detected as much sensitive with Folin-Ciocalteu as a monomer. Since persimmon proanthocyanidin grows linearly in polymer length, each additional degree of polymerization (DP) adds weight but no additional Folin-Ciocalteu reactivity [13]. Therefore, it is not surprising that when PT, PT40 and PT60 were used as standards in the Folin-Ciocalteu reaction, the response would be lower than that of the monomeric standards. As shown in Table 1, the use of GA, C, EGCG and ECG as standards in Folin-Ciocalteu method underestimated the total phenol content of PT40 by about 50 % while using PT20 as a standard overestimated the total phenol content of PT40 by about 320 %. Although PT40 is a complex mixture, it could represent the structural heterogeneity of persimmon proanthocyanidin including high degree of polymerization, high heterogeneity, both A and B type linkages and high galloylation, and the purification of persimmon proanthocyanidin to remove non-tannin materials is not tedious and has a high yield. In addition, the structural features of the fraction can be characterized by thiolysis-HPLC and MALDI-TOF MS as previously reported [17, 18]. Since no commercially available standard is reflective of the complex and unique structure of persimmon proanthocyanidin, we recommend that purified high molecular weight persimmon proanthocyanidin such as PT40 may be a better choice than GA for use as a standard for accurate estimating total phenol content in persimmon products rich in proanthocyanidin with high molecular weight.
Vanillin assay
The vanillin reaction involves reaction of vanillin with metasubstituted ring of flavanols to yield a red adduct [22]. Although the reaction is not specific to condensed tannins, it is also widely used to estimate proanthocyanidins. C is the most commonly used standard in this assay, but under normal conditions for vanillin assay, the rates of the reaction of the polymer differ from that of C [22]. Because only some of the internal flavanol units in the polymer react with vanillin when sulfuric acid or hydrochloric are used as a catalyst, the variable reactivity of the subunits of the tannin polymer was one of limitations of this assay. Lack of appropriate standards for quantification of a specific plant of interest due to the structural heterogeneity of condensed tannins was another big limitation of this assay. In our study, we observed that the color yield of persimmon tannins in vanillin assay is significantly lower than that of C. Considering persimmon proanthocyanidin is a heterogeneous polymer consisting of C, EGCG and myricetin terminal units and EGC, EGCG, C and ECG as extension units, and it contains both A and B type linkages, to explore the possible structural feature which has great influence on the color yield in vanillin assay, we used commercially available monomeric catechins including C, EGCG and ECG as standards in this assay. We also prepared three characteristic structural units of PT including A-type ECG dimer, A-type EGCG dimer, A-type EC dimer and B-type EC dimer and compared their reactivity in vanillin assay. As shown in Fig. 3, on a mass basis, the color yield of C was the highest, and it was 1.6 times higher than that of EGCG and 2.6 times higher than that of ECG. But on a mole basis, the color yield of C was 0.6 times higher than that of EGCG and 1.4 times higher than that of ECG, which meant that the structural variations in monomers greatly affected its color yield with vanillin. The color yields on mass basis of all dimers were about 50–60 % that of their corresponding monomer. If we calculated the color yield on mole basis, we could clearly see that the color yields of B-type EC dimer, A-type EGCG dimer and A-type ECG dimer were 92, 118 and 116 % of those of C, EGCG and ECG, respectively. From these results, it seemed that under the current conditions, only the terminal unit of the dimers reacted with the vanillin and the type of interflavan linkage had little effect on the reaction. Because the reaction was mainly limited to the terminal unit, it is not surprising that as the degree of polymerization (DP) increases, no additional color yielded, as evidenced by the standard curves plotted with PT and its polymeric fractions (Fig. 3). As shown in Fig. 3, on a mass basis, the slope of standard curves from PT, PT40 and PT60 were 1.45, 1.33 and 1.28, respectively. These values were lower than that of the monomers and dimers. The slopes of PT, PT40 and PT60 were 3–4 times lower than that of C, indicating that PT content would be underestimated by three to fourfold if C was used as a standard for the vanillin assay. C was reported to have a much greater slope than other standards in colorimetric assays such as DMAC assay [13]. Similar to Folin-Ciocalteu assay, the slopes of standard curve from PT, PT40 and PT60 were similar, but the slope of standard curve from PT20 was 1.5–2 times lower than that of PT, PT40 and PT60, indicating that the reaction of the low molecular weight fraction of PT with vanillin differed significantly from that of the high molecular polymeric fractions. One explanation for the low color yield of PT20 with vanillin may be that PT20 contains many compounds that could not react with vanillin, since the vanillin reacts only with meta-substituted flavanoids. We calculated the condensed tannin contents of purified PT40 with the above standards and compared the results obtained by formaldehyde-HCl precipitation method, which is used routinely and served as a comparative check for the accuracy of the colorimetric assays [24]. As shown in Table 2, if the results were expressed as mass % equivalents of standards, the condensed tannin content presented in PT40 determined using vanillin assay and formaldehyde-HCl precipitation method were very similar, but using C as a standard underestimated the condensed tannin content of PT40 by 81 %. Compared to C, ECG and EGCG were better standards, for the condensed tannin content of PT40 estimated by ECG and EGCG standards were 69.3 and 49.4 % of the PT40 results, separately. Results from B-type EC dimer, A-type EGCG and A-type ECG dimer standards suggested that A-type EGCG and A-type ECG dimer were superior standards than B-type EC dimer in this assay.
It was obvious that the purified polymeric fraction derived from persimmon was the most suitable standard for accurately measuring proanthocyanidins content in persimmon by vanillin assay. Because in some cases, the polymeric “standard” prepared in this way is not available and there is no simple way to confirm that the purified polymer is similar to that produced in other laboratory from the same plant material. We recommend that adoption of A-type EGCG dimer or A-type ECG dimer as a standard is a better approach for interlaboratory comparisons. Because the A-type EGCG dimer and A-type ECG dimer are defined chemical compounds, interlaboratory comparisons of vanillin analysis based on these standards will be more meaningful than comparisons based on poorly defined, polymeric standards, and the data could be reliably reproduced by any other laboratory. Since the preparation of A-type EGCG dimer and A-type ECG dimer is laborious, if the A-type EGCG dimer or A-type ECG dimer is unavailable, using ECG instead of the commonly used C as a standard is also recommended. Although the ECG standard underestimated the condensed tannin content of PT40 by about 30 %, it is commercially available, which makes the data comparison among different samples and different laboratories easy and believable.
References
Tian Y, Zou B, Yang L, Xu S-f, Yang J, Yao P, Li C-m (2011) High molecular weight persimmon tannin ameliorates cognition deficits and attenuates oxidative damage in senescent mice induced by d-galactose. Food Chem Toxicol 49(8):1728–1736
Tian Y, Zou B, Li C-m, Yang J, Xu S-f, Hagerman AE (2012) High molecular weight persimmon tannin is a potent antioxidant both ex vivo and in vivo. Food Res Int 45(1):26–30
Jang I-C, Jo E-K, Bae M-S, Lee H-J, Jeon G-I, Park E, Yuk H-G, Ahn G-H, Lee S-C (2010) Antioxidant and antigenotoxic activities of different parts of persimmon (Diospyros kaki cv. Fuyu) fruit. J Med Plant Res 4(2):155–160
Borges-Argaez R, Canche-Chay CI, Pena-Rodriguez LM, Said-Fernandez S, Molina-Salinas GM (2007) Antimicrobial activity of Diospyros anisandra. Fitoterapia 78(5):370–372
Gorinstein S, Kulasek GW, Bartnikowska E, Leontowicz M, Zemser M, Morawiec M, Trakhtenberg S (1998) The influence of persimmon peel and persimmon pulp on the lipid metabolism and antioxidant activity of rats fed cholesterol. J Nutr Biochem 9(4):223–227 0955-2863
Kondo S, Yoshikawa H, Katayama R (2004) Antioxidant activity in astringent and non-astringent persimmons. J Hortic Sci Biotech 79(3):390–394 1462-0316
Zou B, C-m Li, J-y Chen, X-q Dong, Zhang Y, Du J (2012) High molecular weight persimmon tannin is a potent hypolipidemic in high-cholesterol diet fed rats. Food Res Int 48(2):970–977
Fukai S, Tanimoto S, Maeda A, Fukuda H, Okada Y, Nomura M (2009) Pharmacological activity of compounds extracted from persimmon peel (Diospyros kaki THUNB.). J Oleo Sci 58(4):213–219
Lee SO, Chung SK, Lee IS (2006) The antidiabetic effect of dietary persimmon (Diospyros kaki L. cv. Sangjudungsi) peel in streptozotocin induced diabetic rats. J Food Sci 71(3):S293–S298 1750-3841
Park YS, Leontowicz H, Leontowicz M, Namiesnik J, Jesion I, Gorinstein S (2008) Nutraceutical value of persimmon (Diospyros kaki Thunb.) and its influence on some indices of atherosclerosis in an experiment on rats fed cholesterol-containing diet. Adv Hortic Scie 22:250–254
Hagerman AE, Butler LG (1994) Assay of condensed tannins or flavonoid oligomers and related flavonoids in plants. Methods Enzymol 234:429–437
Feliciano RP, Shea MP, Shanmuganayagam D, Krueger CG, Howell AB, Reed JD (2012) Comparison of isolated cranberry (Vaccinium macrocarpon Ait.) proanthocyanidins to catechin and procyanidins A2 and B2 for use as Standards in the 4-(dimethylamino) cinnamaldehyde assay. J Agric Food Chem 60(18):4578–4585
Schofield P, Mbugua DM, Pell AN (2001) Analysis of condensed tannins: a review. Anim Feed Sci Tech 91(1):21–40 0377-8401
Sun B, Belchior GP, Ricardo-da-Silva JM, Spranger MI (1999) Isolation and purification of dimeric and trimeric procyanidins from grape seeds. J Chromatogr A 841(1):115–121
Gu H-F, Li C-M, Xu Y-j, Hu W-f, Chen M-h, Q-h Wan (2008) Structural features and antioxidant activity of tannin from persimmon pulp. Food Res Int 41(2):208–217
Li C, Trombley J, Schmidt M, Hagerman A (2010) Preparation of an acid butanol standard from fresh apples. J Chem Ecol 36(5):453–460
Li CM, Leverence R, Trombley JD, Xu SF, Yang J, Tian Y, Reed JD, Hagerman AE (2010) High molecular weight persimmon (Diospyros kaki L.) proanthocyanidin: a highly galloylated, a-linked tannin with an unusual flavonol terminal unit, myricetin. J Agric Food Chem 58(16):9033–9042
S-f Xu, Zou B, Yang J, Yao P, Li C-m (2012) Characterization of a highly polymeric proanthocyanidin fraction from persimmon pulp with strong Chinese cobra PLA2 inhibition effects. Fitoterapia 83(1):153–160
Spranger I, Sun B, Mateus AM, Freitas Vd, Ricardo-da-Silva JM (2008) Chemical characterization and antioxidant activities of oligomeric and polymeric procyanidin fractions from grape seeds. Food Chem 108(2):519–532
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In: Lester P (ed) Methods enzymol, vol 299. Academic Press, London, pp 152–178
Waterman PG, Mole S (1994) Analysis of phenolic plant metabolites, vol 83. Blackwell, New York
Hagerman AE (2002) Tannin analysis. www.users.muohio.edu/hagermae/
McMurrough I, McDowell J (1978) Chromatographic separation and automated analysis of flavanols. Anal Biochem 91(1):92–100
Prior RL, Fan E, Ji H, Howell A, Nio C, Payne MJ, Reed J (2010) Multi-laboratory validation of a standard method for quantifying proanthocyanidins in cranberry powders. J Sci Food Agr 90(9):1473–1478
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 31271833), the Chinese Ministry Program for New Century Excellent Talents in University (NCET-12-0865) Special Fund for Agro-scientific Research in the Public Interest (No. 201203047) and Fundamental Research Funds for the Central Universities (2013PY022).
Conflict of interest
None.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zou, B., Dong, Xq., Ge, Zz. et al. Development of suitable standards for quantitative determination of persimmon phenol contents in Folin-Ciocalteu and vanillin assays. Eur Food Res Technol 239, 385–391 (2014). https://doi.org/10.1007/s00217-014-2232-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-014-2232-x