Abstract
Royal jelly (RJ) fatty acids have recently been shown to possess various pharmacological and biological activities. In this work, we studied the immunomodulatory effects of 10-hydroxy-trans-2-decenoic acid (10-HDA) and 3,10-dihydroxy-decanoic acid (3,10-DDA), isolated from RJ, using a model of phytohaemagglutinin-activated human peripheral blood mononuclear cells (PBMCs). We showed that higher concentrations (500 μM) of both fatty acids inhibited the proliferation of PBMCs, and the process was followed by a decrease in the production of interleukin-2 (IL-2). 10-HDA at the concentration of 500 μM inhibited the production of IL-1β and tumor necrosis factor-α by stimulated PBMCs, whereas the same dose of 3,10-DDA had no effect on the levels of these cytokines. Regarding T helper (Th) cytokine profile, higher concentration of 10-HDA, in contrast to the lower one (50 μM), inhibited both Th1 and Th2 response, whereas Th17 response was not significantly modulated, as judged by the levels of interferon-γ, IL-5 and IL-17A in culture supernatants, respectively. Lower concentration of 3,10-DDA stimulated Th1 and Th17 responses and inhibited IL-10 production, whereas the higher dose augmented the Th2 response. In conclusion, our results showed a significant, dose-dependent, immunomodulatory effect of RJ fatty acids in vitro, which was also associated with their structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Royal jelly (RJ), a milky-white substance excreted by mandibular and hypopharyngeal glands of the worker honeybees (Apis mellifera), is a food essential for both the queen bee and her larvae. RJ has been used as cosmetic and dietary supplement due to the presence of many important compounds, such as proteins, sugars, free amino acids, fatty acids, minerals and vitamins [1, 2].
A number of pharmacological functions of RJ have been reported. These include antitumor [3], immunomodulatory [4–11] and vasodilatative [12] effects; antimicrobial [13], antiallergic [14] and antioxidative [15] properties; and many other biological activities. It is highly likely that the two most important components of RJ, proteins and fatty acids, are responsible for these different activities, especially immunomodulatory. It has been demonstrated that the major royal jelly protein 1 (MRJP1), the most abundant protein component of RJ, exerts immunostimulatory function through the induction of pro-inflammatory cytokine production [9]. Conversely, MRJP3 inhibits the production of pro-inflammatory cytokines [10] and down-regulates T helper 2 (Th2) cytokine production, especially interleukin-4 (IL-4) [8].
Medium-chain fatty acids, the unique RJ components, have attracted much attention of researches due to their chemical structures and pharmacological effects [16, 17]. In our previous works, we showed that higher doses of 10-hydroxy-trans-2-decenoic acid (10-HDA) and 3,10-dihydroxy-decanoic acid (3,10-DDA), the most important RJ fatty acids, suppressed the immune response in rats [4, 5]. Later on, we demonstrated that lower doses, especially of 3,10-DDA, stimulated the function of monocyte-derived dendritic cells (MoDCs) [6, 7], whereas higher doses were inhibitory. These dose- and structure-dependent differences in the biological activities encouraged us to study the immunomodulatory properties of these fatty acids in more detail using a model of phytohaemagglutinin (PHA)-activated human peripheral blood mononuclear cells (PBMCs). PBMCs contain different immune cell types of both innate and adaptive immunity, such as monocytes, T- and B-cells, natural killer cells and DCs, and mimic the in vivo system of immune responses very closely [18]. Therefore, the aim of this study was to analyze and compare the dose-dependent effect of 10-HDA and 3,10-DDA on proliferation and cytokine production by PHA-activated human PBMCs. The study includes innate immunity cytokines (IL-1β, IL-6, tumor necrosis factor-α (TNF-α)) and those of the Th network such as Th1, Th2, Th17 and T regulatory (Treg) cytokines. Similar work has not been performed so far.
Materials and methods
Isolation of royal jelly fatty acids
The complete process of the isolation of RJ fatty acids was previously described [16]. Briefly, fresh RJ was lyophilized and extracted for 48 h with dichloromethane and subsequently with methanol. After evaporation of the solvent from the dichloromethane extract, the residue was submitted to several chromatographic separations and afforded 10-HDA. After evaporation of the solvent from the methanol extract, the residue was diluted with distilled water and extracted with ethyl acetate. The ethyl acetate residue was then submitted to several chromatographic separations, affording 3,10-DDA. Isolated fatty acids were identified by comparison of their nuclear magnetic resonance (NMR) (heteronuclear multiple-quantum coherence, HMQC and heteronuclear multiple-bond coherence, HMBC) and mass spectrometry data with literature values. Purity of these fatty acids was 98 % (analyzed by high-performance liquid chromatography). 10-HDA and 3,10-DDA were dissolved in dimethylsulfoxide (DMSO) (Sigma, Munich, Germany) and then in RPMI 1640 medium (Sigma) at final concentrations of 2 mM. Final concentrations of DMSO did not exceed 0.4 %. The chemical structures of these fatty acids are shown in Fig. 1.
Preparation of peripheral blood mononuclear cell culture
PBMCs were isolated from buffy coats obtained from five healthy volunteers by density gradient centrifugation (Lymphoprep, PAA Vienna, Austria). The Ethical Committee of Military Medical Academy, Belgrade, Serbia, has approved the use of human cells for in vitro research. Cells were washed in phosphate-buffered saline (PBS) and resuspended in RPMI 1640 medium (Sigma, Munich, Germany) supplemented with 2 mM l-glutamine, antibiotics [20 μg/ml of gentamycin (ICN, Costa Mesa, CA, USA), 100 U/ml of penicillin and 100 μg/ml of streptomycin (both from Galenika, Belgrade, Serbia)], 50 μM 2-mercaptoethanol (2-ME) (Sigma, Munich, Germany) and 10 % heat-inactivated fetal calf serum (FCS) (ICN, Costa Mesa, CA, USA).
Viability and apoptosis assay
PBMCs (1 × 105 cells/tube) were collected, washed with PBS, stained with propidium iodide (PI) (10 μg/ml of PBS) (Sigma) and analyzed by flow cytometry (EPICS XL-MCL, Krefeld, Germany). PI-stained cells were considered as dead cells. Viability of the cells was higher than 95 %. Apoptosis was determined after staining of PBMCs with PI (10 μg/ml PI dissolved in hypotonic citrate buffer) and incubation of the cells for 6 h in dark. After that, the cells were analyzed using flow cytometry (EPICS XL-MCL). PBMCs with hypodiploid nuclei were identified as apoptotic cells, and the results were presented as percentages.
Proliferation assay
For the cell proliferation assay, the PBMCs were plated in 96-well plates (ICN Costa Mesa, CA, USA) (2 × 106 cells/ml; 200 μl of PBMC suspension), cultivated with PHA (Serva) (30 μg/ml) alone or PHA with different concentrations of RJ fatty acids and incubated for 3 days in an incubator (Flow, Irvine, UK) with 5 % CO2 at 37 °C. After 72 h, the cultures were pulsed with [3H] thymidine (1 μCi/well, Amersham, Buckinghamshire, UK) during the last 18 h of cultivation. After harvesting, the radioactivity was measured using a scintillation counter (Beckman). The results were expressed as proliferation index.
Cytokine assay
PHA-stimulated PBMCs were cultivated in 96-well flat-bottomed plates (2 × 106 cells/ml; 200 μl of PBMCs suspension) with or without RJ fatty acids, as described previously. After 72 h, the supernatants were collected and stored at −40 °C for later analysis of cytokine levels. The concentrations of IL-2, interferon-γ (IFN-γ), IL-5, IL-17A, IL-10, IL-1β, IL-6 and TNF-α in the supernatants were determined using sandwich ELISA kits (R&D Systems, Minneapolis, USA), according to the manufacturer’s instructions.
Statistical analysis
Data are presented as mean ± standard deviation (SD). Statistical analysis was performed using GraphPad Prism software (GraphPad, San Diego, CA, USA). Significant differences were assessed using one-way ANOVA followed by the Dunnett’s multiple comparison test. Values of p < 0.05 or less were considered as statistically significant.
Results
Effect of RJ fatty acids on the proliferation of lymphocytes
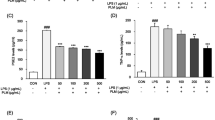
PBMCs were stimulated with PHA and different concentrations of 10-HDA or 3,10-DDA, respectively. PBMCs stimulated with PHA alone served as a control. Results presented in Fig. 2a show that only the highest concentration of both fatty acids (500 μM) significantly inhibited cellular proliferation. Lower concentrations of 3,10-DDA stimulated PHA-induced proliferation of PBMCs, but because of interdonor variability, the differences were not statistically significant. Inhibited proliferation was not a consequence of cell apoptosis (Fig. 2b), but was associated with inhibited production of IL-2 (Fig. 2c).
Effect of 10-HDA and 3,10-DDA on proliferation (a), apoptosis (b) and IL-2 production (c) by PBMCs in culture. PBMCs were cultivated with PHA alone or PHA with different concentrations of RJ fatty acids for 3 days. a Cells were pulsed with [3H]-thymidine for the last 18 h. Proliferation was measured by [3H]-thymidine uptake. Values are presented as proliferation index (mean ± SD; n = 5). b Control and RJ fatty acid-treated PBMCs were collected, washed with PBS, stained with PI and analyzed by flow cytometry. Apoptotic cells were identified as the cells with hypodiploid nuclei. Values are expressed as percentages of apoptosis ± SD (n = 5). c Supernatants of PBMC cultures were collected and used for IL-2 detection by ELISA. Results are presented as mean ± SD (n = 5). *p < 0.05 compared to the control
Effect of RJ fatty acids on the production of pro-inflammatory cytokines
Based on cell proliferation studies, we decided to examine a lower (50 μM) and the highest (500 μM) dose of RJ fatty acids on cytokine production of PHA-activated PBMCs. At first, we studied the production of three pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α). Results are shown in Fig. 3. It can be seen that neither concentration of 3,10-DDA modulates the secretion of all these cytokines. However, higher concentration of 10-HDA significantly suppressed the production of TNF-α and IL-1β.
Effect of 10-HDA and 3,10-DDA on the production of pro-inflammatory cytokines by PBMCs. Supernatants of PBMC cultures with PHA and with or without RJ fatty acids were collected after 3 days of cell cultivation and used for TNF-α, IL-1β and IL-6 detection by ELISA. Values are expressed as mean ± SD (n = 5). **p < 0.01 compared to the control
Effect of RJ fatty acids on the Th profile of cytokines
The supernatants of PHA-stimulated PBMCs were tested for the presence of four key cytokines produced by effector CD4+ cells: IFN-γ (a dominant Th1 cytokine), IL-5 (a main representative of Th2 cytokines), IL-17A (a key cytokine of Th17 cells) and IL-10 (a key immunoregulatory cytokine). As presented in Fig. 4, lower concentration of 3,10-DDA stimulated the production of IFN-γ and IL-17A, but down-regulated the production of IL-10. In contrast, higher concentration of this fatty acid stimulated the production of IL-5. Lower concentration of 10-HDA did not significantly modulate the production of all tested Th cytokines, in contrast to the higher one, which inhibited the production of IFN-γ and IL-5.
Th polarizing capability of 10-HDA and 3,10-DDA. PBMCs were stimulated with PHA alone or with two different concentrations of 10-HDA and 3,10-DDA for 72 h. Production of IFN-γ, IL-5, IL-17A and IL-10 was measured by ELISA in the culture supernatants. Cytokine levels are expressed as mean ± SD (n = 5). *p < 0.05; **p < 0.01 compared to the control
Discussion
In this work, we studied the effects of 10-HDA and 3,10-DDA, free fatty acids isolated from RJ, on proliferation and cytokine production by PHA-activated human PBMCs in vitro. We found that these fatty acids exhibited different immunomodulatory activities, which were associated with their concentrations and structure.
Both RJ fatty acids, at higher concentrations (500 μM), significantly suppressed PBMC proliferation, and the effect was not associated with cellular apoptosis. Since PHA is a T-cell mitogen, it can be postulated that RJ fatty acids suppressed the proliferation of T cells. Antiproliferative effect has already been described for RJ components including 10-HDA [4, 5]. In this context, it was logical to test the level of IL-2 of PBMC cultures bearing in mind that IL-2 is a dominant T-cell growth factor [19]. We showed that the inhibited proliferation of PBMCs correlated with a decreased production of IL-2, suggesting that both fatty acids interfere with signaling required for IL-2 production. However, it remains to be studied weather these fatty acids also inhibited IL-2 receptor alpha expression, as we demonstrated in our previous work on rat spleen T cells using the same concentration of 10-HDA [5].
Although anti-inflammatory properties of RJ have been reported in several studies [11, 20–22], very few of them have been focused on the involvement of RJ fatty acids in these processes. Up to now, it has been shown that 10-HDA inhibited TNF-α production induced by IFN-β and IFN-γ [23, 24] and IL-6 production, induced by LPS, by murine macrophages [25]. We showed that higher concentration of 10-HDA inhibited the production of TNF-α and IL-1β by human PBMCs stimulated with PHA, whereas the secretion of IL-6 was unimpaired. In contrast, 3,10-DDA had no detectable anti-inflammatory properties as judged by this experimental approach. Since production of both TNF-α and IL-1β requires activation of nuclear factor-kappa B (NF-κB) [26], it can be postulated that this transcription factor could be a target of 10-HDA. Based on the experiments in murine macrophages, it seems that 10-HDA inhibits certain NF-κB-dependent genes, rather than the whole activity of NF-κB. This hypothesis is based on the finding that 10-HDA had no effect on LPS-induced IFN-β gene expression in RAW264 cells [23, 25]. The mechanism by which 10-HDA inhibits LPS-induced IL-6 production by macrophages has been recently studied by Sugiyama et al. [25]. They hypothesized that 10-HDA inhibits a subset of NF-κB that activates the promoter of inhibitor of NF-κB zeta, a transcription factor activating the κB promoter sequence, which may be regulated by post-translational modification of NF-κB subunits. The differences, regarding inhibited TNF-α production versus unimpaired production of IL-6 in our culture system, could be explained by different activators of PBMCs and different cell sources of IL-6. For example, although monocytes/macrophages are the main IL-6-producing cells, the subsets of T cells also secrete this cytokine [27].
Our study is the first one examining the modulatory effect of RJ fatty acids on Th responses, simultaneously, in a model of PHA-activated PBMCs. There are at least four types of Th cells, Th1, Th2, Th17 and Tregs. Th1 cells produce IFN-γ as their signature cytokine, whereas Th2 cells secrete IL-4, IL-5 and IL-13. The third major effector population of CD4+ T cells, Th17 cells, produces IL-17A and IL-22. Th1, Th2 and Th17 cells play a protective role against intracellular pathogens, helminths and extracellular bacteria/fungi, respectively. Moreover, Th1 and Th17 cells have been thought to be responsible for many autoimmune diseases, whereas Th2 cells have been shown to be crucial in pathogenesis of allergic inflammation. Treg cells mainly produce IL-10 and transforming growth factor beta (TGF-β), which are critical in maintaining self-tolerance and in modulating immune responses to infections [19, 28].
To our surprise, we found that 10-HDA and 3,10-DDA differ substantially in their influence on Th cytokine levels and Th polarizing capability. Lower concentration of 3,10-DDA increased the levels of IFN-γ and IL-17A, but decreased the level of IL-10. In contrast, higher concentration of 3,10-DDA stimulated the Th2 response, as judged by the increased level of IL-5. Lower concentration of 10-HDA did not modify any of these responses compared with higher concentration, which significantly reduced Th1 and Th2. Although modulatory effect of 10-HDA and 3,10-DDA on Th1 and Th2 responses has already been investigated in our previous papers [6, 7] using an allogeneic T-cell system, this is the only report related to the effect of RJ and its components on the Th17 response.
The effect of RJ fatty acids on Th subsets may be direct by targeting T cells or indirect via antigen-presenting cells (APCs). DCs, as a key population of APCs, are the main regulators of Th responses [29] and a target of the action of both 10-HDA and 3,10-DDA [6, 7]. In this context, it can be postulated that the stimulatory activity of lower concentration of 3,10-DDA on IFN-γ and IL-17A production may be dependent on IL-12 and IL-23 produced by DCs within PBMCs, which drive Th1 and Th17 responses, respectively [19]. Such a phenomenon has not been recognized so far and may be relevant if 3,10-DDA will be considered as an immunostimulator for conditions where both Th1 and Th17 immune responses need to be enhanced, such as tumors and various infections. This is of particular interest because lower doses of 3,10-DDA decreased IL-10 production. By inhibiting the down-modulatory activity of IL-10 on the immune response [30], the activity of Th1 and Th17 cells will be additionally potentiated.
Stimulation of the Th2 response by higher concentrations of 3,10-DDA is an interesting finding that has not been described yet for any RJ components and may be specific for 3,10-DDA. The increased Th2 response was not a consequence of lower Th1 response, bearing in mind the mutual antagonistic effect between Th1 and Th2 cytokines [19]. The only paper that might be relevant to our work is related to a study of mice fed with dietary fish oil containing various saturated fatty acids, including those of medium chain [31]. The authors demonstrated an enhanced secretion of Th2-type cytokines, which was indirectly mediated by CD11b+ cells, belonging to macrophages and DCs. If this hypothesis is true in our culture model, the molecular mechanisms involved in interactions between DCs and T cells responsible for Th2 polarization remain to be elucidated. Biologically, promotion of Th2 response may have beneficial effect on eradication of helminth infections, but at the same time, it can exaggerate reactions in individuals suffering from allergy.
The inhibitory effect of higher concentration of 10-HDA on Th1 and Th2 responses may also be dependent on accessory cells within PBMCs, since we observed the same phenomenon when MoDCs were pretreated with 500 μM of 10-HDA before their co-cultivation with allogeneic CD4+ T cells [7]. In this context, this finding may be relevant considering 10-HDA as an immunosuppressive agent for the treatment of undesired immune reactions in autoimmune diseases, allergy or transplantation.
The mechanisms by which 10-HDA and 3,10-DDA perform their immunomodulatory and anti-inflammatory activities are not clear. Previous reports demonstrated that NF-κB [23–25], p38 kinase and c-Jun N-terminal kinase-AP-1 [32] signaling pathways mediated the effects of 10-HDA. However, studies showing direct evidence of 10-HDA and 3,10-DDA binding sites are lacking. It is well documented that fatty acids exert much of their effects acting as natural ligands of G protein-coupled receptors (GPR40, GPR43, GPR84 and GPR120) and several transcription factors including peroxisome proliferator-activated receptors (PPARs), sterol regulatory element-binding proteins and liver X receptors [33]. PPARγ is a member of the nuclear receptor family of transcription factors that are activated by a variety of dietary and endogenous fatty acids [34]. Malapaka et al. [35] have recently demonstrated that decanoic acid, a 10-carbon fatty acid, is a direct ligand of PPARγ and partially activates this receptor. It has been shown that PPARγ agonists inhibit the production of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) in phorbol myristyl acetate-activated peripheral blood monocytes [36] and decrease TNF-α expression and NF-κB activity in porcine PBMCs [37]. Ligation of PPARγ might also suppress IL-2, IFN-γ, IL-4 and IL-5 production in T cells [34]. In this context, we speculate that 10-HDA achieves its immunomodulatory effects predominantly through PPARγ-dependent pathway. In our study, we did not observe any changes in the production of IL-6 and IL-17A although PPARγ agonists decrease the secretion of these cytokines [34, 36, 38]. An explanation could be that medium-chain fatty acids are low-potency partial agonists of PPARγ [39]. Therefore, it will be important to evaluate the direct effect of 10-HDA on PPARγ activity in the next experiments.
The difference in the activity between 10-HDA and 3,10-DDA may be hypothetically explained by the hypothesis that 3,10-DDA predominantly acts through another signaling pathway. Wang et al. [40] showed that GPR84, a cell-surface G protein-coupled receptor, functions as a receptor for C9-14 free fatty acids, especially those of C10. GPR84 is activated by medium-chain fatty acid with the hydroxyl group at the 2 or 3 position more effectively than monohydroxylated fatty acids [41]. In addition, activation of GPR84 in LPS-stimulated RAW264.7 cells by medium-chain fatty acids increased the secretion of IL-12 [40]. In our previous paper, we showed that 3,10-DDA-treated MoDCs significantly augmented the production of IL-12 [6]. As already mentioned, this cytokine plays a crucial role in promoting differentiation of naïve CD4 + T cells into Th1 effector cells, which primarily secrete IFN-γ. It is possible that 3,10-DDA at low concentrations mostly binds to and activates GRP84, whereas at high concentrations interferes with other receptors such as PPARγ and GPR120.
In conclusion, we showed that 10-HDA and 3,10-DDA, RJ fatty acids, exerted immunomodulatory activities using an in vitro model of PHA-activated PBMCs. Higher doses of 10-HDA inhibited the proliferation of human PBMNC in vitro, decreased IL-1β and TNF-α production and suppressed Th1 and Th2 immune responses. Based on these activities, the compound might be an effective immunosuppressive agent. In contrast, lower concentrations of 3,10-DDA favored Th1 and Th17 immune responses, which is desired for the stimulation of immune system against tumor and infectious agents.
References
Ramadan MF, Al-Ghamdi A (2012) Bioactive compounds and health-promoting properties of royal jelly: a review. J Funct Foods 4:39–52
Viuda-Martos M, Ruiz-Navajas Y, Fernández-López J, Pérez-Álvarez JA (2008) Functional properties of honey, propolis, and royal jelly. J Food Sci 73:R117–R124
Bincoletto C, Eberlin S, Figueiredo CA, Luengo MB, Queiroz ML (2005) Effects produced by Royal Jelly on haematopoiesis: relation with host resistance against Ehrlich ascites tumour challenge. Int Immunopharmacol 5:679–688
Gasic S, Vucevic D, Vasilijic S, Antunovic M, Chinou I, Colic M (2007) Evaluation of the immunomodulatory activities of royal jelly components in vitro. Immunopharmacol Immunotoxicol 29:521–536
Vucevic D, Melliou E, Vasilijic S, Gasic S, Ivanovski P, Chinou I, Colic M (2007) Fatty acids isolated from royal jelly modulate dendritic cell-mediated immune response in vitro. Int Immunopharmacol 7:1211–1220
Dzopalic T, Vucevic D, Tomic S, Djokic J, Chinou I, Colic M (2011) 3,10-Dihydroxy-decanoic acid, isolated from royal jelly, stimulates Th1 polarising capability of human monocyte-derived dendritic cells. Food Chem 126:1211–1217
Mihajlovic D, Rajkovic I, Chinou I, Colic M (2013) Dose-dependent immunomodulatory effects of 10-hydroxy-2-decenoic acid on human monocyte-derived dendritic cells. J Funct Foods 5:838–846
Okamoto I, Taniguchi Y, Kunikata T, Kohno K, Iwaki K, Ikeda M, Kurimoto M (2003) Major royal jelly protein 3 modulates immune responses in vitro and in vivo. Life Sci 73:2029–2045
Majtan J, Kovacova E, Bilikova K, Simuth J (2006) The immunostimulatory effect of the recombinant apalbumin 1-major honeybee royal jelly protein-on TNFalpha release. Int Immunopharmacol 6:269–278
Kohno K, Okamoto I, Sano O, Arai N, Iwaki K, Ikeda M, Kurimoto M (2004) Royal jelly inhibits the production of proinflammatory cytokines by activated macrophages. Biosci Biotechnol Biochem 68:138–145
Mannoor MK, Shimabukuro I, Tsukamotoa M, Watanabe H, Yamaguchi K, Sato Y (2009) Honeybee royal jelly inhibits autoimmunity in SLE-prone NZB × NZW F1 mice. Lupus 18:44–52
Matsui T, Yukiyoshi A, Doi S, Sugimoto H, Yamada H, Matsumoto K (2002) Gastrointestinal enzyme production of bioactive peptides from royal jelly protein and their antihypertensive ability in SHR. J Nutr Biochem 13:80–86
Fontana R, Mendes MA, de Souza BM, Konno K, Cesar LM, Malaspina O, Palma MS (2004) Jelleines: a family of antimicrobial peptides from the Royal Jelly of honeybees (Apis mellifera). Peptides 25:919–928
Oka H, Emori Y, Kobayashi N, Hayashi Y, Nomoto K (2001) Suppression of allergic reactions by royal jelly in association with the restoration of macrophage function and the improvement of Th1/Th2 cell responses. Int Immunopharmacol 1:521–532
Jamnik P, Goranovic D, Raspor P (2007) Antioxidative action of royal jelly in the yeast cell. Exp Gerontol 42:594–600
Melliou E, Chinou I (2005) Chemistry and bioactivity of royal jelly from Greece. J Agric Food Chem 53:8987–8992
Isidorov VA, Czyżewska U, Jankowska E, Bakier S (2011) Determination of royal jelly acids in honey. Food Chem 124:387–391
Jenny M, Klieber M, Zaknun D, Schroecksnadel S, Kurz K, Ledochowski M, Schennach H, Fuchs D (2011) In vitro testing for anti-inflammatory properties of compounds employing peripheral blood mononuclear cells freshly isolated from healthy donors. Inflamm Res 60:127–135
Zhu J, Yamane H, Paul WE (2010) Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol 28:445–489
Erem C, Deger O, Ovali E, Barlak Y (2006) The effects of royal jelly on autoimmunity in Graves’ disease. Endocrine 30:175–183
Karaca T, Bayiroglu F, Yoruk M, Kaya MS, Uslu S, Comba B, Mis L (2010) Effect of royal jelly on experimental colitis Induced by acetic acid and alteration of mast cell distribution in the colon of rats. Eur J Histochem 54:e35
Sugiyama T, Takahashi K, Mori H (2012) Royal jelly acid, 10-hydroxy-trans-2-decenoic acid, as a modulator of the innate immune responses. Endocr Metab Immune Disord Drug Targets 12:368–376
Sugiyama T, Takahashi K, Kuzumaki A, Tokoro S, Neri P, Mori H (2013) Inhibitory mechanism of 10-hydroxy-trans-2-decenoic acid (royal jelly acid) against lipopolysaccharide- and interferon-beta-induced nitric oxide production. Inflammation 36:372–378
Takahashi K, Sugiyama T, Tokoro S, Neri P, Mori H (2012) Inhibition of interferon-gamma-induced nitric oxide production by 10-hydroxy-trans-2-decenoic acid through inhibition of interferon regulatory factor-8 induction. Cell Immunol 273:73–78
Sugiyama T, Takahashi K, Tokoro S, Gotou T, Neri P, Mori H (2012) Inhibitory effect of 10-hydroxy-trans-2-decenoic acid on LPS-induced IL-6 production via reducing IkappaB-zeta expression. Innate Immun 18:429–437
Vallabhapurapu S, Karin M (2009) Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol 27:693–733
Zubiaga AM, Munoz E, Merrow M, Huber BT (1990) Regulation of interleukin 6 production in T helper cells. Int Immunol 2:1047–1054
Romagnani S, Maggi E, Liotta F, Cosmi L, Annunziato F (2009) Properties and origin of human Th17 cells. Mol Immunol 47:3–7
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392:245–252
Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG (2011) Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol 29:71–109
Petursdottir DH, Hardardottir I (2009) Dietary fish oil decreases secretion of T helper (Th) 1-type cytokines by a direct effect on murine splenic T cells but enhances secretion of a Th2-type cytokine by an effect on accessory cells. Br J Nutr 101:1040–1046
Yang XY, Yang DS, Wei Z, Wang JM, Li CY, Hui Y, Lei KF, Chen XF, Shen NH, Jin LQ, Wang JG (2010) 10-Hydroxy-2-decenoic acid from Royal jelly: a potential medicine for RA. J Ethnopharmacol 128:314–321
Georgiadi A, Kersten S (2012) Mechanisms of gene regulation by fatty acids. Adv Nutr 3:127–134
da Rocha Junior LF, Dantas AT, Duarte AL, de Melo Rego MJ, Pitta Ida R, Pitta MG (2013) PPAR gamma agonists in adaptive immunity: what do immune disorders and their models have to tell us? PPAR Res 2013:519724
Malapaka RR, Khoo S, Zhang J, Choi JH, Zhou XE, Xu Y, Gong Y, Li J, Yong EL, Chalmers MJ, Chang L, Resau JH, Griffin PR, Chen YE, Xu HE (2012) Identification and mechanism of 10-carbon fatty acid as modulating ligand of peroxisome proliferator-activated receptors. J Biol Chem 287:183–195
Jiang C, Ting AT, Seed B (1998) PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature 391:82–86
Kim DI, Kim KH, Kang JH, Jung EM, Kim SS, Jeung EB, Yang MP (2011) Trans-10, cis-12-conjugated linoleic acid modulates NF-kappaB activation and TNF-alpha production in porcine peripheral blood mononuclear cells via a PPARgamma-dependent pathway. Br J Nutr 105:1329–1336
Klotz L, Burgdorf S, Dani I, Saijo K, Flossdorf J, Hucke S, Alferink J, Nowak N, Beyer M, Mayer G, Langhans B, Klockgether T, Waisman A, Eberl G, Schultze J, Famulok M, Kolanus W, Glass C, Kurts C, Knolle PA (2009) The nuclear receptor PPAR gamma selectively inhibits Th17 differentiation in a T cell-intrinsic fashion and suppresses CNS autoimmunity. J Exp Med 206:2079–2089
Liberato MV, Nascimento AS, Ayers SD, Lin JZ, Cvoro A, Silveira RL, Martinez L, Souza PC, Saidemberg D, Deng T, Amato AA, Togashi M, Hsueh WA, Phillips K, Palma MS, Neves FA, Skaf MS, Webb P, Polikarpov I (2012) Medium chain fatty acids are selective peroxisome proliferator activated receptor (PPAR) gamma activators and pan-PPAR partial agonists. PLoS ONE 7:e36297
Wang J, Wu X, Simonavicius N, Tian H, Ling L (2006) Medium-chain fatty acids as ligands for orphan G protein-coupled receptor GPR84. J Biol Chem 281:34457–34464
Suzuki M, Takaishi S, Nagasaki M, Onozawa Y, Iino I, Maeda H, Komai T, Oda T (2013) Medium-chain fatty acid-sensing receptor, GPR84, is a proinflammatory receptor. J Biol Chem 288:10684–10691
Acknowledgments
This study has been supported by the following grants: Ministry of Education and Science, R. Serbia (Project No.: 175102); Military Medical Academy, Belgrade, Serbia (Project No: VMA 07-10/B.23); Serbian Academy of Science and Arts; The General Secretariat of Research and Technology of Greece (PENED project). The authors thank S. Vasilijic, S. Gasic, I. Majstorovic and Z. Stojic-Vukanic for their assistance in this work.
Conflict of interest
None.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mihajlovic, D., Vucevic, D., Chinou, I. et al. Royal jelly fatty acids modulate proliferation and cytokine production by human peripheral blood mononuclear cells. Eur Food Res Technol 238, 881–887 (2014). https://doi.org/10.1007/s00217-014-2154-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-014-2154-7